- *Corresponding Author:
- Zunyue Li
Department of Pulmonary Medicine, Fushun County Hospital of Traditional Chinese Medicine, Fushun, Sichuan Province 643200, China
E-mail: lizunyue527@163.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “133-140” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To systematically evaluate the efficacy of adrenocorticotropic hormone in the treatment of respiratory diseases is the objective of the study. The literature related to controlled trials on the application of adrenocorticotropic hormone drugs for the treatment of patients with respiratory diseases was searched in Cochrane library, Web of science, PubMed, ProQuest and Chinese biomedical literature databases Wanfang, Wipu and China Knowledge Network from the time of establishment to August 2022, and the software review manager 5.3 was used to analyze the 10 included literatures and the heterogeneity of the studies was explored. This meta-analysis showed that adrenocorticotropic hormone improved the efficiency of treatment for patients with respiratory diseases [Odds ratio: 0.17, 95 % confidence interval (0.13, 0.21), p<0.00001] and improved patients lung function [Odds ratio: 2.98, 95 % confidence interval (2.63, 3.32), p<0.00001], with all of the above differences being statistically significant. In addition, 3 papers reported safety findings, 1 paper reported no adverse reactions in either group and 2 papers mentioned a lower incidence of adverse reactions in the experimental group than in the control group [Odds ratio: -0.17, 95 % confidence interval (-0.27, -0.07), p=0.001]. The funnel plots for each study suggested no publication bias. The use of adrenocorticotropic hormone drugs in the treatment of patients with respiratory diseases can significantly improve their clinical outcomes and lung function, and has a good safety profile, which is worth promoting in clinical practice. However, the quality of the literature included in this study is low and the quality of the sample is variable, so the precision of the argument is limited, and there is a need to conduct randomised controlled studies of higher quality and similar sample size for further analysis.
Keywords
Adrenocorticotropic hormone, respiratory diseases, chronic obstructive pulmonary diseases, bronchial asthma
Respiratory diseases are infectious and non-infectious diseases of the respiratory system such as bronchitis, pharyngitis, emphysema and chronic bronchopathy[1]. Most respiratory diseases, such as bronchitis and asthma, are common and frequent[2]. The pathogenesis of respiratory diseases is complex and patients are often affected by pathogens or lifestyle factors that lead to inflammatory reactions in the respiratory system, poor ventilation and insufficient oxygen levels in the blood vessels[3]. Most patients with respiratory diseases have a long and even lifelong course and frequent recurrent episodes, especially in elderly patients, who have bronchiectasis, but in the early stages of the disease there is no specific manifestation other than a mild cough, which is insidious and develops gradually into chronic bronchitis, which seriously affects the quality of life of patients. In addition, the increasing misuse of antibiotics has led to serious resistance to antibiotics in some patients, which has made treatment of the disease much more difficult[4,5]. Therefore, there is an urgent need to find an effective drug to improve the poor ventilation and inflammation in respiratory patients and to improve their blood oxygen levels.
Adrenal Cortex Hormone (ACH) has the main role of regulating the body’s metabolism of water, salt, sugar and cholesterol and is anti-infective and anti-immune[6]. In recent years, it has been frequently used in the treatment of respiratory diseases. A number of studies have been conducted over a long period of time to confirm that clinical treatment with ACH can improve the outcome of respiratory diseases, however, due to the variability of interventions in various studies, as well as the different evaluation methods and the small sample sizes in the current published studies, this has led to a lack of validity, comprehensiveness and systematization of the studies[7]. Therefore, this study provides evidence-based data for the treatment of respiratory diseases by searching the literature on the use of ACH in patients with respiratory diseases at home and abroad and analysing the effects of its use.
Materials and Methods
Literature search:
The library’s online resources are used to access relevant literature and keep abreast of domestic and foreign research progress. Database sources include PubMed, Cochrane library, Web of science, ProQuest and the Chinese biomedical literature databases Wanfang, China Knowledge Network and Wipu. Searches were conducted using a combination of subject terms and free words and manual searches were used to track down relevant references when necessary. Randomised controlled trials on the use of ACH drugs for the treatment of respiratory diseases were searched in the library from the time of establishment to December 2022. In addition, references to the included literature were traced to obtain relevant literature as a supplement. The search was conducted using a combination of subject terms and keywords, and the English search terms were: "Adrenal cortex hormone", "Respiratory diseases", and "Chronic obstructive pulmonary diseases", "Bronchial asthma", etc. Chinese keywords include adrenal cortex hormone, respiratory diseases, chronic obstructive pulmonary diseases, bronchial asthma, etc.
