- *Corresponding Author:
- R. D. Jangle
Department of Pharmaceutical Biotechnology, Poona College of Pharmacy, Bharati Vidyapeeth University, Pune–411 038
E-mail: rdjangle@gmail.com
| Date of Submission | May 25, 2011 |
| Date of Revision | March 01, 2012 |
| Date of Acceptance | March 06, 2012 |
| Indian J Pharm Sci, 2012, 74 (2): 91‑100 |
Abstract
Vacuum foam drying is evaluated as an alternative for lyophilization for enhanced process and storage stability of bovine serum albumin. The protein protective efficiency of different stabilizers was compared in vacuum foam drying and lyophilization. Sucrose mixtures produced better foam characters than mannitol. Unlike calcium lactate, incorporation of polyvinyl pyrrolidone to sucrose synergistically enhanced the recovery of bovine serum albumin. The conformational stability and bovine serum albumin content further increased with sodium phosphate as compared to potassium phosphate. All sucrose mixtures, except calcium lactate showed large αâ??helix amideâ??I band at approximately 1656 cmâ??1. The amorphous powder diffraction in case of sodium phosphate monobasic mixture retained maximum bovine serum albumin content. The crystallization of similar mixtures in lyophilization reduced its bovine serum albumin content. Vacuum foam drying showed better processing and storage stability of bovine serum albumin than lyophilization process. Hence vacuum foam drying is short, simple and industrially economical process for biomolecules preservation.
Keywords
Bovine serum albumin, inorganic phosphates, lyophilization, saccharides, vacuum foam drying
Recent advances in recombinant DNA technology have resulted in a great number of pharmacologically active proteins with a potential to enter pharmaceutical formulations. Pharmaceutically acceptable shelf-life is often imparted to the therapeutic protein by formulating in solid dosage form using lyophilization [1]. It is necessary to use appropriate amorphous excipient (e.g. sucrose) to avoid denaturation of proteins after long-term storage in the dried solid form or lyophilization and final reconstitution [2-4]. With increasing complexity of biological materials with regard to structure, activity and metabolism, prospects for successful preservation by freeze drying are decreasing. Lyophilization is extensively applied, but generates variety of stresses to unfold/denature proteins. These include low temperature stresses, freezing stresses (formation of dendritic ice crystals) increased ionic strength, changed pH and drying stresses due to the removal of protein hydration shell. Cold denaturation (i.e. structural change) was evident after incubation of frozen ovalbumin solution, which increased with decreasing temperature between ‑10 and ‑40° [5]. Morana et al. showed that the S‑adinosyl‑L‑methionine (SAM) was completely damaged during lyophilization. The addition of trehalose protected the enzyme during lyophilization [6]. Trehalose glasses surrounded SAM and arrested their conformational changes and thus protected the enzyme. Remmele et al. investigated the role of sucrose in stabilization of the protein structure [7]. Sucrose minimized the perturbations of protein structure caused by freezing and dehydration stresses encountered during lyophilization. Martin et al. demonstrated that stabilization of lysozyme depends on excipient to enzyme mass ratio. The freeze dried lysozyme appears to be less effectively stabilized compared to the spray dried form [8]. Carpenter et al. showed phosphofructokinase purified from rabbit skeletal muscle, is fully inactivated after freeze drying. The addition of trehalose or maltose to the enzyme solution prior to freeze drying resulted in recovery up to 80% [9]. The addition of zinc to the enzyme‑sugar mixture prior to freeze drying greatly enhanced the stability. Although, previous studies on optimizing additives for virus protection during lyophilization have increased the titer, the process was time consuming and caused loss of virus potency [10].
Vacuum foam drying (VFD) is the potential approach coming in to the use recently for the preservation of sensitive biological materials. Research in our laboratory has shown its applicability for preservation of virus. The concept arrived is applied in processing of sugar‑phosphate mixture by vacuum foam drying to obtain amorphous glassy matrix for model protein (Bovine Serum albumin, BSA). In VFD process, liquid samples are dehydrated by boiling under vacuum to form stable foams. This is performed under vacuum above freezing point of solution, but significantly below 100°. These suspensions of sensitive biologicals are transformed into mechanically stable dry foams. The foams consist of thin films, from which water can be efficiently removed during subsequent stability drying. Bronshtein, has patented a scalable long‑term preservation technique for sensitive biomolecules. Biotherapeutics stable at higher pressures (erythropoietin, enzymes, vaccines) can be stabilized with pharmaceutical excipients using vacuum foam drying [11]. Sucrose, a nonreducing and chemically inert sugar, has been frequently added to protein formulations as stabilizer. Its stabilizing ability was assumed to be a function of hydrogen bonding ability and transformation into glassy state. Sucrose tends to crystallize out during freeze or spray drying [12]. Herein, we have studied the effect of the physical state of sucrose on BSA stability during processing and storage. Inorganic phosphates, having similar chemical structure to borate could arrest nucleation and hence crystallization of sucrose. The effect of sucrose‑phosphate mixtures and sucrose‑borate mixtures with glass forming agents on stability of vacuum foam dried and lyophilized BSA was investigated.
