- *Corresponding Author:
- J. A. Al-Maghrabi
King Faisal Specialist Hospital and Research Center, Jeddah 21589, Saudi Arabia
E-mail: jalmgrabi@kau.edu.sa
| Date of Received | 11 September 2022 |
| Date of Revision | 27 February 2023 |
| Date of Acceptance | 11 October 2023 |
| Indian J Pharm Sci 2023;85(5):1241-1247 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
5'-Adenosine monophosphate activated protein kinase has a significant role in tumor growth, progress and metastasis, so the study of 5'-Adenosine monophosphate activated protein kinase's association with cancer risk is of important value. The aim of this study is to describe 5'-Adenosine monophosphate activated protein kinase expression and investigates its possible association with the clinicopathological factors of urinary bladder cancer to estimate its clinical usefulness in diagnosis and prognosis. This retrospective study utilized previously diagnosed 128 cases of bladder cancer and twenty-three specimens of normal bladder, which were assembled from the pathology archive, and were employed in the construction of tissue microarrays in this study. An immunohistochemistry staining has been applied to tissue microarrays slides for the revealing of 5'-Adenosine monophosphate activated protein kinase expression. Brown cytoplasmic staining were presented in malignant urothelial cells of 106 (82.8 %) bladder neoplasms of which 98 (92.5 %) cases showed weak staining. On the other hand, out of 23 positive normal bladder mucosa 18 (78.3 %) cases revealed moderate to strong 5'-Adenosine monophosphate activated protein kinase expression. Positive 5'-Adenosine monophosphate activated protein kinase immunohistochemistry staining in malignant urothelial cells was significantly more prevalent in low grade bladder tumors (p=0.035). A Cox proportional hazards model showed that high grade tumors had a significant effect on the hazard/survival in comparison with tumors of low grade (p=0.032). Furthermore, Kaplan-Meier survival distribution curves showed that survival behavior is better in low grade tumors and cases with negative vascular invasion. The outcomes of this study suggest that 5'-Adenosine monophosphate activated protein kinase immunohistochemistry staining could be a supportive tool to predict grade and prognosis of urinary bladder cancer.
Keywords
5'-Adenosine monophosphate activated protein kinase, urinary bladder, cancer, immunohistochemistry
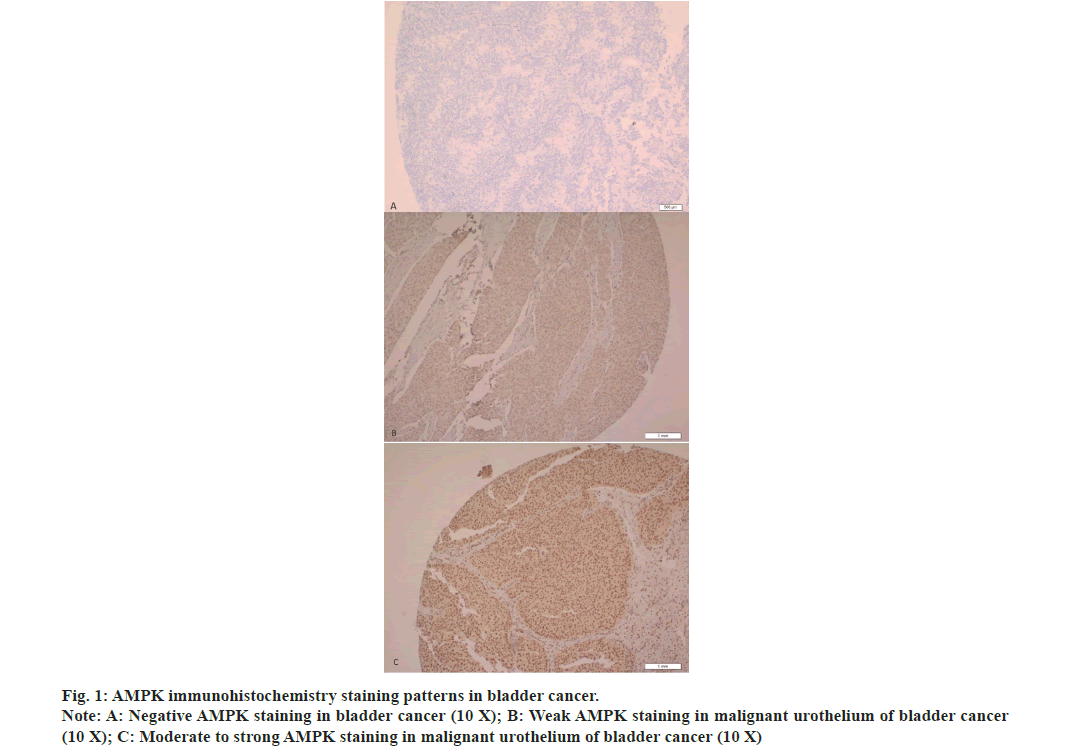
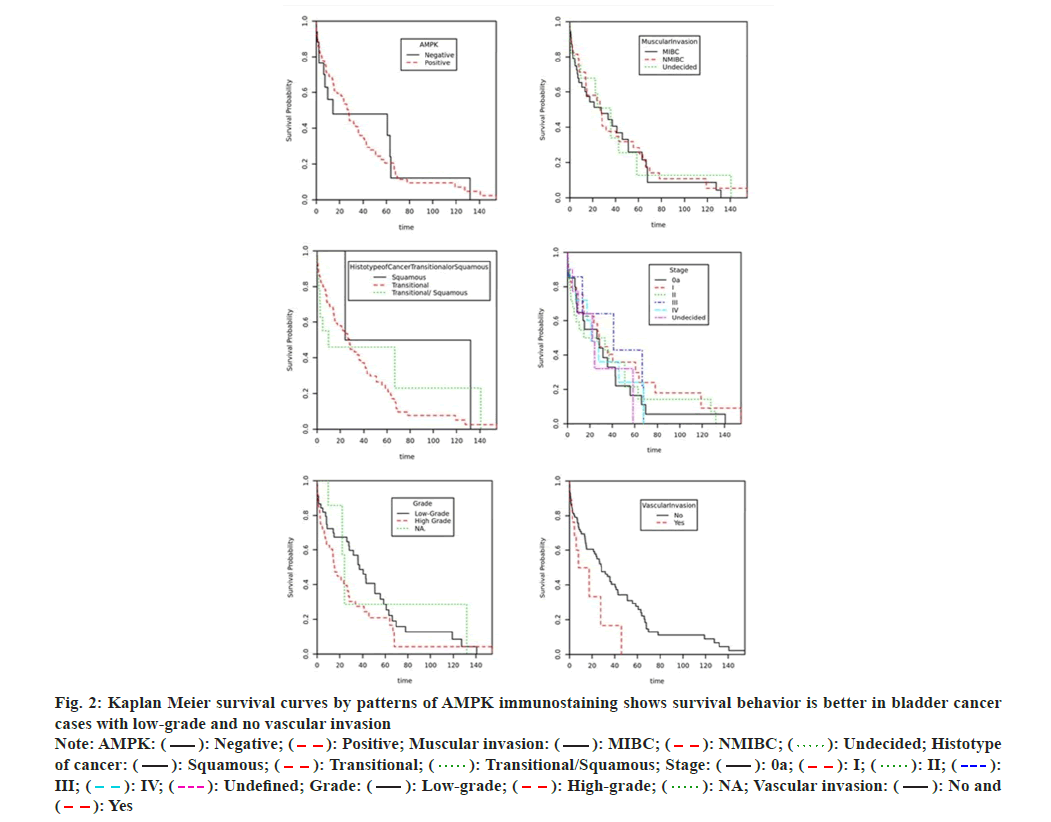
Urinary bladder carcinomas are harmful disorders and significant bases of death worldwide[1]. In recent study, Siegel et al.[2] stated that malignant tumors of the urinary bladder scored six in the directory of the most widespread tumors not including skin cancer with approximately 81.4 thousand cancer patients recently recorded and greater than 18 thousand related death in the United States of America. Despite significant improvements in tumor therapy, bladder cancer keeps on presenting an enormous contest to physicians because of high relapse rate. For instance, nearly 70 % of a new registered bladder tumors recrudesce in 5 y, plus a huge chance of progressing to aggressive invasive tumors[3,4]. The medication of carcinoma of bladder rests extremely on the clinicopathological parameters of patients like indications of bad or good prognosis. Existing clinical information are still insufficient to assess the tumor outcomes due to significant discrepancies at the same grade or stage, commonly because of neoplastic cell heterogeneousness[5]. So, it is significant to gain new investigational tools which meet the requirements of clinicians for the treatment of bladder carcinomas. Significant efforts have been made to obtain innovative markers to facilitate tumor identification and prediction of prognosis, improve high risk patient stratification and boost cancer treatment[6,7]. A large amount of these existing markers was ineffectively sensitive or specific making it essential to explore other biomarkers which may be more precise and better predictors of clinical consequences. 