Literature inclusion and exclusion criteria:
Inclusion criteria: Type of study-Chinese or English randomized controlled trials published in China and abroad on ACH for respiratory diseases; study population-Meeting the diagnostic criteria for respiratory internal medicine diseases in the internal medicine[8], with relative consistency between the two groups in terms of age, gender, duration of disease and education level; interventions-The control group mainly consisted of sputum treatment, antibiotics to prevent infection, effective oxygen therapy and other symptomatic treatments. As patients with respiratory diseases are all infected to varying degrees after the onset of illness, sputum should be retained before giving them antibiotics and saline gargles should be given to them before and after they cough. The experimental group should be given the same conventional treatment as the control group and at the same time choose appropriate ACH drugs such as fluticasone as adjuvant treatment, with the exact dosage, administration and duration of treatment depending on the patient’s own condition; outcome indicators-Clinical efficacy, lung function and overall incidence of adverse reactions after the patients received ACH drugs.
Exclusion criteria: Documents where the content of the article is mutilated or where the original text cannot be extracted; documents belonging to the types of conference proceedings, summaries of experience and reviews; the design of the article is not sufficiently rigorous; literature in languages other than Chinese and English; repeated publications limited to 1 article and literature on the experimental group combined with other interventions in addition to the application of ACH drugs.
Literature screening and data extraction:
The literature that met the study objectives and inclusion and exclusion criteria was screened by reading its title and abstract and removing those that did not fit with the current analysis. Literature with poor study design protocols, poor quality and no usable data were removed by further reading of the full text. The literature data were extracted and collated using Microsoft excel software, including first author, publication date, sample size, intervention method and outcome indicators for each trial. The literature was screened independently by two investigators (trained in full systematic evaluation) and any disagreements were negotiated on the basis of the screening criteria, consulting a third party expert with expertise in the field if necessary.
Evaluation of the quality of the literature:
The included literature was independently evaluated by two researchers based on the Cochrane risk of bias assessment tool version 5.1[9,10]. The risk of bias of the included literature was assessed, items were classified into three categories of "high", "low" and "unclear" and consisted of the following seven items. They are random assignment method; allocation protocol concealment; blinding of study subjects and treatment protocol implementers; blinding of the study outcome measure; completeness of outcome data; selective reporting of study results and other sources of bias. If low risk was fully met, the study was assigned "Grade A", if partially met, "Grade B", if not met, "Grade C" and all entries were assigned "High risk bias". High-risk bias" was excluded.
Statistical methods:
Statistical processing was performed using ReviewManager (RevMan) 5.3 for meta-analysis, primarily using I2 to evaluate the magnitude of heterogeneity. When I2<50 % and p>0.1, this suggested low or no heterogeneity between studies, and a fixed-effects model was used for the meta-analysis. However, when I2≥50 % and p≤0.1, it suggested high heterogeneity and the meta-analysis used a random-effects model and finally a funnel plot for bias evaluation.
Results and Discussion
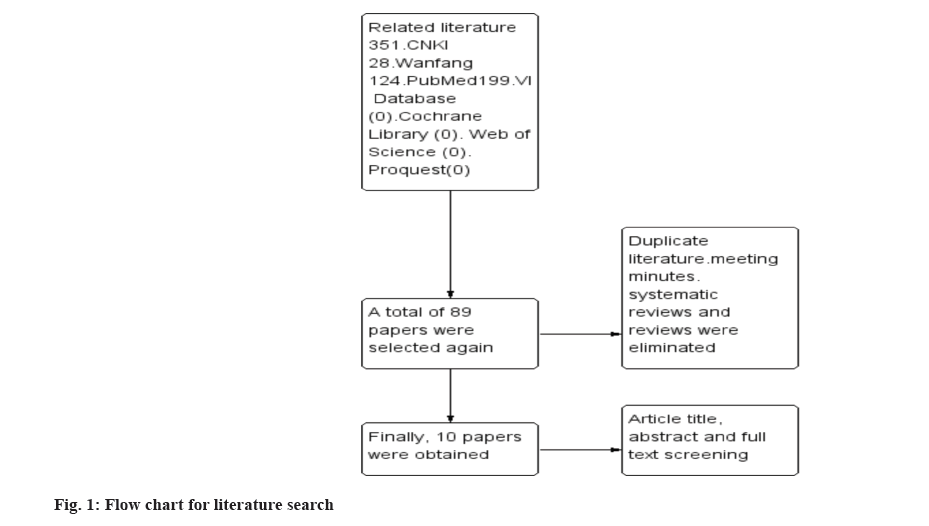
Results of literature search were evaluated here. The search of each database according to the above keywords yielded a total of 351 papers. 89 papers were obtained after removing duplicates, conference proceedings, systematic evaluations and reviews, and then 10 papers were finally obtained after reading the titles, abstracts and full texts of the articles. The specific search process was shown in fig. 1.