Materials and Methods
Albumin, Bovine fraction extrapure grade and polyvinyl pyrrolidone K30 (PVP, M.W. 40 kDa) were obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. Sodium hydrogen orthophosphate (anhydrous), potassium dihydrogen orthophosphate (anhydrous) were purchased from Merck Ltd. Mumbai, India. Disodium tetraborate (Borax), calcium lactate and sucrose (GR) were from Loba Chemie Pvt. Ltd., Mumbai, India. Deionized water obtained using NANOpure Diamond water purification system (Barnstead Thermolyne, Dubuque, IA).
Effect of pH on BSA solutions
The buffered BSA solutions were prepared aseptically. BSA, 1 mg/ml was dissolved in buffer solutions of pH 2.5, 4.5, 5.5, 7.5 and 8.5 separately [13]. Five milliliters of each solution was stored in rubber stoppered, crimped glass vial wrapped in aluminum foil. The vials in triplicate were kept 25° and 2‑4° for 7 and 14 days. BSA content was evaluated at the end by Lowry method [14].
Screening of protectants for vacuum foam dried BSA
Aqueous solutions were prepared containing sucrose or mannitol (15, 20, 22.5, 25 and 30%) and 10 mg/ml BSA, each separately, in 50 ml beaker. Accurately measured 2 ml of each solution was filled in clean and dry vials. The rubber closures were half‑closed and the solutions were vacuum foam dried using Heto LyoPro 3000 lyophilizer at temperature 25‑30° (Pressure 0.06 hPa, 24 h). The vacuum sealed dried products were evaluated for product features, BSA content and moisture content.
Effect of PVP and calcium lactate on VFD processed products
Aqueous solutions containing 10 mg/ml of BSA, sucrose 25% w/v (optimized), PVP and Ca‑lactate in the concentration range of 0.5‑2.5% w/v, each separately, were prepared. Accurately measured 2 ml of final solution was filled in 10 ml glass vials. The rubber closures were positioned as half‑closed. The solutions were processed as per VFD. The dried products were analyzed for residual BSA content along with product features and residual moisture.
Inorganic phosphates and sodium tetraborate
The bulk solution of sucrose (25% w/v) containing BSA (10 mg/ml), 1.5% w/v PVP (optimized) were prepared in deionized water. Accurately weighed amount of sodium phosphate (monobasic), potassium phosphate (monobasic), each separately at 0.25‑1.25 M, and sodium tetraborate at 25‑175 mM concentrations were added to sucrose solution. The pH of resultant solutions or dispersions were measured and then subjected to VFD. The dried products were analyzed for residual BSA content and residual moisture.
Evaluation of vacuum foam dried mixtures
BSA (protein) content in foam was estimated by Lowery method used for total protein estimation [14]. After rehydration, sample was centrifuged to remove any aggregate formed in the process and supernatant was taken for analysis. The method is sensitive as it involves two color‑forming reactions. In the Biuret reaction, Cu2+ (in the presence of base) reacts with the peptide bond to produce a deep blue color. Here a complex mixture of inorganic salts reacts with tyrosine and tryptophan residues of peptides to produce intense blue‑green color. The reaction was initiated by addition of 100 μl of BSA solution (1 mg/ml) to reagent solutions as given in the standard procedure of the method. The absorbance of each sample was measured at 662 nm using UV/Vis spectrophotometer (JascoV‑530). The percentage protein content was determined from the standard curve and was measured relative to fresh samples. Each sample was assayed in triplicate.
Residual moisture
Moisture content of the dried product was determined by Karl Fischer titrator. The amount of moisture was determined equivalent to the Karl Fischer reagent. Twenty microlitre of anhydrous methanol was transferred to titration vessel. To this, accurately weighed sample was added and titration was carried out to the electromagnetic end point. Ten microlitres of water were accurately measured and titrated to the end point as reference [15].
XRPD analysis
The morphology of the vacuum foam dried product was studied by X‑ray powder diffraction pattern using X‑Ray diffractometer, Philips PW 3710, Holland, (2θ range of 2–50o, scan speed of 0.1o 2θ/sec). The radiation of (l) 1.524 Ao wavelength was used [16].
Stability studies
The dried mixtures with optimum BSA content were subjected to short‑term stability [17]. Stability was studied with respect to BSA content retention, moisture content and morphological changes in the product after stored at particular conditions for specific period. Twenty five vials of optimized finished vacuum foam dried products were placed in stability ovens at 25° and 40°/75% RH for stability assessment. Stability samples were pulled periodically for analysis. All stability formulations were assayed by Lowry method for protein content and characterized using Karl Fischer titrator and XRPD.
Lyophilization of optimized mixtures
An aqueous BSA (10 mg/ml) solution containing plain 25% w/v sucrose, plain 25% w/v mannitol, 25% w/v sucrose and 1.5% w/v PVP, previous solution replacing PVP with 1.5% w/v calcium lactate and 25% w/v sucrose, 1.5% w/v PVP, 0.5 M sodium phosphate monobasic (SPM) and other two solutions prepared by replacing sodium phosphate with 0.5 M potassium phosphate (monobasic) and 100 mM sodium tetraborate were prepared in deionized water. Accurately measured 2 ml of each solution was filled in glass vials. The rubber stoppers were placed and the solutions were frozen at –40° for three hours. Ten vials of each solution were lyophilized using Virtis Advantage lyophilizer (primary drying at –50° condenser temperature, 10 hPa pressure, 48 h; secondary drying at –25° shelf temperature, 48 h, 0.06 hPa pressure) [10]. The vials were vacuum‑sealed and dried cakes were analyzed identical to vacuum foam dried mixtures.