5'-Adenosine Monophosphate-activated protein Kinase (AMPK) is a significant cellular sensor and regulator of energy. It is a complex enzyme composed of 3 subunits (α is a catalyst, β and γ are regulative), every single subunit is encrypted by two or three different genes so there is about 12 likely AMPK isoforms in human[8,9]. Many reports have reviewed AMPK role in tumor growth, development and therapy[9-19]. This kinase inhibits tumor growth and development through controlling cellular proliferation and assessing the checkpoints of the cell cycle of tumor cells after energy resource depletion. AMPK reacts to cellular stress by controlling the growth of cells and synthetic process via reducing mammalian target of rapamycin activity and synthesis of proteins by directly phosphorylating Raptor[9-12]. Additional anti-cancer influences of AMPK might engage increasing deoxyribonucleic acid repair and autophagy to correct the damage caused by ultraviolet[13]. This view was supported and approved by some studies which showed that AMPK activates and phosphorylate p21 and p53[14,15], thus arrests cell cycle and supports the survival of cells. In the last 5 y, two studies had identified functional deficit of AMPK activity in several cancers[20,21]. Liu et al.[22] reported that AMPK expression was considerably found in cell lines and malignant tissues and AMPK phenotype has been associated with poor survival. Moreover, data from several studies showed that AMPK stimulates cell proliferation and glycolysis in tumors[21,22]. Nevertheless, considering some published literature, AMPK may have a protective function in tumor development by saving malignant cells from toxic compound impacts, nutrient deficiency and hypoxia. Therefore, activators of AMPK could be risky in tumor chemotherapy[23,24], since they fight the growth of tumor in a few other cancer types and presents a valuable role in tumor therapy[25]. Still, little is identified concerning the clinical importance of AMPK phenotype in urinary bladder cancer. So certain validation is needed to clarify AMPK’s exact roles in the emergence and evolution of bladder carcinoma. Detection of the association between AMPK and carcinoma of bladder may enhance people's perception of bladder cancer and aid developing better treatment and preventive approaches. This study aims to describe the AMPK expression in urinary bladder cancer and to investigate a possible association between this expression and disease clinicopathological factors and follow-up data to estimate its diagnostic and prognostic usefulness. This project was accomplished in the lab of pathology over a period of 2 y ended on 15th Apr 2022 and was allowed by the Biomedical Ethics Committee (Reference No 1127-13). Informed signed consent was waived by the Biomedical Ethics Committee as the research project used archived material. The practices followed were consistent with the 1975 Helsinki Declaration, as amended in 2000. Written informed consent is routinely obtained from all patients who underwent a surgical procedure at our institution to use tissue samples for lab studies. This is a retrospective study that utilized a convenience series of tissues samples (paraffin-embedded) of previously diagnosed 128 bladder cancers and 23 normal bladder mucosa, which have been gathered from the stores of pathology department. We excluded all cases with history of intravesical chemotherapy. 