Basic features of inclusive research were shown in Table 1[11-20]. A total of 10 articles were included in the literature, published between 2016 and 2022 and the cases were all sourced from China. There were 1094 patients with respiratory diseases in the literature, including 547 cases in both the control and experimental groups, with a minimum sample size of 54 cases and a maximum sample size of 180 cases. Interventions-The experimental group in all five studies was treated with adrenocorticosteroids and the control group was treated with conventional Western medicine. The characteristics of the literature are shown in Table 1.
| First author | Published (year) | Sample size (control/experimental group) | Grouping method | Intervention methods | Closing indicators | |
|---|---|---|---|---|---|---|
| Control group | Experimental group | |||||
| Zhao J[11] | 2016 | 60/60 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1 |
| Wang XD[12] | 2022 | 30/30 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1, 2, 3, 4 |
| Wei ZQ[13] | 2019 | 48/48 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1, 2 |
| Yao YF et al.[14] | 2020 | 90/90 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1 |
| Xie C et al.[15] | 2019 | 54/54 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1, 2, 4 |
| Huang YG[16] | 2017 | 65/65 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1 |
| Liu CY[17] | 2021 | 63/63 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+inhaled fluticasone | 1, 3 |
| Zhao TZ[18] | 2019 | 50/50 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1, 3 |
| Wang YX[19] | 2018 | 60/60 | Randomised controlled trials | Conventional drug treatment | Conventional drugs+ACH | 1, 4 |
| Zhou YX[20] | 2017 | 27/27 | Randomised controlled trials | Conventional drug treatment | Conventional medicine+fluticasone propionate aerosol | 1, 3 |
Note: 1: Clinical efficacy; 2: Clinical indicators, 2, 3: Lung function and 4: Occurrence of adverse reactions or complications