Results and Discussion
Among the critical factors affecting the solution stability of BSA are pH and temperature. The results of pH stability of BSA (1 mg/ml) in buffered solutions of pH 2.5‑8.5 at 25° and 2‑4° are shown in Table 1. Aqueous solutions of BSA at all pH were stable at cold storage (7 days), while higher temperature showed loss of BSA after 14 days. The result revealed that BSA solutions are stable in the pH range of 4‑6. The degradation of BSA in solution occurs by aggregation, deamidation, and oxidation. Moving the solution pH away from the isoelectric point denatures many proteins. BSA has an isoelectric point at pH 5.4.
| pH range | After 7 days protein | After 14 days protein | ||
|---|---|---|---|---|
| content (%) | content (%) | |||
| 2-4° | 25° | 2-4° | 25° | |
| 2.5 | 82.8±2 | 72.6±4 | 61.5±2 | 46.9±2 |
| 4.5 | 86.7±3 | 70.1±1 | 64.5±3 | 50.7±1 |
| 5.5 | 98.1±2 | 79.4±3 | 73.5±2 | 57.1±3 |
| 7.5 | 87.2±1 | 48.9±2 | 50.2±3 | 46.4±2 |
| 8.5 | 86.7±3 | 56.6±3 | 56.6±1 | 51.7±1 |
Table 1: pH Study for bsa solution
Kanal et al. have investigated that the pH is responsible for net charge on BSA molecule. Above or below isoelectric point, electrostatic repulsion from “like” charges favors the denatured state [18]. The acidic solution at pH <5.4 results in increased positive charges on the surface of the BSA molecule and this causes strong electrostatic repulsive forces forcing components to move away from one another so that “unfolding” or “denaturing” of the BSA molecule occurs [19]. Molecules also repulse one another so that little aggregation is observed. In the pH range of 4.0 to 6.0, BSA molecule shows compactness. Further increase in pH forms intermediates, which is also a denatured state but less compared to acidic pH [18].
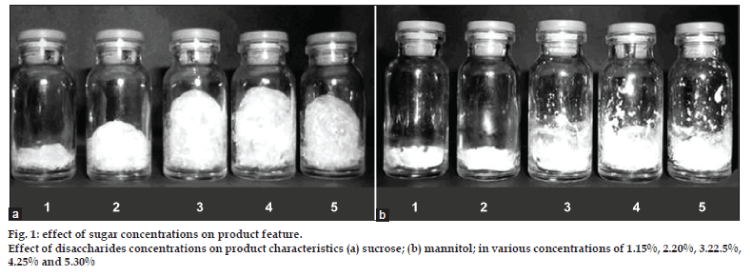
Disaccharides are widely used as protectant in preservation of protein pharmaceuticals. The extent of preservation effect varies with the amount of sugar added as well as with the specific type of sugar used [20,21]. The effect of sucrose concentrations on product feature is shown in fig. 1a. The amount of solid content is important for foam formation and our study revealed that minimum 20% w/v sucrose is required for desired foam formation and preservation of BSA content. The uniform foams obtained were brittle and hence collapsed during handling and storage. The nature of the foam has significant effect on residual moisture in product. The moisture content of 15% and 20% w/v sucrose products were in the range of 7‑9% and that for higher sucrose products were 3.0‑4.5%. Hence the amount of solid content is important for foam properties. The uniformly foamed product retained less moisture during dehydration drying. The preservation effect of the sugar generally increases with increasing amounts of sugar used up to the certain level, but an excess addition of sugar results in lowering the stability of protein. The VFD involves removal of moisture under vacuum. The higher amount of sucrose contributed to total solid content during VFD.
The effect of mannitol concentrations on product features is shown in fig. 1b. Mannitol as protectant has been reported for preservation of protein structure [22]. Mannitol has shown poor foam forming capacity, as it is not dissolved in aqueous solution of protein above 20% w/v.
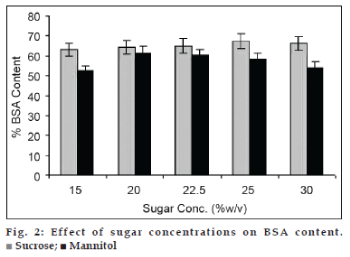
The mannitol mixture was mostly seen deposited to sidewall of vials. Due to this poor foamability, less surface area is available for moisture to be removed and the final product contains around 6% of moisture. The effect of sucrose and mannitol concentrations on BSA content of VFD processed mixtures is shown in fig. 2.
The VFD processing of BSA showed 35±2.5% loss of BSA content. The product with higher sucrose retained slightly better content. Similarly the loss of BSA was significant in mannitol containing mixtures. The pH of mixtures was in the range of 5‑5.5. Sugars alone did not stabilize BSA molecule.
