- *Corresponding Author:
- H. V. Hegde
Regional Medical Research Centre, Nehru Nagar, Belgaum‑590 010, India
E-mail: harshavh@rediffmail.com
| Date of Submission | 17 March 2014 |
| Date of Revision | 24 January 2015 |
| Date of Acceptance | 03 August 2015 |
| Indian J Pharm Sci, 2015;77(4):446-452 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Spermacoce hispida L. is one of the important medicinal plants used in traditional systems of medicine. It is observed that, several times it is difficult to differentiate the plant from the other allied species from the same genus, Spermacoce, especially, when they are in drug form. Therefore, the present study aims to document the differences in the pharmacognostic characters, preliminary phytochemical analysis and polyphenolic contents from the leaves of four species belonging to the genus Spermacoce, viz. S. hispida L., S. mauritiana O. Gideon, S. stricta L. and S. ocymoides Burm. Transverse section passing through the midrib with lamina on either sides, epidermal characters, leaf constants, organoleptic characters, physicochemical analysis, extractive values and preliminary phytochemical analysis were carried out for all these species. Total phenolic content by Folin-Ciocalteu method and total flavonoids by AlCl3 method were also estimated from the leaves of all these species. The results indicated that S. hispida can be clearly differentiated from the other selected species on the basis of size and number of epidermal cells, size of trichomes, leaf constants, physicochemical analysis and extractive values. However, it is also found that S. hispida possess total phenolic content at 6.88±0.34 mg CAE/g and 9.17±0.46 mg TAE/g. Total flavonoids was at 5.98±0.30 mg QE/g. The study will provide information with respect to identification and differentiation amongst selected species of genus Spermacoce.
Keywords
Traditional medicine, Spermacoce, pharmacognostic study, phytochemical, total phenolic content, total flavonoids
Plants are utilized extensively as raw drugs for many formulations in traditional systems of medicine. Often, pharmacognosy is employed as a tool to check the genuineness of the raw drugs and to detect adulteration/substitution of these plant materials. Despite of several modern techniques, the QC of the raw drugs still rely upon the pharmacognostic studies by and large. According to the World Health Organization, the macroscopic and microscopic description of a medicinal plant is the first step towards establishing the identity and the degree of purity of such materials and should be carried out before any tests are undertaken [1]. Usually, the identification of the medicinal plants in the field is based on morphological features or other traditionally known characteristics. In several such instances, there is a chance of selecting incorrect raw drugs/adulterants, especially because of their similar morphological features with other plants. Therefore, an extensive microscopical and phytochemical screening is needed for raw drug to avoid any ambiguity and such a study will serve also as a reference for further studies [2].
Traditionally, leaves of several species of Spermacoce, belonging to family Rubiaceae, are being used in various Indian systems of medicine. The major species among them is Spermacoce hispida L., which is locally known as ‘Madanaghanti’ [3]. The leaves S. hispida are reported to be used in treating conjunctivitis, haemorrhoids, gallstones and to relieve headache [4]. Generally, most of these activities are attributed to the secondary metabolites in the plant [5]. In recent past, it is understood that, the phenolic compounds present in plants have a great deal of biologically active constituents and therefore have been studied extensively. One of the prominent properties of the phenolics is their excellent radical scavenging activity [6], whereas flavonoids, a group of polyphenolic compounds, are known for series of properties such as free radical scavenging activity, inhibition of hydrolytic and oxidative enzyme and antiinflammatory action [7]. However, no information is available on the total phenolic contents and flavonoids in the genus Spermacoce.
Meantime, it was observed that, it is difficult to differentiate the plants and the leaves morphologically, both in the field and in raw form, due to its similarity with other allied species of same genus viz. S. mauritiana O. Gideon., S. stricta L. and S. ocymoides Burm. [3,8]. Therefore, the present study is designed to provide information on pharmacognostical characters, preliminary phytochemical analysis and polyphenolic contents from the leaves of selected species from the genus Spermacoce, in the process of standardizing parameters for correct identification of S. hispida leaves and for their differentiation from other allied species of the same genus.
Materials and Methods
Fresh leaves of S. hispida, S. mauritiana, S. stricta and S. ocymoides were collected from Belgaum, India (N 15.71369o, E 074.37958o) and were authenticated. Herbaria were prepared and deposited in herbarium repository (Voucher No. RMRC‑530, RMRC‑531, RMRC‑532, RMRC‑533, respectively) at RMRC Belgaum for future reference.