Table 1: Basic Characteristics of the Included Literature
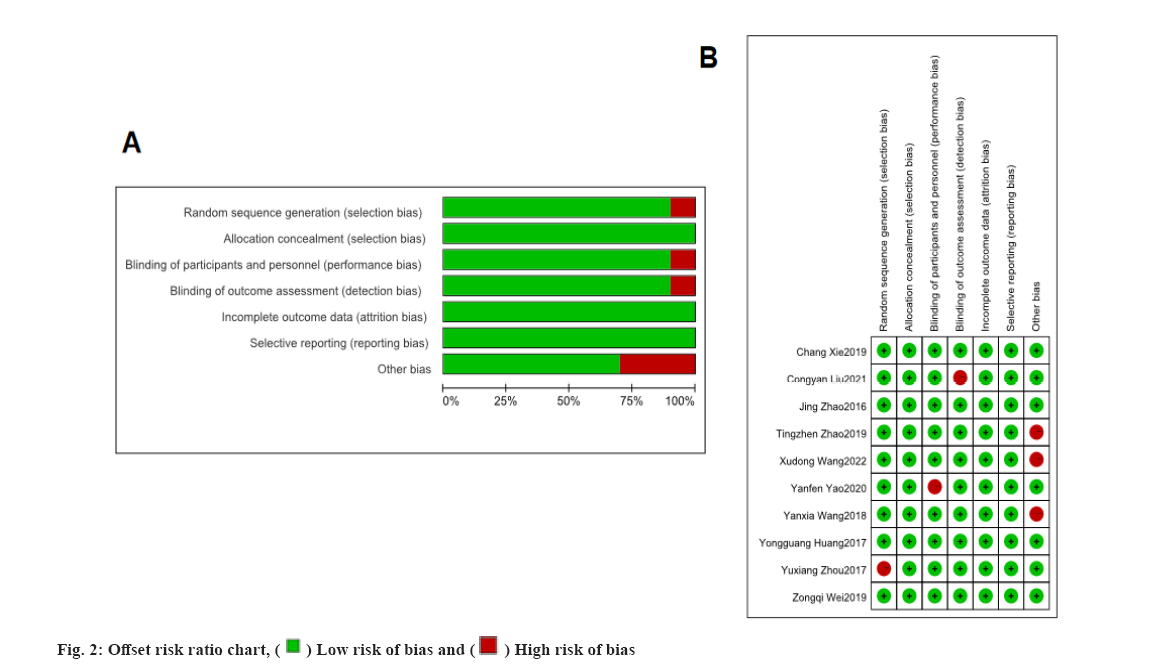
All 10 included papers were randomised controlled trials and none of the studies described allocation concealment, blinded selective reporting and told other sources of bias. Each of these papers reported efficacy outcomes, four referred to pulmonary function index outcomes, three referred to adverse effects or complication outcomes and three reported clinical index outcomes. The results of the risk of bias assessment are detailed in fig. 2.
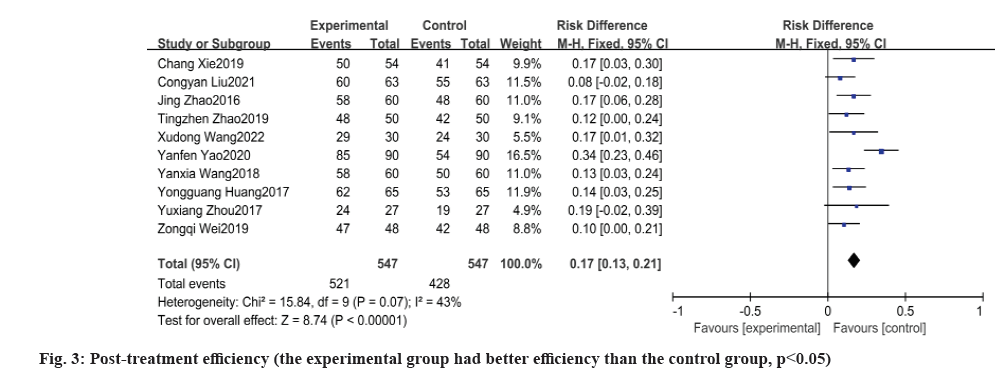
Meta-analysis of efficacy results was discussed as follows. Post-treatment efficiency was calculated here. All 10 papers reported the total effective rate after treatment. Total effective rate=(significant+effective)/total number of cases×100 % or total effective rate=(significantly improved+improved)/total number of cases×100 %. Heterogeneity between the experimental and control groups was tested yielding p=0.07, I2=43 %, there was no heterogeneity and the results of meta-analysis by fixed effects model showed that the experimental group had a higher effective rate after treatment than the control group and the difference between the two groups was statistically significant [Odds Ratio (OR): 0.17, 95 % Confidence Interval (CI): 0.13, 0.21, p<0.00001] as shown in fig. 3.
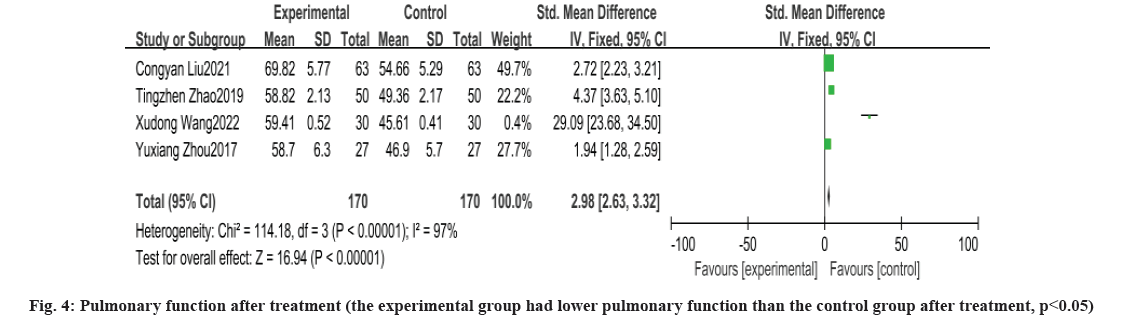
Pulmonary function after treatment was shown in fig. 4. A total of four papers reported changes in lung function after treatment with heterogeneity between the experimental and control groups tested yielding p<0.00001, I2=97 %, with heterogeneity, and a random effects model for meta-analysis showed that percentage of Forced Expiratory Volume in the 1 s (FEV1%) was higher in the experimental group than in the control group after treatment, with a statistically significant difference between the two groups [OR: 2.98, 95 % CI: 2.63, 3.32, p<0.00001].