Bovine serum albumin, a large globular protein (66,000 Da), has 580 amino acids in its chain. The BSA molecule is an alpha (55%) plus beta protein structure. Disaccharides like sucrose are an abundant source of hydrogen forming groups (–OH). They protect native protein conformation against dehydration stresses by replacing essential water molecules through molecular interaction e.g., hydrogen bonding with amino acids. Many factors affect the physical and chemical stability of proteins during processing and storage. An amorphous state of proteins and stabilizers allows maximal H‑bonding between them and crystallization of any one of the above during lyophilization often causes protein destabilization due to an inefficient hydrogen bonding. Mannitol can easily be crystallized and its crystallization is apparently responsible for the destabilization of some proteins during lyophilization. Lueckel et al. have concluded that the aggregation of interleukin‑6 during lyophilization could not be inhibited effectively in a formulation containing only mannitol [23]. Mannitol at 10 or 20% stabilized a co‑spray‑dried anti‑IgE monoclonal antibody during storage at 5 or 30°, but mannitol at 30% crystallized and drastically destabilized the protein during storage [24]. Considering the foam characteristics and BSA content, sucrose was selected for further studies. The result also demonstrated the need for multivalent stabilizer for preservation of BSA.
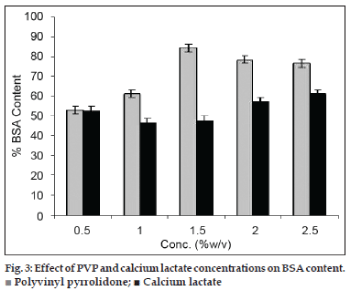
The products obtained with sucrose were brittle and collapsed during handling. Also the segmental motion of such a large molecule (BSA) was not arrested by use of sugars alone. This is expected to increase the protein aggregation and water adsorption during storage (increased surface area). The concentration of sucrose was kept constant at 25% w/v. The products with PVP concentration less than 1.5% w/v were brittle and collapsed similar to plain sucrose batches. The PVP amount with 2‑2.5% w/v being more viscous produced denser foam with reduced foam height. The optimum foamability and foam stability was evident using 1.5% w/v PVP. The moisture content of sucrose‑PVP mixtures decreased from 4.5 to 2.0 with polymer concentration. The mixtures had a pH of 5.5‑5.81. The effect of PVP concentration on BSA content is shown in fig. 3. A polymer concentration of 1.5% w/v (in presence of sucrose) enhanced BSA content from 60 to 84%.
All the mixtures containing calcium lactate (0.5‑2.5% w/v) solution showed foam formation. The foams were dense and non porous. The moisture content of calcium lactate mixtures was higher (5.25±1.21) than PVP‑sucrose mixtures. The mixtures had a pH of 4.91‑5.35. The effect of sucrose‑calcium lactate on retained BSA content is shown in fig. 3. Unlike PVP, the calcium lactate did not improve stability of BSA. The BSA content ranged between 46‑57%.
Sucrose is crystalline in nature and it was found that it crystallizes out, when used alone, after VFD process [data not shown] and it also lacks the required mechanical strength. The PVP K‑30 has been previously reported to increase the glass transition temperature of sucrose products [25]. The polymer has ability to form three‑dimensional network in presence of sucrose. This increases the mechanical strength and hence stability of dried foams. It increases the viscosity of sucrose solutions during VFD. The effect is found to be dependent on the concentration of polymer. The low concentrations are inadequate for establishing three‑dimensional structures. The results also reveal that PVP in higher concentrations (hence higher viscosity) has contributed to resistance to foam formation. Calcium lactate has not effective in forming hydrogen bonds. Hence the net result is molecular mobility of BSA causing loss of its content due to dehydration‑induced stresses.
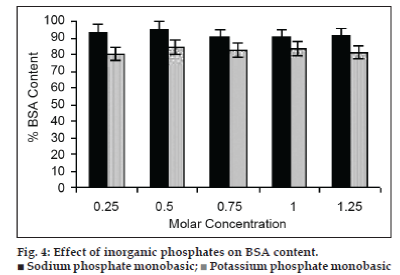
Although stabilizing effect of sucrose was synergistic with PVP, it is of formulation interest to improve their performance. The screening of inorganic water‑soluble phosphates was aimed to improve processing and storage stability of BSA. These phosphates are expected to alter foam characters. Closed foam structures of small bubbles and desired foamability were obtained with all the products containing sodium phosphate monobasic. The moisture content of these products was in the range of 1.5‑2.5 whereas potassium phosphate monobasic did not form foam and the residual moisture content of products was found to be 3.15±0.82. The effect of water‑soluble inorganic phosphates with sucrose (25% w/v) on preservation of BSA during VFD is shown in fig. 4.
Results showed that all water soluble phosphates improved the stability of VFD processed sucrose‑PVP‑BSA mixtures. The protection afforded by phosphates was dependent on the salt used and their concentrations. Sodium phosphate monobasic has retained maximum BSA content at all concentrations whereas potassium phosphate monobasic mixtures with sucrose and PVP showed BSA content of 82.38±2.09%. The BSA protection efficiency was greater with sodium phosphate monobasic as compared to potassium phosphate monobasic.