4 µm slides were prepared from paraffin tissue blocks and stained with hematoxylin and eosin, then were reassessed. Tumor characters and patient clinical data (patient's age and gender, stage, histotype of tumor, and metastasis) were collected from the medical records unit of our university hospital. All normal samples were obtained from individuals who were tasted for non-cancerous diseases. World Health Organization recommendation has been adapted in bladder cancer staging processes. TMA was made similarly to what we illustrated in our previous report[26]. Carcinoma samples of 128 bladder patients and twenty-three normal mucosa samples have been employed in this procedure. Blocks of TMA were cut into 4 µm slides and placed on aminosilane coated slides. This protocol has been operated employing autostainer (BenchMark ULTRA, Ventana, Arizona, USA) as it has been described earlier[27]. AMPK polyclonal antibody has been diluted to a ratio 1:100 (Santa Cruz Biotechnology, USA) and employed in immunohistochemistry stain, then were visualized by ULTRAVIEW TM DAB system. Placenta specimen slide with 4 µm thickness has been employed like a positive control. A slide handled with Tris buffer in place of AMPK antibody was employed as a negative control. Every case that revealed cytoplasmic brown color in more than 5 % of malignant cells has been counted positive. Two pathologists analyzed immunohistochemical staining. The number of positive cells has been approximated by means of a semiquantitative technique in three lenses fields of 40x. A grading protocol for immunohistochemistry staining has been used to measure the intensity of AMPK staining (1, 2, 3 and 0 for negative). Grades of staining are displayed as two groups, negative (0) and positive (1, 2 and 3) in the current study. All information has been assessed employing software from Statistical Package for Social Sciences (version 21). All outcomes have been displayed as ratios and incidences. The relationship between bladder carcinoma clinical parameters and AMPK immunoexpression has been examined by chi-square and Fisher tests. A Cox Proportional Hazards model has been conducted to determine if any of the clinical parameters have an important consequence on the survival. Evaluation of survival distributions for different intensity of AMPK immunostaining grades has been performed by making use of the survival curves of Kaplan Meier. The significance level has been counted at p less than 0.05. All 128 carcinoma cases of bladder (105 males and 23 females) were revised. Both males and females exhibited an almost alike distribution pattern of immunohistochemistry stain. Clinical data of these patients is listed in Table 1. The commonest category of cancer was urothelial carcinoma (82 %), then squamous differentiation variant (13.3 %), and less frequently squamous cell carcinoma (4.7 %). The median age is 62 y. 118 bladder cancer cases were staged and 116 cases were graded. Overall mortalities from cancer were 38. Muscularis propria invasion was present in 64 cases, 19 tumors showed blood vessel invasion, 22 tumors invaded lymph nodes and 25 tumors showed distant metastases (Table 1). AMPK has been found downregulated during the evolution of malignancy, it was present in 106 (82.8 %) bladder carcinomas of which 98 (92.5 %) cases revealed weak immunostaining (fig. 1A-fig. 1C). Approximately 68 % of malignant tumor displayed