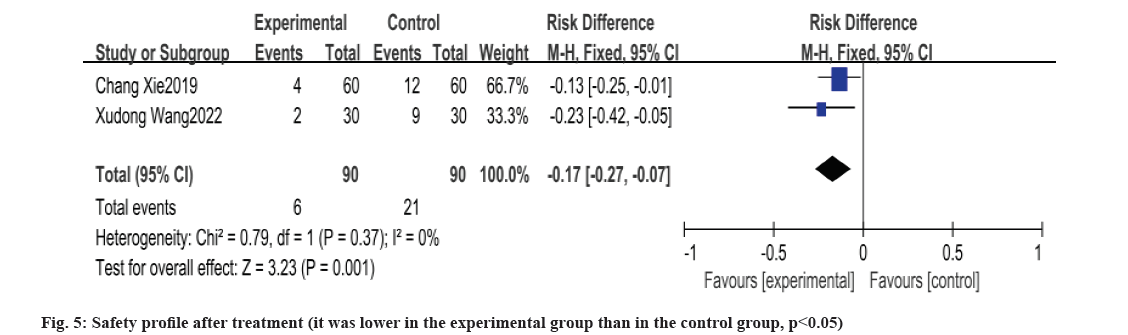
Safety profile after treatment was shown in fig. 5. In this study, three papers reported safety-related findings, of which, one reported no adverse reaction situation after treatment in both groups, the remaining two papers had a lower incidence of adverse reactions in the experimental group than in the control group and the test of heterogeneity between the experimental and control groups yielded p=0.37, I2=0 %, no heterogeneity, and the results of meta-analysis by fixed-effects model showed that the experimental group had the lower incidence of adverse reactions in the experimental group than in the control group, and the difference between the two groups was statistically significant [OR: -0.17, 95 % CI: -0.27, -0.07, p=0.001].
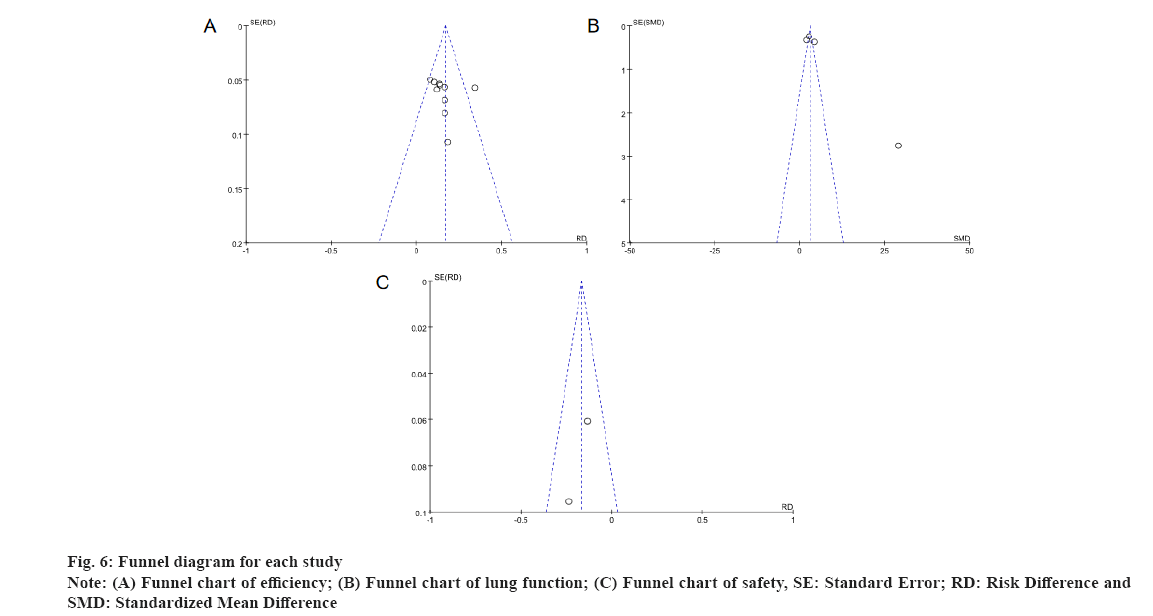
Publication bias evaluation was shown in fig. 6. The individual study which includes the funnel plots, suggest that the scatter is essentially symmetrical and distributed in a funnel shape, so the funnel plots suggest no publication bias.