Vacuum foam drying stresses include dehydration, surface adsorption and aggregation. A protein structure can be stabilized by combined principle of glassy state and water substitution hypothesis. Research in the last decade has showed that disaccharides can provide significant protection for labile structures during lyophilization. Kinetic and specific interactions are involved in restricting the molecular mobility and retarding the chemical degradation due to the formation of glassy matrix. Specific interactions involving hydrogen bonding of the protectants and the labile molecules help to preserve the structure of the latter in the absence of water. However, disaccharides are reported to devitrify on storage and with absorption of moisture [26]. The use of phosphate‑sugar mixtures raises the interesting prospects of using disaccharide matrices for maintaining relatively high glass transition temperatures.
The pH of the mixture subjected for VFD and hydrogen bonding with BSA to form a stable glassy state are the two important effects during processing of BSA in sucrose‑phosphate mixtures. Sucrose has also been reported to form a glassy matrix with borate ions [27]. However, effective borate concentrations are toxic and not regulated. The interaction of sugars with additives in solutions will have profound effect on peptide in solid state. Ohtake et al. have reported glass state formation of sucrose with inorganic phosphates [28]. In aqueous solutions and at pH range of 4‑9, the water‑soluble phosphates are predominantly in the HPO4 2‑ form. This form of phosphate interacts strongly with sucrose giving rise to formation of network that upon dehydration exhibit higher Tg. The complex formation between sucrose‑phosphate is supported by a three dimensional polymeric network of PVP. The pH of aqueous solutions is expected to affect BSA stability as well as the strength of interaction between phosphate ion and sucrose during glass formation in VFD. The 25% w/v sucrose is used in all the VFD products. This product also consists of uniform strength of PVP (1.5% w/v).
The effective hydrogen bonding of sucrose and pH of optimum BSA (5.4) with sodium phosphate monobasic produced higher content. It is important to note that this pH was also suitable for sucrose–phosphate interaction for stable glass formation. Potassium phosphate monobasic is less effective as compared to sodium phosphate monobasic, owing to more affinity for sodium than potassium salt. The acidic pH supported the retention of BSA content. The reported study has confirmed that ~0.35 M concentration is optimum to produce an interpenetrated network of sucrose and soluble phosphates. At higher concentration, phosphates have been crystallized, affecting adversely on BSA content. The complexation between sucrose and phosphate is affected by pH of the solution. The loss of protein content at higher concentration of phosphate is attributed to salting out of excess phosphate, reducing protein content by ionic interactions.
The BSA stabilizing efficiency of sucrose‑ phosphates mixtures after VFD was compared with established composition of sucrose‑sodium tetraborate. Products showed good foamability to all the borate concentrations. Foam consists of closed cell structures. Moisture content of these mixtures was found to be 2.6‑3.6%. The pH of the sodium tetraborate mixtures was in the range of 8.40‑8.90. Sodium tetraborate has shown concentration dependant effect on BSA content (Table 2).
| Sodium tetraborate (mM) | pH | % Moisture content | % BSA content |
|---|---|---|---|
| 25 | 8.42 | 3.67±0.11 | 84.35±2 |
| 50 | 8.49 | 3.43±0.07 | 89.48±1 |
| 75 | 8.56 | 2.76±0.09 | 92.77±3 |
| 100 | 8.66 | 2.65±0.08 | 93.82±2 |
| 125 | 8.78 | 2.95±0.11 | 90.29±3 |
| 150 | 8.80 | 2.76±0.12 | 91.73±1 |
| 175 | 8.86 | 2.85±0.09 | 85.85±2 |
Table 2: Effect of sodium tetraborate on bsa content
The 100 mM of sodium tetraborate has shown maximum BSA content. Further increase in concentration has not shown any significant increase in BSA content. It is well known that borate forms chemical complexes with polyhydroxy compounds [29]. The increase in molecular size and changes in the molecular interactions due to complexation reduces mobility of BSA in the amorphous state. Such a phenomenon has been earlier reported during freeze drying where borate‑sugar combinations improved the protein stability at higher temperature [27]. The BSA stabilizing effect of sucrose‑sodium phosphate mixtures during VFD was found to be equivalent to sucrose‑borate mixtures.