brown color in more than 50 % of the malignant cells. On the other hand, 5 (21.7 %) cases of normal bladder mucosa showed weak AMPK expression and 18 (78.3 %) cases revealed moderate to strong staining. All positive stained cases of both malignant tumors and control groups showed nuclear and cytoplasmic staining. AMPK immunohistochemical staining has been found significantly associated with the grade of urinary bladder tumors, i.e., AMPK expression is significantly more prevalent in low-grade cancers of the bladder (p=0.035) (Table 1). A significant portion of low-grade carcinomas showed low AMPK staining grades, while strong staining of AMPK was present in only 5 (11 %) cases of low-grade tumors. More than 90 % of bladder carcinomas, which did not show muscularis propria invasion (non-muscle invasive bladder cancer) expressed AMPK, however, no significant association was observed between muscular invasion and AMPK expression (p value=0.146). Regarding the histologic type of bladder cancer, almost 85 % of urothelial carcinoma cases were observed to have positive AMPK expression, but the variance was of no statistical significance (p value=0.219). No significant association was observed between stage, lymph node involvement, vascular invasion, metastasis and AMPK expression (Table 1). A Cox proportional hazards model has been conducted to decide which clinicopathological variables have a significant effect on the hazard/survival. The outcomes were not important according to an alpha of 0.05, p=0.581, showing AMPK, gender, age, muscular invasion, histologic type of cancer, stage, lymph node, vascular invasion and metastasis were not able to adequately predict the hazard/survival. However, only survival distribution of cases with high grade is considerably different in comparison with cases of low grade (p=0.032). Kaplan Meier survival curves exhibited that the survival behavior is better in bladder cancer cases with low-grade and no vascular invasion (fig. 2). Carcinoma of bladder still counted as a serious task to doctors due to bad prognosis and large relapse rate irrespective of substantial developments in tumor diagnosis and treatment[3,4]. At the present time, there is neither a particular marker that can foresee the consequences of bladder carcinoma, nor biomarkers developed from the bladder carcinoma pathogenesis could be appreciated to identify those at risk for cancer development. In the last two decades, great considerations have been given to the significant function of AMPK in malignant tumors. AMPK works as a tumor suppressor that defends against the development of cancer; this role of anti-cancer is enriched by the activator of AMPK (biguanide phenformin)[20,28]. Other studies suggested that as soon as the tumor has evolved, AMPK converts to act as a tumor promoter that protects tumor cells and enhance their survival, thus promoting tumor formation, by defending against genotoxic, oxidative and metabolic stresses[29,30]. Investigating the AMPK association with the risk of carcinoma is of great importance. The data collected from previous studies draw attention to AMPK as a main controller of the metabolism of energy, AMPK supports producing energy and inhibits consumption pathways of energy[31,32]. This molecule also triggers arrest of cell cycle, induces apoptosis and controls growth rate of tumor[33]. However, some studies reported that the AMPK role is still debatable and requires further investigation because they found that AMPK was overexpressed in tumors of various organs, including breast[22], lung[23] and prostate[16]. Furthermore, AMPK acted as a suppressive molecule of tumor