In recent decades, as global environmental pollution has become more serious and hazy weather has gradually increased, the number of people suffering from respiratory diseases has increased and the age of onset has become younger. At the same time, respiratory diseases are showing many new features[21], the pathogenesis is becoming more complex-In addition to viral and bacterial infections, other external factors may also cause or even aggravate respiratory diseases, which makes the targeting of disease treatment a serious challenge[22]; rapid onset-Due to increasing environmental pollution, the onset of respiratory diseases is becoming shorter and shorter, therefore, medical staff often do not have enough time to determine the mechanism and cause of the patient’s illness, and are unable to formulate individualized treatment plans, resulting in missed treatment timing and delayed treatment; more prone to relapse-The recurrence of the disease will lead to the mutation of pathogenic bacteria in the organism, which greatly increases the difficulty of treatment, causing a vicious circle, making it difficult to eradicate the disease and often accompanying the patient’s condition for life, seriously affecting the patient's quality of life[23]; increased complications-As the disease persists and progressively deteriorates, it is often complicated by cervical lymphadenitis, stomatitis and even sepsis in the later stages of the disease. Therefore, the current treatment of respiratory diseases should be based on a strict and rapid grasp of the mechanisms of the disease, and the right medicine should be used to actively prevent and treat complications and slow down the progress and deterioration of the disease[24].
At this stage, many diseases in respiratory medicine such as infectious diseases, hemorrhagic shock and autoimmune diseases are clinically treated with ACH to relieve clinical symptoms such as asthma, cough and hemorrhagic shock. It is also effective in the treatment of diseases related to respiratory medicine. For patients suffering from respiratory diseases, ACH can play an anti-inflammatory and anti-fibrotic role. For example, ACH can alleviate the symptoms of acute exacerbation and severely impaired lung function in patients with chronic obstructive pulmonary disease and can also largely down-regulate the mortality rate and complication rate of pulmonary tuberculosis and improve the quality of life of patients. Overseas reports have shown that ACH can bind to cells in respiratory organs to exert anti-inflammatory effects, while the lipocortin produced can also stimulate the activity of neutrophils to break down necrotic cells, with a dual anti-inflammatory effect[25].
Meta-analysis is a quantitative analysis of the results of multiple studies that share a common object of study and are independent of each other, to analyze the differences in the characteristics of the research questions and to evaluate the results of the studies in a comprehensive manner. This method of analysis is more scientific and rigorous than traditional reviews, and can improve the efficacy of statistical tests and the consistency of results evaluation, making the findings more reliable and comprehensive[26]. Therefore, this study is a meta-analysis of the effectiveness of autologous blood therapy in the treatment of bronchiectasis, to analyze the effectiveness and safety of ACH in the treatment of respiratory diseases, and to provide a medical basis for clinical work.
A total of 10 papers were included in this study, with 547 study subjects in both the control and experimental groups. The treatment efficiency, lung function and adverse reaction rate of patients in the experimental group were significantly better than those in the control group and the data were statistically significant (p<0.05).