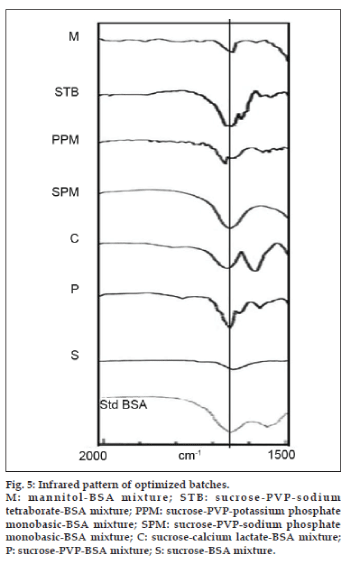
At the end of screening of stabilizers for BSA, the mixtures with maximum BSA content from each set of mixtures were selected for further analysis (Table 3). The BSA content of these mixtures was in accordance with the results obtained earlier. An attempt is made to correlate the BSA content of optimized mixtures with infrared spectra and X‑ray powder diffraction pattern. The stabilizing effect of mixtures on structure and conformation of peptide can be evaluated using amide‑I region in infrared spectra. The IR spectra of optimized mixtures of BSA are shown in fig. 5.
| Batch code | Composition | % Moisture content | % BSA content |
|---|---|---|---|
| S | 25% Sucrose | 4.21±1 | 72.2±3 |
| P | 25% S + 1.5% PVP | 2.51±2 | 83.9±2 |
| C | 25% S + 1.5% Ca-Lactate | 4.78±3 | 69.1±4 |
| SPM | 25% S + 1.5% PVP + SPM 0.5 M | 2.05±1 | 95.4±3 |
| PPM | 25% S + 1.5% PVP + PPM 0.5 M | 3.42±2 | 86.2±2 |
| STB | 25% S + 1.5% PVP +100 mM Sod. tetraborate | 2.43±1 | 93.8±2 |
| M | 25 % Mannitol | 5.11±1 | 71.2±3 |
Table 3: Bsa content of optimized batches
Hazare et al. have reported infrared spectra with their second derivative of human serum albumin with sucrose, calcium lactate, PVP, sodium phosphate and potassium phosphate [30]. The native BSA shows a large α‑helix band at approximately 1656 cm‑1 indicated by vertical line. The amide‑I peak in all the mixtures were observed at or around 1656 cm‑1. The spectra of sucrose-BSA mixture (S) shows small amide‑I peak with broadening and peak was shifted to 1648 cm‑1. The loss of peptide conformation has been reflected in BSA content. A sharp amide‑I band is evident in sucrose‑PVP mixture (P). The peak has been broadening laterally. The nature of amide‑I band in sucrose‑calcium lactate is different from other mixtures. The amide-I peak has been split into two and peak was shifted to 1669 cm‑1. This reflects perturbation of protein secondary structure. A single sharp peak without tailing (1656 cm‑1) indicating maximum BSA protection was observed with sodium phosphate monobasic. An intense amide‑I peak with lateral broadening was observed with sucrose‑potassium phosphate monobasic mixture (PPM). IR peak of BSA formulation with sucrose‑potassium phosphate monobasic mixture was shifted slightly to 1668 cm-1. The mixture containing sodium tetraborate (STB) showed 1656 cm‑1 peak similar to sodium phosphate monobasic. The low intensity amide‑I band in mannitol mixture reveals loss of conformational stability of BSA. The sharp amide‑I peak without shift or broadening indicates retention of maximum BSA content. The observed amide‑I peak characteristics and obtained BSA content correlated well. Izutsu et al. have similarly correlated BSA content of freeze dried samples with amide‑I peak characters [31].
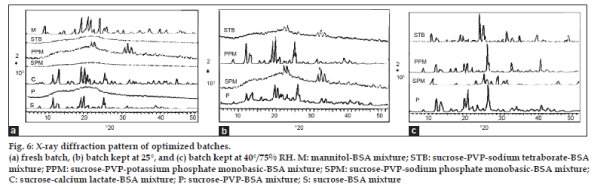
The X‑ray diffraction analysis is reported for pharmaceuticals to analyze the physical state of mixtures. The X‑ray diffraction pattern of optimized mixtures is shown in fig. 6a. The amorphous behavior was evident with sucrose‑PVP; sucrose‑sodium phosphate monobasic‑PVP, and sucrose‑sodium tetraborate‑PVP mixtures. The reduction in crystallinity of sucrose was observed in sucrose‑potassium phosphate monobasic‑PVP mixtures. Crystallization of excipients during dehydration is an important factor in the stability of protein pharmaceuticals. The changes in amorphous or crystalline nature of formulations significantly affect the performance and stability of protein pharmaceuticals [22,32]. Sucrose alone failed to protect BSA, and calcium lactate and mannitol also has crystallized out in the VFD process. PVP formed glassy matrix with sucrose and inhibited sugar crystallization. The results confirmed a glassy state formation due to network formation between sucrose and inorganic phosphates. The PVP has network‑forming ability and has strengthened the sucrose‑phosphate glassy matrix. The glassy state is responsible for increasing effective molecular size of sucrose thus making it difficult for devitrification. The mixtures with amorphous glassy state were subjected for accelerated stability study.