Transverse section of midrib and epidermal characters
Fresh leaves of selected species of Spermacoce were used for microscopic sectioning. Sharp razor blades were used to take transverse section passing through midrib with lateral extensions of lamina on either side. Phloroglucinol and conc. HCl were used to observe lignified tissues. Upper and lower epidermal layers were peeled to study the epidermal characters.
Numerous temporary and permanent mounts of the sections were made and examined [9,10].
Determination of leaf constants
Leaf constants were determined according to standard methods with the help of camera lucida after calibrating the microscope using stage and ocular micrometers. Leaf constants were observed from leaves collected from different plants to ensure the variations of characters and average result of characters were mentioned [10].
Powder characteristics
The collected leaves were washed, shade dried and pulverized. The powder was then passed through # 85 mesh and collected fine powder is used for powder microscopy. Fragments of powder were cleared with chloral hydrate. Phloroglucinol and conc. HCl were used to observe lignified tissues, iodine to observe starch grains, acetic acid and dil. H2SO4 to detect calcium oxalate crystals and glycerine was used as mountant. The diagrams of different structures were drawn with the help of camera lucida mentioning the scale [10].
Microphotographs
Microscopic descriptions of tissues were justified with microphotographs mentioned with appropriate scale bars, photographs were taken with Olympus BX‑41 microscope.
Organoleptic characters, physicochemical analysis and extractive values
Fine powder obtained by pulverization was used for organoleptic characters to evaluate color, texture, odour and taste. Physicochemical parameters were done to evaluate the percentage of loss on drying, total ash content, acid insoluble ash and water soluble ash. Extracts of powdered leaves were prepared with different solvents for the study of extractive values [11].
Preliminary phytochemical analysis
Aqueous and ethanol extracts were prepared to find out the presence of secondary metabolites performing various tests [11].
Total phenolic content
Total phenolic content (TPC) was quantified using modified Folin–Ciocalteu method [6]. The assay mixture was prepared using 0.5 ml of distilled water, 0.125 ml different concentrations of standard tannic acid and/or caffeic acid with 0.125 ml of Folin–Ciocalteu reagent, incubated for 10 min in dark. After 10 min 1.25 ml 7% aq. sodium carbonate and 1 ml of distilled water was added and the reaction mixture was incubated in dark for 90 min at 37°. The absorbance of blue colour was read at 760 nm using distilled water instead of standards in the reaction mixture as blank on double beam spectrophotometer. Similarly, extracts prepared (10% w/v in methanol) were also quantified and the results were compared to the standard curves and expressed as mg/tannic or caffeic acid equivalent per gram dry powder for the samples.
Total flavonoids
Total flavonoid (TF) contents were quantified using method given by Luxmon‑Ramma et al. [12]. One milliliter of 2% methanolic AlCl3 was reacted with 1 ml of different concentrations of standard quercetin for 10 min in dark. Absorbance was measured at 367 nm on double beam spectrophotometer using 2% methanolic AlCl3 as blank. Standard was replaced with extracts prepared (10% w/v in methanol) and results were compared to the standard curves obtained. The results were expressed as mg/quercetin equivalent per gram dry powder for the samples and the data was represented as showed by Sandeep et al [13].
Results
Transverse section of midrib
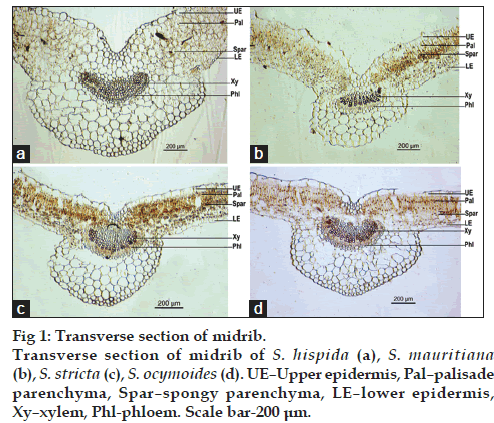
The characters of transverse section (TS) of midrib with lateral extensions of the lamina on its either sides are shown in fig. 1a‑d. All the species were dorsiventral in nature, plano convex shaped with a depression on middle of dorsal side. Detailed TS showed rectangular shaped upper and lower epidermis. Lamina showed two layers of palisade parenchyma underneath the upper epidermis and 3 to 5 rows of spongy parenchyma were found beneath palisade layer. The rest of the midrib was occupied by the cortical parenchyma with collateral vascular bundle embedded in the middle. Xylem was towards center and phloem towards periphery. Parenchymatous tissue was thin walled with prominent intercellular spaces.