and was correlated with better prognosis and concluded that this molecule could be utilized as a predictive marker and therapeutic target[12,22,23,34]. While other studies, including present one showed decreased expression of AMPK in some tumors including urinary bladder cancer. They also found a significant association between AMPK phenotype and some clinical parameters like stage and grade, lymph node metastases, blood vessel invasion and tumor relapse which gives sign for bad clinical consequences[35-40]. The observations of the present study are consistent with the findings of some reports[38-40], which described decreased level of AMPK expression in urinary bladder cancer. Also, the current findings are in alignment with the results of other studies which reported that the presence of AMPK expression considerably shows better prognosis and survival in malignant tumors[34]. Accordingly, proposing that AMPK reactivation might show a possible therapeutic usefulness in urinary bladder cancer like breast cancer[22]. The disparities between the present report and earlier ones could be clarified by the methods sensitivity, variance between patients, and sample size differences. Still more inclusive investigations are indisputably of big value for evaluating the capabilities of this marker to diagnose carcinoma of bladder and to predict its prognosis. The present report and other studies, which slowed the significance of AMPK expression in predicting the prognosis of tumors, had some weak points, for example a relatively insignificant sample size employed in these studies and immunostaining semi-quantitative analyses. In summary, our findings showed that the positive immunohistochemistry staining of AMPK was significantly correlated with low grade urinary bladder cancer. The association of AMPK with some clinical data proposes the engagement of this marker in bladder tumor progression. The outcomes of this study suggest that AMPK immunohistochemistry staining could be a supportive tool in predicting grade and prognosis of urinary bladder cancer.
| AMPK expression in urothelial cells | p value | |||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||
| n | (%) | n | (%) | |||||
| Gender | Female | 4 | -17.4 | 19 | -82.6 | 0.999 | ||
| Male | 18 | -17.1 | 87 | -82.9 | ||||
| Age at diagnosis (y) | < 60 y | 8 | -15.4 | 44 | -84.6 | 0.812 | ||
| ≥60 y | 14 | -18.4 | 62 | -81.6 | ||||
| Histologic type of tumors | Urothelial carcinoma | 16 | -15.2 | 89 | -84.8 | 0.219 | ||
| Squamous differentiation variant | 4 | -23.5 | 13 | -76.5 | ||||
| Squamous cell carcinoma | 2 | -33.3 | 4 | -66.7 | ||||
| Stage | 0 | 1 | -4.8 | 20 | -95.2 | 0.086 | ||
| I | 3 | -9.7 | 28 | -90.3 | ||||
| II | 8 | -22.2 | 28 | -77.8 | ||||
| III | 0 | 0 | 7 | -100 | ||||
| IV | 7 | -30.4 | 16 | -69.6 | ||||
| Undecided | 3 | -30 | 7 | -70 | ||||
| Grade | High grade | 12 | -18.2 | 54 | -81.8 | 0.035 | ||
| Low grade | 5 | -10 | 45 | -90 | ||||
| NA | 5 | -41.7 | 7 | -58.3 | ||||
| Muscular invasion | MIBC | 14 | -21.9 | 50 | -78.1 | 0.146 | ||
| NMIBC | 4 | -8.7 | 42 | -91.3 | ||||
| Undecided | 4 | -22.2 | 14 | -77.8 | ||||
| Lymph node | No | 16 | -15.1 | 90 | -84.9 | 0.212 | ||
| Yes | 6 | -27.3 | 16 | -72.7 | ||||
| Vascular invasion | No | 18 | -16.5 | 91 | -83.5 | 0.742 | ||
| Yes | 4 | -21.1 | 15 | -78.9 | ||||
| Metastasis | No | 16 | -15.5 | 87 | -84.5 | 0.366 | ||
| Yes | 6 | -24 | 19 | -76 | ||||
Note: NA: Undetermined grade; NMIBC: Non-Muscle Invasive Bladder Cancer and MIBC: Muscle Invasive Bladder Cancer
Table 1: Distribution of various clinicopathological variables with AMPK immunostaining in urinary bladder cancer.