The BSA content of stability batches (25° and 40°/75% RH) is shown in Table 4. The moisture content of products was variable and was found to be in the range of 2.9‑7.9%. The moisture uptake of sucrose, PVP and calcium lactate was quite high as compared to sodium phosphate monobasic, potassium phosphate monobasic, sodium tetraborate and mannitol. The moisture absorption pattern is indicative of hygroscopicity of sucrose‑additives glassy matrix. The absorbed moisture can potentiate protein degradation by hydrolysis and oxidation at higher temperatures. It is also expected to reduce the glass transition temperature of the mixture. This reduced Tg and causes crystallization of sucrose. The retention of higher BSA content correlates with amorphous structure during storage. The crystalline products of sucrose, calcium lactate and mannitol have showed a loss of more than 50% BSA content after stability.
| Batch code | 25º | 40º/75% RH |
|---|---|---|
| S | 72.24±3 | 45.1±3 |
| P | 83.91±2 | 69.2±1 |
| C | 69.13±4 | 42.4±3 |
| SPM | 95.45±3 | 83.1±2 |
| PPM | 86.21±2 | 73.4±1 |
| STB | 93.13±2 | 82.4±1 |
| M | 71.21±3 | 42.3±1 |
Table 4: Bsa content of stability batches
The sucrose with PVP (P) has shown amorphous nature after VFD but after stability it showed initiation of crystallization at 25° and complete crystallization at 40° (as shown in fig. 6b and 6c, respectively). Potassium phosphate monobasic (PPM) has also shown somewhat similar effect with PVP. Sodium phosphate monobasic (SPM) as well as sodium tetraborate (STB) were mostly amorphous at 25°. But during storage at 40°, all the products were crystallized, hence loss of BSA content was observed. The sucrose‑sodium phosphate monobasic and sucrose‑sodium tetraborate products absorbed moisture up to 6% but the product retained 82±2% BSA content at all the storage conditions. This reveals that sucrose‑sodium phosphate and sucrose‑sodium tetraborate glasses in this case have been stabilized in presence of absorbed moisture. Hence sodium phosphate monobasic and sodium tetraborate formed stable glasses during vacuum foam drying. The BSA has not much stable at higher temperatures.
Therapeutic proteins are often formulated in the solid state, e.g., lyophilized, to yield a pharmaceutically acceptable shelf life. The storage stability of solid protein pharmaceuticals is affected by temperature and absorbed moisture in the products. The stability of optimized mixtures was established at higher temperature (40°) and higher humidity (75% RH).
Proteins are stabilized mainly by two mechanisms; one is formation of hydrogen bonding with sucrose and second by arresting the peptide mobility in glassy state. The gradual loss of glassy state during storage is reported in literature. This is because of relaxation of glass due to absorbed moisture. The glass relaxation is slow and gradual process. This corresponds to proportionate loss of conformation or structure of biomolecules. In this study, a high molecular BSA is effectively stabilized using sucrose‑sodium phosphate mixtures. The stabilizing ability is equivalent to sucrose‑sodium tetraborate mixtures.
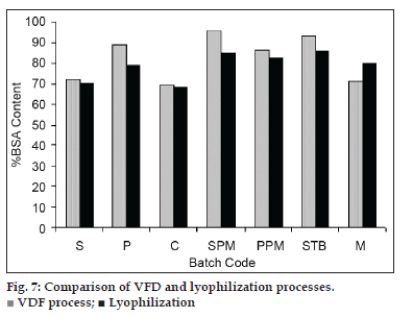
Lyophilization is widely used process for preservation of sensitive biomolecules. Therefore, the efficiency of VFD is compared with conventional lyophilization. The optimized mixtures were processed through both the processes. BSA content in dried mixtures of the two processes is shown in fig. 7.
VFD products showed higher BSA content than that of lyophilized mixtures. Lyophilized products showed higher moisture content than that of VFD products. Lyophilization process takes more than 24 h for dehydration drying. Whereas, VFD is devoid of freezing step and causes cryoinjuries that can reduce BSA content. The formation of sucrose‑phosphate glass during lyophilization is reported [28]. The weak glassy matrix of sucrose and phosphates formed due to salting out of phosphates in the lyophilization.
Sugar alone did not provide sufficient protection to BSA during drying. Minimum moisture content and foam formation were visible at and above 20% w/v of sucrose. Water soluble phosphates have shown higher stabilization efficiency for BSA. Sodium phosphate monobasic provided more protection to BSA than that of the potassium phosphate monobasic. Sodium phosphate product was amorphous after stability studies at 25°. However sodium phosphate monobasic and sodium tetraborate products were amorphous at room temperature. Denaturation at higher temperature and humidity may be due to larger molecular size of BSA. VFD is short, simple and economic drying process for biomolecules and can be a very good alternative to lyophilization.
References
- Pikal MJ. Freeze drying of proteins. In: Lee VHL, editor. Peptide and Protein Delivery. New York: Marcel Dekker; 1998.
- Prestrelski S, Tedeschi N, Arakawa T, Carpenter J. Dehydration-induced conformational changes in proteins and their inhibition by stabilizers. Biophys J 1993;65:661-71.
- Chang B, Beauvais R, Dong A, Carpenter J. Physical factors affecting the storage stability of freeze-dried interleukin-1 receptor antagonist: Glass transition and protein conformation. Arch BiochemBiophys1996;331:249-58.
- Allison S, Dong A, Carpenter J. Counteracting effects of thiocyanate and sucrose on chymotrypsinogen secondary structure and aggregation during freezing, drying and rehydration. Biophys J 1996;71:2022-32.
- Koseki T, Kitabatake N, Doi E. Freezing denaturation of ovalbumin at acid pH. J Biochem 1990;107:389-94.