Epidermal characters
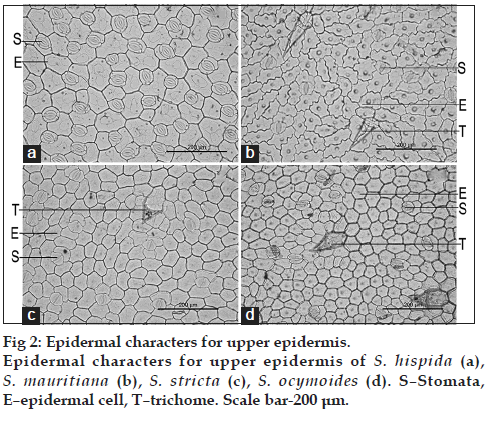
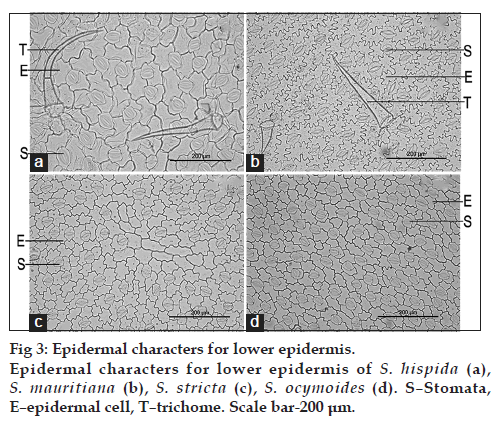
The comparative results of upper epidermis are mentioned in Table 1 (fig. 2) and lower epidermis are mentioned in Table 2 (fig. 3). The results revealed size and type of epidermal cells, size and type of trichomes, type of stomata and number of epidermal cells. The upper epidermal cells of S. hispida were rectangular to polygonal shaped which were similar in S. stricta and S. ocymoides whereas S. mauritiana possessed irregular shape with papillae. S. hispida has shown variation in size of epidermal cells and number of epidermal cells as compared to other species. Trichomes were absent in S. hispida and were present in other species. The lower epidermal cells were irregular shaped with wavy margin in all species, S. hispida has shown variation in size of epidermal cells and number of epidermal cells as compared to other species. Trichomes were present in S. hispida and S. mauritiana and were absent in other two species. All species possessed Rubiaceous (paracytic) stomata in both upper and lower epidermal layer.
| Characters | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides |
|---|---|---|---|---|
| Type of epidermal cells | Rectangle to | Irregular shaped with | Rectangle to polygonal | Rectangle to polygonal |
| polygonal shaped | papillae | shaped | shaped | |
| Size of epidermal cells (µ) | 149.86–368.42 | 200.67–367.17 | 164.11–228.22 | 98.54–213.63 |
| Type of stomata | Rubiaceous | Rubiaceous | Rubiaceous | Rubiaceous |
| Type of trichome | ‑ | Uniseriate | Thick walled, uniseriate | Thick walled, uniseriate |
| Size of trichome (µ) | ‑ | 50.99–202.62 | 59.09–82.25 | 85.90–110.28 |
| Epidermal cells number | 175–208 | 192–218 | 325–349 | 400–430 |
UE: Upper epidermis
Table 1: Epidermal Characters For Ue
| Characters | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides |
|---|---|---|---|---|
| Type of epidermal cells | Irregular shaped, wavy | Irregular shaped wavy | Irregular shaped, wavy | Irregular shaped wavy |
| Size of epidermal cells (µ) | 133.10-369.89 | 165.33-391.64 | 93.50-280.59 | 100.25-209.11 |
| Type of stomata | Rubiaceous | Rubiaceous | Rubiaceous | Rubiaceous |
| Type of trichome | Uniseriate | Uniseriate | ‑ | ‑ |
| Size of trichome (µ) | 200.39-479.60 | 64.55-355.50 | ‑ | ‑ |
| Epidermal cells number | 236-252 | 350-380 | 496-520 | 530-560 |
LE: Lower epidermis
Table 2: Epidermal Characters For Le
Leaf constants
The comparative results of leaf constants are mentioned in Table 3. The leaf constants of S. hispida showed differences with respect to stomatal number, stomatal index, vein islet number, vein termination number and palisade ratio comparing with other species.