ACH improves the efficiency of treatment of patients with respiratory diseases. In this study, the control group was treated with conventional Western medicine drugs which were able to promote recovery through anti-inflammatory, wheezing, antispasmodic and acid-base balance, but in more severe respiratory diseases, due to the progression of the disease, the airway resistance gradually increases and irreversible airflow is blocked in the airway, when the treatment with conventional drugs such as anti-infection, wheezing and relief of airway spasm alone is ineffective. In contrast, in this study, the use of ACH drugs was found to significantly increase the efficiency of treatment of patients with respiratory diseases. The reason for this may be that the use of ACH drugs in addition to conventional treatment improves the anti-inflammatory capacity of the airways to a certain extent, stabilizes the development of the disease and reduces the chance of acute exacerbation, relieves the inflammatory response of the airways and prevents the deterioration of the patient’s condition, thus improving the efficiency of treatment[27].
ACH also improves lung function in patients with respiratory disease. ACH is a lipid-soluble drug, which can bind to hormone receptors in cells after rapid diffusion in human body and activate hormone receptors in body, produce binding substances, and also activate anti-inflammatory genes to improve the anti-inflammatory ability and effect of the body. Macrophages and T cells can secrete pro-fibrotic cytokines to accelerate lung fibrosis in respiratory medicine patients, and inhibit macrophages from continuously producing cytokines to effectively relieve lung fibrosis. ACH can not only greatly improve the efficacy of respiratory diseases, but also have a better effect on the improvement of lung function. For example, beclomethasone propionate aerosol is an ACH drug with strong anti-inflammatory and anti-sensitizing effects, thus helping to alleviate airway mucosal swelling and bronchospasm; in addition, beclomethasone propionate aerosol can reduce tissue inflammation and fluticasone aerosol is also an ACH drug, which can act directly on the lungs to control asthma symptoms quickly with its strong anti-inflammatory effects and down-regulate the use of other drugs. The dose used is better for improving lung function levels[28].
There are few studies on the differences in safety of ACH after treatment of patients with respiratory diseases and studies on the safety of medication can be added at a later stage.
Only three papers in this study reported adverse reaction findings but one reported no adverse reactions in either group, while the other two mentioned a lower incidence of adverse reactions in the experimental group than in the control group. This indicates that the safety of ACH drugs in the treatment of respiratory diseases is good. However, if ACH drugs are used inappropriately, psychiatric adverse effects and even cardiac arrhythmias may occur in severe cases, and even though the duration of administration was short in the trial, strict 24 h Electrocardiogram (ECG) monitoring should be performed during treatment. Whether administered intravenously, orally or by inhalation, drug contraindications should be strictly controlled according to the patient’s actual situation and the appropriate route of administration should be selected on merit.
Limitations of this study are as follows-The age, duration and condition of the subjects in the included studies differed to a certain extent, thus causing statistical heterogeneity and clinical heterogeneity that may affect the results of the meta-analysis in this paper; individual literature has flaws in the study methodology and low study quality, which is likely to have potential bias and affect the reliability of the results; the specific dose, usage and duration of conventional Western medicine treatment in the control group of study subjects as well as there were differences in the specific doses, usage, duration of treatment and measurement of outcome indicators in the control subjects, which were clinically heterogeneous, resulting in the possibility of bias in the results of the meta-analysis; the quality of the 10 included papers was low and the sample quality was variable, limiting the precision of the argumentative results.
Available evidence suggests that the use of ACH in the treatment of respiratory medicine patients can significantly improve clinical outcomes and lung function, and has a good safety profile, making it worthwhile to promote its use in the clinical setting. However, due to the limitations of this study, a multicenter, randomised, double-blind, large sample randomised controlled trial is still needed to further validate the results.
Conflict of interests:
The authors declared no conflict of interest.
References
- Si XL, Luan XD, Xu I. Analysis of the effect of clinical nursing protection on nursing management of respiratory medicine. China Health Ind 2022;19(17):74-7.
- Zhang B, Lu HY, Gu Y, Bian J, Yang JF, Lu CL, et al. Analysis on nutritional status and its influencing factors in elderly patients with chronic obstructive pulmonary disease. J N Sichuan Med Coll 2021;36(10):1319-22.
- Simkovich SM, Goodman D, Roa C, Crocker ME, Gianella GE, Kirenga BJ, et al. The health and social implications of household air pollution and respiratory diseases. NPJ Prim Care Respir Med 2019;29(1):12.
[Crossref] [Google scholar] [PubMed]
- Liu WP, Yang YF, Zhang K, Xing HM, Yang LF, Li HX, et al. Investigation and analysis of nosocomial infections in hospitalized patients of respiratory medicine department from 2015 to 2018. Chinese J Disinfect 2020;37(11):851-4.