- Morana A, Stiuso P, Colonna G, Lamberti M, Carteni M, Rosa M. Stabilization of S-adenosyl L-methionine promoted by trehalose. BiochimBiophysActa 2002;1573:105-8.
- Rammele R, Stuchnoff C, Carpenter J. Real-time in situ monitoring of lysozyme during lyophilization using IR spectroscopy: Dehydration stress in the presence of sucrose. Pharm Res 1997;14:1548-55.
- Martin G, Liao Y, Brown M. Investigation of the stabilization of freeze-dried lysozyme and the physical properties of the formulations. Eur J Pharm Biopharm 2004;58:15-24.
- Carpenter J, Crowe L, Crowe J. Stabilization of phosphofructokinase with sugars during freeze drying: characterization of enhanced protection in the presence of divalent cations. BiochimBiophysActa1987;923:109-15.
- Kadam SS, Pisal SS, Mane JJ, Shah MH. Effect of excipients on product characteristics and structure of lyophilized lasota vaccine. Indian J Biotech 2005;4:106-14.
- Bronshtein V. Scalable long-term shelf preservation of sensitive biological solutions and suspensions. US Patent 6509146B1, 2003.
- Davidson P, Sun W. Effect of Sucrose/Raffinose Mass Ratios on the Stability of Co-Lyophilized Protein during Storage above the Tg. Pharm Res 2001;18:474-9.
- Anonymous. Reagents and solutions. In, Indian Pharmacopoeia. 3rd ed. New Delhi: Government of India, Ministry of wealth and family welfare, The Controller of Publications; 1985. p. A1.
- Lowry O, Rosbrough N, Farr A, Randall R. Protein measurement with the folin phenol reagent. J BiolChem 1994;193:265-75.
- Anonymous, Karl Fischer reagent. British Pharmacopoeia. Appendix A2. London: Her Majesty Stationary Office; 2000. p. 104.
- Izutsu K, Yoshioka S, Terao T. Effect of mannitolcrystallinity on the stabilization of enzymes during freeze-drying. Chem Pharm Bull 1994;42:5-8.
- ICH steering committee. Stability testing of new drug substances and products. Q1A [R2]; 2003, 1-5.
- Kanal K, Fullerton G, Cameron I. A Study of the Molecular Sources of Nonideal Osmotic Pressure of Bovine Serum Albumin Solutions as a Function of pH.Biophys J 1994;66:153-60.
- Creighton T. Proteins: Structures and Molecular Principles. New York: W. H. Freeman and Co.; 1984.
- Izutsu K, Yoshioka S, Takeda Y. The effects of additives on the stability of freeze-dried beta-galactosidase stored at elevated temperature. Int J Pharm 1991;71:137-46.
- Liu R, Langer R, Klibanov A. Moisture-induced aggregation of lyophilized proteins in the solid state. BiotechnolBioeng1991;37:177-84.
- Izutsu K, Ocheda S, Aoyagi N, Kojima S. Effect of sodium tetraborate and boric acid on non isothermalmannitol crystallization in frozen solutions and freeze dried solids. Int J Pharm 2004;273:85-93.
- Lueckel B, Helk B, Bodmer D, Leuenberger H. Effects of formulation and process variables on the aggregation of freeze-dried interleukin-6 [IL-6] after lyophilization and on storage. Pharm DevTechnol 1998;3:337-46.
- Costantino H, Andya J, Nguyen P, Dasovich N, Sweeney T, Shire S, et al. Effect of mannitol crystallization on the stability and aerosolperformance of a spray-dried pharmaceutical protein, recombinant humanized anti-IgE monoclonal antibody. J Pharm Sci 1998;87:1406-11.
- Pisal S, Wawde G, Salvankar S, Lade S, Kadam S. Vacuum foam drying for preservation of Lasota virus: Effect of additives. AAPS PharmSciTech 2006;73:Article 10.
- Roser BJ, De Castro AG. Amorphous glasses for stabilizing sensitive products. PCT Patent WO 99/047174, 2001.
- Pezron E, Leibler L, Ricard A, Lafuma F, Audebert R. Complex formation in polymer-ion solution. 1. Polymer concentration effects. Macromolecules 1989;22:1169-74.
- Ohtake S, Schebor C, Palecek S, Pablo J. Effect of pH, counter ion, and phosphate concentration on the glass transition temperature of freeze-dried sugar-phosphate mixtures. Pharm Res 2004;21:1615-21.
- Miller D, Anderson R, de Pablo J. Stabilization of lactate dehydrogenase following freeze-thawing and vacuum-drying in the presence of trehalose and borate. Pharm Res 1998;15:1215-21.
- Hazare A, More H, Pisal S. Effect of sugar additives on stability of human serum albumin during vacuum foam drying and storage. CurrDrug Deliv 2011;8:678-90.
- Izutsu K, Aoyagi N, Kojima S. Protection of protein secondary structure by saccharides of different molecular weights during freeze drying. Chem Pharm Bull 2004;52:199-3.
- Imamura K, Ogawa T, Sakiyama T, Nakanishi K. Effect of types of sugars on the stabilization of protein in the dried state. J Pharm Sci2003;92:266-73.