| Characters | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides |
|---|---|---|---|---|
| Stomatal number | ||||
| UE | 47-55 | 26-28 | 23-28 | 32-38 |
| LE | 65-74 | 80-92 | 105-130 | 150-170 |
| Stomatal index | ||||
| UE | 20.45-21.88 | 11.86-12.32 | 5.69-8.61 | 7.07-9.09 |
| LE | 20.96-22.69 | 18.18-20.81 | 17.47-20.00 | 21.73-24.28 |
| Vein islet number | 2-4 | 2-5 | 6-8 | 4-6 |
| Vein termination number | 2-5 | 4-7 | 3-4 | 4-6 |
| Palisade ratio | 7 | 7 | 5 | 6 |
UE: Upper epidermis, LE: lower epidermis
Table 3: Leaf Constants
Powder characteristics
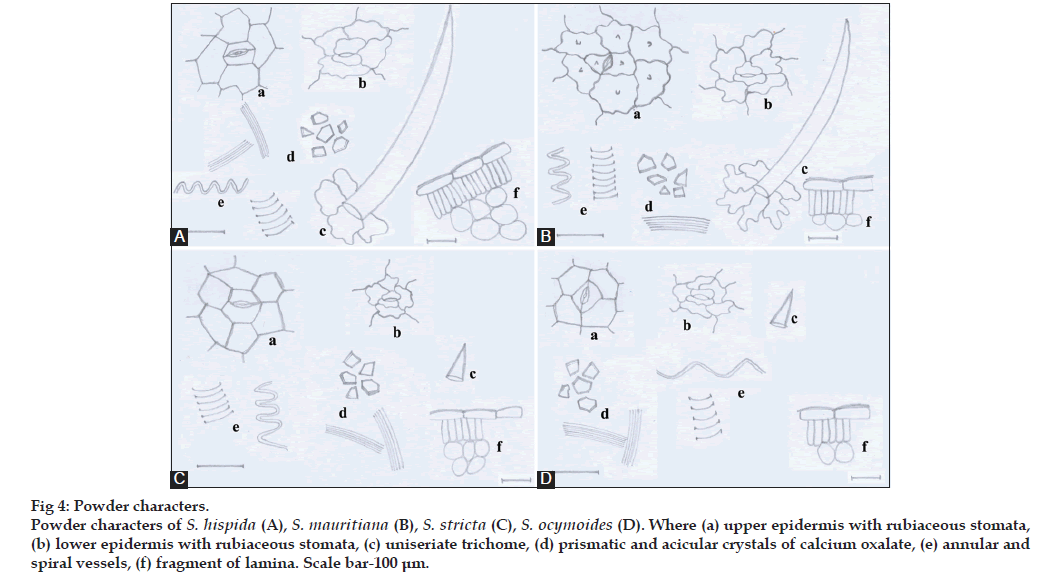
Powders of all the species revealed the presence of fragments of upper and lower epidermal cells, lamina, mesophyll tissue, palisade parenchyma, trichomes, annular and spiral vessels and prismatic and acicular crystals of calcium oxalate (fig. 4).
Figure 4: Powder characters. Powder characters of S. hispida (A), S. mauritiana (B), S. stricta (C), S. ocymoides (D). Where (a) upper epidermis with rubiaceous stomata, (b) lower epidermis with rubiaceous stomata, (c) uniseriate trichome, (d) prismatic and acicular crystals of calcium oxalate, (e) annular and spiral vessels, (f) fragment of lamina. Scale bar-100 μm.