- Chen JX, Yang S, Wu G. Impact of pharmacist intervention on rational use of antibiotics in the inpatients of respiratory medicine. J Navy Med 2021;42(2):192-5.
- Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs 2019;38(5):336-9.
[Crossref] [Google scholar] [PubMed]
- Li ZX, Feng GL, Li XH. Research progress on the effect of traumatic hemorrhagic shock on the level of adrenocorticotropic hormone. Chin Emerg Med 2020;40(1):85-9.
- Ye R, Lu ZY. Internal medicine. 6th ed. People’s Health Press, 2004.
- Tian Y, Wang Z. Meta-analysis of the efficacy and safety of adding reed stem soup in the treatment of bronchiectasis. Clin Res Chin Med 2020;12(11):5.
- Han WH, Xie Y, Wang JJ, Li JS. Systematic evaluation and meta-analysis and grade evaluation of clinical efficacy and safety of phlegm-heat-clearing injection for bronchiectasis. Chin J Gerontol 2019;39(16):3949-57.
- Zhao J. The clinical value of adrenocorticotropic hormone in respiratory medicine. Everybody Health 2016;10(7):111-2.
- Wang XD. Efficacy of Adrenocorticotropic Hormone (ACH) in the treatment of respiratory medicine diseases. China Contemp Med 2022;28(4):121-3.
- Wei ZQ. Analysis of adrenocorticotropic hormone treatment in respiratory medicine diseases. Jilin Med J 2019;40(9):2074-5.
- Yao YF, Li Y, Rong MS. Analysis of the effect of clinical application of adrenocorticotropic hormone in respiratory medicine. Pregnancy Parent 2020;2(8):115.
- Xie C, Sun SB. Clinical experience of adrenocorticotropic hormone treatment in respiratory medicine diseases. Electron J Clin Med Lit 2019;6(56):38-9.
- Huang YG. Analysis of the clinical application effect of adrenocorticotropic hormone in respiratory medicine. Dkgest Latest Med Inf 2017;17(56):151.
- Liu CY. Clinical application and curative effect observation of adrenocortical hormone in respiratory medicine. J Math Med 2021;34(2):264-5.
- Zhao TZ. Exploring the clinical application and efficacy observation of adrenocorticotropic hormone in respiratory medicine. Health Guide 2019(35):81.
- Wang YX. Therapeutic effects of adrenocorticotropic hormones in respiratory medicine. Diet Health 2018;5(34):40.
- Zhou YX. Analysis of clinical effect of adrenal cortical hormone in the department of respiratory medicine. Chin Community Doct 2017;33(5):34-5.
- Joshi M, Goraya H, Joshi A, Bartter T. Climate change and respiratory diseases: A 2020 perspective. Curr Opin Pulm Med 2020;26(2):119-27.
[Crossref] [Google scholar] [PubMed]
- Aghasafari P, George U, Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm Res 2019;68:59-74.
[Crossref] [Google scholar] [PubMed]
- Liu Y, Yu YN, Yu XW. Analysis of pathogenic bacteria detection and drug resistance in patients with respiratory diseases. South China J Prev Med 2021;47(2):266-9.
- Chen X, Sun RY, Zhang BW. Clinical study of acetylcysteine solution combined with terbutaline in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Mod Drug Clin 2021;36(12):2611-6.
- Chellappan DK, Yee LW, Xuan KY, Kunalan K, Rou LC, Jean LS, et al. Targeting neutrophils using novel drug delivery systems in chronic respiratory diseases. Drug Dev Res 2020;81(4):419-36.
- Yao J, Yao C, Yang SX. Systematic evaluation of norepinephrine in shock treatment/meta-analysis of methodological quality evaluation. China Pharm Her 2021;18(7):99-103.
- Ma Y. Clinical application and efficacy of adrenocorticotropic hormone in respiratory medicine. China Healthcare Nutr 2021;31(16):102.
- Shi XM, Gao YD. Clinical study on the combination of sulforaphane cough capsules with salbutamol sulfate and fluticasone propionate aerosol in the treatment of children with cough variant asthma. China Drug Clin 2020;20(4):512-4.