Acknowledgments:
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, under grant number (G: 49-828-1440). The authors acknowledge with thanks DSR for technical and financial support.
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69-90.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30.
[Crossref] [Google Scholar] [PubMed]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49(3):466-77.
[Crossref] [Google Scholar] [PubMed]
- Prout Jr GR, Barton BA, Griffin PP, Friedell GH, Group NB. Treated history of noninvasive grade 1 transitional cell carcinoma. J Urol 1992;148(5):1413-9.
[Crossref] [Google Scholar] [PubMed]
- Youssef RF, Lotan Y. Predictors of outcome of non–muscle-invasive and muscle-invasive bladder cancer. Sci World J 2011;11:369-81.
[Crossref] [Google Scholar] [PubMed]
- Compérat EM, Burger M, Gontero P, Mostafid AH, Palou J, Rouprêt M, et al. Grading of urothelial carcinoma and the new “World Health Organisation classification of tumours of the urinary system and male genital organs 2016”. Eur Urol Focus 2019;5(3):457-66.
[Crossref] [Google Scholar] [PubMed]
- Kelloff GJ, Sigman CC, Scher HI. Biomarker development in the context of urologic cancers. Urol Oncol 2015;33(6):295-301.
[Crossref] [Google Scholar] [PubMed]
- Vara-Ciruelos D, Russell FM, Hardie DG. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol 2019;9(7):190099.
[Crossref] [Google Scholar] [PubMed]
- Cheng J, Zhang T, Ji H, Tao K, Guo J, Wei W. Functional characterization of AMP-activated protein kinase signaling in tumorigenesis. Biochim Biophysica Acta 2016;1866(2):232-51.
[Crossref] [Google Scholar] [PubMed]
- Dasgupta B, Chhipa RR. Evolving lessons on the complex role of AMPK in normal physiology and cancer. Trends Pharmacol Sci 2016;37(3):192-206.
[Crossref] [Google Scholar] [PubMed]
- Hadad SM, Fleming S, Thompson AM. Targeting AMPK: A new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol 2008;67(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem 2006;75:137-63.
[Crossref] [Google Scholar] [PubMed]
- Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: A novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther 2014;15(2):156-69.
[Crossref] [Google Scholar] [PubMed]
- Caraci F, Chisari M, Frasca G, Chiechio S, Salomone S, Pinto A, et al. Effects of phenformin on the proliferation of human tumor cell lines. Life Sci 2003;74(5):643-50.
[Crossref] [Google Scholar] [PubMed]
- Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 2004;4(10):793-805.
[Crossref] [Google Scholar] [PubMed]
- Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun 2004;321(1):161-7.
[Crossref] [Google Scholar] [PubMed]
- Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem 2005;280(47):39582-93.
[Crossref] [Google Scholar] [PubMed]
- Hardie DG, Schaffer BE, Brunet A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol 2016;26(3):190-201.
[Crossref] [Google Scholar] [PubMed]
- Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: Mechanisms of action and physiological activities. Exp Mol Med 2016;48(4):e224.
[Crossref] [Google Scholar] [PubMed]
- Pineda CT, Ramanathan S, Tacer KF, Weon JL, Potts MB, Ou YH, et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 2015;160(4):715-28.