Organoleptic characters, physicochemical analysis and extractive values
The results of organoleptic characters are mentioned in Table 4, they were similar in all species. Physicochemical analysis and extractive values results are mentioned in Table 5. The results of S. hispida shown difference in terms of loss on drying (5.7263% w/w), total ash content (9.6457% w/w), acid insoluble ash (0.0794% w/w) and water soluble ash (7.0412% w/w) with other selected species. The results of extractive values revealed, S. hispida shown difference in terms of water soluble extractive (21.9649% w/w) and ethanol soluble extractive (6.9464% w/w) with other selected species.
| Name of the test | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides |
|---|---|---|---|---|
| Color | Green | Green | Green | Green |
| Texture | Fine | Fine | Fine | Fine |
| Odour | Characteristic | Characteristic | Characteristic | Characteristic |
| Taste | Pungent | Pungent | Pungent | Pungent |
Table 4: Organoleptic Characters
| Name of thetest (% w/w) | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides |
|---|---|---|---|---|
| Loss on drying | 5.7263 | 4.1420 | 6.7941 | 4.8378 |
| Total ash content | 9.6457 | 9.0938 | 10.6420 | 11.3207 |
| Acid insoluble ash | 0.0794 | 1.7031 | 2.7908 | 2.0841 |
| Water soluble ash | 7.0412 | 2.6844 | 2.4599 | 4.2453 |
| Water soluble | 21.9649 | 27.5432 | 13.5298 | 15.0250 |
| extractive | ||||
| Ethanol soluble | 6.9464 | 9.0976 | 10.2009 | 12.8647 |
| extractive |
Table 5: Physicochemical Analysis And Extractive Values
Preliminary phytochemical analysis
The results of tests for the detection of phytochemicals are mentioned in Table 6. Ethanol and aqueous extracts were treated with various reagents to detect the phytochemicals. All the species showed the presence of alkaloids and glycosides, tannins and phenolic compounds, carbohydrates and amino acids. S. hispida and S. mauritiana showed presence of flavonoids and in other two species it was absent. Steroids and proteins were absent in all species.
| Secondary metabolite | Tests | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous extract | Ethanol extract | Aqueous extract | Ethanol extract | Aqueous extract | Ethanol extract | Aqueous extract | Ethanol extract | ||||
| Alkaloids | Dragendorff’s | −ve | −ve | −ve | −ve | −ve | −ve | −ve | −ve | ||
| Hager’s | +ve | +ve | +ve | +ve | +ve | +ve | +ve | −ve | |||
| Wagner’ | +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |||
| Glycosides | Legal’s | +ve | +ve | +ve | −ve | +ve | −ve | +ve | −ve | ||
| Deoxysugars | +ve | +ve | +ve | +ve | −ve | +ve | +ve | +ve | |||
| Flavonoids | Shinoda’s | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | ||
| Tannins and phenolic | 5% FeCl3 | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | ||
| compounds | Lead acetate | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | ||
| Bromine water | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | |||
| Dilute HNO3 | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | |||
| Steroids | Salkowski reaction | −ve | −ve | −ve | −ve | −ve | −ve | −ve | −ve | ||
| Liebermann‑Burchard | +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve | |||
| Carbohydrates | Molisch’s | −ve | +ve | +ve | +ve | −ve | +ve | −ve | +ve | ||
| Fehling’s | −ve | +ve | +ve | +ve | −ve | +ve | −ve | +ve | |||
| Benedict’s | −ve | +ve | +ve | +ve | −ve | +ve | −ve | +ve | |||
| Barfoed’s | +ve | +ve | +ve | −ve | −ve | +ve | −ve | +ve | |||
| Selwinoff’s | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | |||
| Proteins | Biuret’s | −ve | −ve | −ve | −ve | −ve | −ve | −ve | −ve | ||
| Million’s | −ve | −ve | −ve | −ve | −ve | −ve | −ve | −ve | |||
| Amino acids | Ninhydrin | −ve | +ve | −ve | +ve | −ve | +ve | −ve | +ve | ||
| Test for tryptophan | −ve | +ve | −ve | +ve | −ve | +ve | −ve | -ve | |||
Table 6: Preliminary Phytochemical Analysis
Total phenolic content
The TPC of selected Spermacoce species were expressed in terms of caffeic acid/tannic acid equivalents using the standard curve equation as shown in Table 7. Results of TPC are presented in Table 8. TPC of S. hispida was 6.88±0.34 mg CAE/g and 9.17±0.46 mg TAE/g. The selected Spermacoce species can be arranged on basis of TPC from lowest as in S.mauritiana<S. hispida<S. stricta<S. ocymoides to highest. Tannic acid equivalent TPC were higher than the caffeic acid equivalent TPC in all species.
| Activity | Concentrationrange (μg/ml) | Regression equation | Correlation coefficient (R2) |
|---|---|---|---|
| TPC | |||
| Caffeic acid | 10–800 | y=0.0029x−0.0458 | 0.9940 |
| Tannic acid | 10–400 | y=0.0022x+0.0225 | 0.9977 |
| TF | |||
| Quercetin | 10–400 | y=0.0059x−0.0506 | 0.9914 |
TPC: Total phenolic content, TF: total flavonoids
Table 7: standard curve equation of tpc and Tf
| Activity | Spermacoce hispida | Spermacoce mauritiana | Spermacoce stricta | Spermacoce ocymoides |
|---|---|---|---|---|
| TPC | ||||
| CAE (mg/g) | 6.88±0.34 | 6.67±0.33 | 7.08±0.35 | 8.17±0.41 |
| TAE (mg/g) | 9.17±0.46 | 8.90±0.45 | 9.44±0.47 | 10.88±0.54 |
| TF | ||||
| QE (mg/g) | 5.98±0.30 | 5.62±0.28 | 6.18±0.31 | 6.31±0.32 |
Figures in tables are represented as mean of three readings±SD. TPC: Total phenolic content, TF: total flavonoids, TAE: tannic acid equivalent, CAE: caffeic acid equivalent, QE: quercetin equivalent, SD: standard deviation
Table 8: Tpc And Tf From Leaves
Total flavonoids
The TF of selected Spermacoce species are expressed in terms of quercetin equivalent using the standard curve equation as shown in Table 7 and results of TF are presented in Table 8. TF of S. hispida was 5.98±0.30 mg QE/g. The selected Spermacoce species can be arranged on basis of TF from lowest as in S. mauritiana<S. hispida<S. stricta<S. ocymoides to highest.
Discussion
S. hispida is widely used in traditional systems of medicine for various ailments [8]. It is also reported to be useful in reducing obesity, to control bloody diarrhea, urinary infections, oliguria, bone disease and fracture healing [14]. The methanol extract of the plant was also reported for its antibacterial activity [15] and hydroxyl radical scavenging and nitric oxide radical scavenging activities [16]. Efforts were also made earlier to identify the chemical constituents of S. hispida [17], S. ocymoides [18] and other non‑Indian species [19] of the genus.
In spite of its wider and known utility, the pharmacognostic information on S. hispida in comparison with other allied species is not available. Hence, the present study forms the first report on comparative pharmacognostic and phytochemical analysis of leaves from the genus Spermacoce.
The results of pharmacognostic studies revealed that, TS of midrib, leaf constants (Table 3) organoleptic characters (Table 4) and preliminary phytochemical analysis (Table 6) remains almost same for all the investigated species of Spermacoce. The results are comparable with the earlier reports for S. hispida [20] and S. ocymoides [21]. However, significant differences were observed in the epidermal characters (Tables 1 and 2), physicochemical analysis and extractive values (Table 5) and polyphenolic contents (Table 8).
The highest TPC and TF were observed in the leaves of S. ocymoides. The present study gains importance, as the TF content was linked to the antioxidant activity in S. hispida [22].
The present study is an effort to fill the gaps in the pharmacognostic studies of the genus Spermacoce, along with providing the differentiating features between the allied species for their correct identification. These parameters, hopefully, shall serve as quality control parameters and also help in further studies on better and correct utility of the intended plant from the genus Spermacoce according to traditional system of medicine.
Acknowledgements
Authors are indebted to the Indian Council of Medical Research (ICMR) for funding the study through the intramural funds of Regional Medical Research Centre, Belgaum. Authors are thankful to Mr. Bhoopal Talawar, Lab Attendant, RMRC Belgaum, for his assistance.
Financial support and sponsorship
The Indian Council of Medical Research for funding the study through the intramural funds of Regional Medical Research Centre, Belgaum.
Conflicts of interest
There are no conflicts of interest.
References
- Balakrishnan M, Dhanapal R, Mohan VM, Chandra Sekhar KB. Studies on pharmacognostical specifications of Azima tetracantha Lam. Int J Phytopharmacol 2010;1:35‑42.