[Crossref] [Google Scholar] [PubMed]
- Brown KA, Samarajeewa NU, Simpson ER. Endocrine-related cancers and the role of AMPK. Mol Cell Endocrinol 2013;366(2):170-9.
- Liu P, Ye F, Xie X, Li X, Tang H, Li S, et al. mir-101-3p is a key regulator of tumor metabolism in triple negative breast cancer targeting AMPK. Oncotarget 2016;7(23):35188-98.
[Crossref] [Google Scholar] [PubMed]
- O’Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, et al. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochemi J 2015;469(2):177-87.
[Crossref] [Google Scholar] [PubMed]
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J 2012;279(15):2610-23.
[Crossref] [Google Scholar] [PubMed]
- Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015;6:7365-78.
[Crossref] [Google Scholar] [PubMed]
- Al-Maghrabi J, Emam E, Gomaa W, Saggaf M, Buhmeida A, Al-Qahtani M, et al. c-MET immunostaining in colorectal carcinoma is associated with local disease recurrence. BMC Cancer 2015;15(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Khabaz MN, Butt NS, Al-Maghrabi B, Anfinan N, Sait K, Al-Maghrabi J. Leptin expression in stromal cells of endometrial carcinomas is associated with advanced stage and disease recurrence. Int J Clin Exp Pathol 2016;9(11):11774-80.
- Vila IK, Yao Y, Kim G, Xia W, Kim H, Kim SJ, et al. A UBE2O-AMPKα2 axis that promotes tumor initiation and progression offers opportunities for therapy. Cancer Cell 2017;31(2):208-24.
[Crossref] [Google Scholar] [PubMed]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012;485(7400):661-5.
- Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, et al. Genetic analysis reveals AMPK is required to support tumor growth in murine Kras-dependent lung cancer models. Cell Metab 2019;29(2):285-302.
[Crossref] [Google Scholar] [PubMed]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1(1):15-25.
[Crossref] [Google Scholar] [PubMed]
- Ha J, Guan KL, Kim J. AMPK and autophagy in glucose/glycogen metabolism. Mol Aspects Med 2015;46:46-62.
[Crossref] [Google Scholar] [PubMed]
- Sanduja S, Feng Y, Mathis RA, Sokol ES, Reinhardt F, Halaban R, Gupta PB. AMPK promotes tolerance to Ras pathway inhibition by activating autophagy. Oncogene 2016;35(40):5295-303.
[Crossref] [Google Scholar] [PubMed]
- William WN, Kim JS, Liu DD, Solis L, Behrens C, Lee JJ, et al. The impact of phosphorylated AMP-activated protein kinase expression on lung cancer survival. Ann Oncol 2012;23(1):78-85.
[Crossref] [Google Scholar] [PubMed]
- Al-Maghrabi J, Al-Sakkaf K, Qureshi IA, Butt NS, Damnhory L, Elshal M, et al. AMPK expression patterns are significantly associated with poor prognosis in breast cancer patients. Ann Diagn Pathol 2017;29:62-67.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Li X, Xie X, Ye F, Chen B, Song C, Tang H, Xie X. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast 2016;30:39-46.
[Crossref] [Google Scholar] [PubMed]
- Guo C, Hao C, Shao R, Fang B, Correa AM, Hofstetter WL, et al. RNA-dependent protein kinase (PKR) depletes nutrients, inducing phosphorylation of AMP-activated kinase in lung cancer. Oncotarget 2015;6(13):11114-24.
[Crossref] [Google Scholar] [PubMed]
- Kopsiaftis S, Hegde P, Taylor III JA, Claffey KP. AMPKα is suppressed in bladder cancer through macrophage-mediated mechanisms. Transl Oncol 2016;9(6):606-16.
[Crossref] [Google Scholar] [PubMed]
- Kopsiaftis S, Sullivan KL, Garg I, Taylor III JA, Claffey KP. AMPKα2 regulates bladder cancer growth through SKP2-mediated degradation of p27. Mol Cancer Res 2016;14(12):1182-94.
[Crossref] [Google Scholar] [PubMed]
- Massari F, Ciccarese C, Santoni M, Iacovelli R, Mazzucchelli R, Piva F, et al. Metabolic phenotype of bladder cancer. Cancer Treat Rev 2016;45:46-57.
[Crossref] [Google Scholar] [PubMed]