- Koilpillai B, Sabesan GS, Sadananda R. Comparative pharmacognostic studies on the barks of four Ficus species. Turk J Bot 2010;34:215‑24.
- Yadav SR, Sardesai MM. Flora of Kolhapur District. Kolhapur: ShivajiUniversity; 2002. p. 234.
- Parrotta JA. Healing Plants of Peninsular India. Wallingford: CAB International Publishing; 2001. p. 614‑5.
- Subramanya MD, Pai SR, Upadhya V, Ankad GM, Bhagwat SS, Hegde HV. Total polyphenolic contents and in vitro antioxidant properties of eight Sida species from Western Ghats, India. J Ayurveda Integr Med 2015;6:24‑8.
- Upadhya V, Pai SR, Ankad G, Hurkadale PJ, Hegde HV. Phenolic contents and antioxidant properties from aerial parts of Achyranthescoynei Sant. Indian J Pharm Sci 2013;75:483‑6.
- Atanassova M, Georgieva S, Ivancheva K. Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. J Univ Chem Technol Metall 2011;46:81‑8.
- Bhat GK. Flora of Udupi. Udupi: Indian Naturalist Publisher; 2003. p. 294.
- Adedeji O, Jawoola OA. Importance of leaf epidermal characters in the Asteraceae family. Not Bot Hort Agrobot Cluj 2008;36:7‑16.
- Kalaskar MG, Saner SY, Pawar MV, Rokade DL, Surana SJ. Pharmacognostical investigation and physicochemical analysis of Celastrus paniculatus willd. Leaves. Asian Pac J Trop Biomed 2012;2 (3, Suppl 1):S1232‑6.
- Daya CL, Patel NM. Preliminary phytochemical screening, pharmacognostic and physicochemical evalution of leaf of Gmelinaarborea. Asian Pac J Trop Biomed 2012; 2 (3, Suppl 1):S1333-7.
- Luximon‑Ramma A, Bahorun T, Soobrattee MA, Aruoma OI. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J Agric Food Chem 2002;50:5042‑7.
- Pai SR, Nimbalkar MS, Pawar NV, Patil RR, Dixit GB. Seasonal discrepancy in phenolic content and antioxidant properties from bark of Nothapodytes nimmoniana (Grah.) Mabb. Int J Pharma Bio Sci 2010;1:1‑17.
- Vinayak M, Chandrashekhar K, Shishir M. Pharmacological activities of Spermacoce hispida Linn: A review. Int J Res Ayurveda Pharm 2013;4:18‑22.
- Kottai MA, Penugonda S, Satheesh KD, Anton SA, Manavalan R. Evaluation of antibacterial activity of various extracts of whole plant of Borreria hispida (Linn). Int J Pharm Sci Res 2010;1:127‑30.
- Shajiselvin CD, Kottai MA. In vitro free radical scavenging activity of various extracts of whole plant of Borreria hispida (Linn). Arch Appl Sci Res 2010;2:54‑60.
- Rathi MA, Meenakshi P, Kumar DG, Arul RC, Sunitha M, Gopalakrishnan VK. High performance thin layer chromatography analysis of Spermacoce hispida. Pharmacologyonline 2011;3:961‑8.
- Jeyachandran M, Anantha KT, Gandhimathi S. Phytochemical investigation of ethnomedicinal Spermacoce ocymoides roots. J Pharmacogn Phytochem 2013;2:86‑8.
- Keat NB, Umar RU, Lajis NH, Chen TY, Li TY, Rahmani M, et al. Chemical constituents from two weed species of Spermacoce (Rubiaceae). Malays J Anal Sci 2010;14:6‑11.
- Patel AJ, Patel JR, Macwan CP, Patel MA, Soni AK. Pharmacognostical and proximate analysis of leaves of Borreria hispida. Asian J Biochem Pharm Res 2011;1:157‑61.
- Pravat KP, Prithwiraj M. Pharmacognostical profile of Spermacoceocymoides (Burm. F) DC. – A study on a medicinal botanical. PharmLett 2012;4:1414‑25.
- Kaviarasan K, Kalaiarasi P, Pugalendi P. Antioxidant efficacy of flavonoid‑rich fraction from Spermacoce hispida in hyperlipidemic rats. J Appl Biomed 2008;6:165‑76.