- *Corresponding Author:
- Ying Zhang
Department of Otolaryngology, Beijing University of Chinese Medicine, Dongzhimen Hospital, Dongcheng, Beijing 100700, China
E-mail: Zyingying0130@126.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “108-113” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The purpose of this study is to evaluate the efficacy of the Chinese herbal ointment Qingluo San in preventing phlebitis in patients with peripheral venous catheters. We conducted a single-center randomized controlled trial, in which 127 eligible patients were assigned randomly to one of two groups; the experimental group or the control group. The degrees of pain intensity and phlebitis were measured at baseline, 24 h, 48 h and 72 h. Demographic information (age, gender, body mass index) and phlebitis sites, catheter dwell time, hemoglobin and white blood cells were obtained from all patients. Compared with the control group, the experimental group showed significantly lower incidence of phlebitis and pain intensity at 24 h, 48 h and 72 h after intervention. However, there were no significant differences about the demographic information (age, gender, body mass index) and phlebitis sites, catheter dwell time, hemoglobin and white blood cells between these two groups. In patients with peripheral venous catheters, external treatment with Qingluo San was an effective method for decreasing the incidence of phlebitis.
Keywords
Peripheral venous catheter, phlebitis, Qingluo San, catheterization
Over 70 % of inpatients require at least one catheterization during their hospital stay, with peripheral venous catheters being the most frequently used vascular device for infusion therapy[1]. The peripheral venous catheter is an invasive procedure, which frequently fails before the treatment is complete. It has been reported that general catheter failure rates range from 35 % to 50 %, regardless of how rigorously controlled the environment[2]. Failure of a catheter generally requires its removal or replacement along with treatment of related sequelae, which can result in venous depletion, a disruption of therapy and an increase in the workload of nurses, adversely affecting the hospital experience for the patient and increasing the cost of healthcare for the hospital[3]. Various types of catheter failure may occur, including mechanical (dislodges, occlusions, infiltrations or extravasations), vascular (phlebitis) and infectious (local infections or bloodstream infections)[4]. There is a significant discrepancy between the results of previous studies[5]. Studies have reported that phlebitis is the most common complication, but other studies have found that the incidence varies widely between 2.3 % and 63.3 %[6].
It has been shown that Traditional Chinese Medicine (TCM) has a positive impact on external therapies as well as on health care in general[7]. Over the past few decades, traditional herbal medicines have received considerable attention for the treatment of different types of phlebitis[8,9]. There has been recent research on the effects of herbal extracts such as Qing Luo Tong Mai pills and aloe vera on preventing and treating phlebitis in both animal and human models[9,10]. Typically, Qingluo Yin is composed of four main ingredients; rhizoma Dioscorea tokoro, green wind vine, Sophora flavescens (S. flavescens) radix and Huang boon. Qingluo San, also known as Qingluo Yin, is a Chinese medicine which aims to clear heat and dampness, dredge collaterals and relieve arthralgia[11]. As of yet, little is known about the effects of Qingluo San externally applied to peripheral venous catheter phlebitis. Qingluo San was evaluated for its effectiveness in treating peripheral venous catheter-associated phlebitis in the present study.
Materials and Methods
Design of study:
During the period December 2020 through November 2021, a randomized, controlled, single-center trial is being conducted in our hospital. Randomly assigned patients with a peripheral venous catheter were divided into two groups; those receiving an experimental catheter and those receiving a control catheter.
Inclusion and exclusion criteria:
This study included patients who met the following criteria for inclusion in the study; aged 18 y or older; patients undergoing hospitalization; having the ability to communicate orally; an arterial catheter is placed in the peripheral vein and is capable of carrying out normal coagulation functions. Those who were excluded from the study included those with contraindications to tubes, such as a history of vascular surgery and a rash on the skin or abnormal coagulation function.
Randomization:
A convenience sampling method was used to select participants and those who met the inclusion criteria were randomly assigned to two equal groups; experimental (n=62) and control (n=65) by using the table of random numbers. During the course of the trial, the first research assistant was responsible for handling randomization and was the only member of the research team who had access to the random numbers.
Ethical approval:
A protocol for the study was approved by both the ethics committee and institutional review board of our hospital before the start of the study. Throughout all the procedures, the Declaration of Helsinki was followed as a guideline. Informed consent was obtained from all patients before enrollment in the study and the study methods and objectives were explained to them. In addition, patients were assured that their personal information would be kept confidential and their records would remain anonymous. According to the findings of the pilot study, all the patients were entitled to withdraw from the study at any time and could be assured of the safety of the intervention at any time.
Intervention:
Qingluo San was externally applied to the sites of phlebitis in the experimental group and contained the following ingredients; among the medicines given were S. flavescens radix (9 g), Huang boon (9 g), green wind vine (9 g), rhizoma Dioscoreae tokoro (10 g), fructus forsythiae (9 g), rhubarb (9 g), corydalis rhizoma (9 g) and scorpion (5 g). Chinese herbal medicine is applied externally by mixing powdered Chinese herbal medicine with adjuvants (70 % black vinegar and 30 % honey) and then rubbing on the skin. The paste was then applied externally to the sites of phlebitis; with a thickness of approximately 4 mm (the scope of applying externally should be greater to the area of phlebitis). It is recommended to avoid external application if there is a skin ulcer. Fixation was achieved by applying a gauze bandage and an elastic bandage externally. During the course of treatment, the external application was conducted once per day for 3 d, for a total of 6 h.
Visual Infusion Phlebitis Scale (VIPS) and Visual Analogue Scale (VAS):
In patients receiving IV infusions, the VIPS are a validated visual tool that is used to determine the severity of phlebitis. According to this scale, phlebitis symptoms (such as swelling, erythema, pain, pallor and induration) are numerically rated based on the presence of certain symptoms. Using this scale, health-care providers are advised to take specific actions based on their rating scores. As a result of the standardization of this scale, the catheter dwell time is eliminated as an important variable during peripheral IV site changes. A score of 0 indicates the absence of phlebitis and a score of 5 indicates advanced thrombophlebitis. A VAS measure was used to assess the pain intensity at the affected area. This measure includes a continuous scale with two end points of zero and ten (0: no pain, 1-3: mild pain, 4-7: moderate pain and 8-10: severe pain).
The demographic data (age, gender, Body Mass Index (BMI), phlebitis sites, catheter dwell time, Hemoglobin (HB) and White Blood Cells (WBC) count) were collected from each patient.
Data analysis:
An analysis of the data was conducted using Statistical Package for the Social Sciences (SPSS), version 21.0, (IBM Corp., Armonk, New York, United States of America (USA)). It has been determined that both demographic variables as well as outcome variables were examined by analyzing the frequency distributions based on categorical variables as well as the means and standard deviations based on continuous variables. Using the Chi-square (χ2) test, categorical variables were compared against continuous variables, while independent sample t tests were used to assess differences between them. It was considered statistically significant if the p<0.05.
Results and Discussion
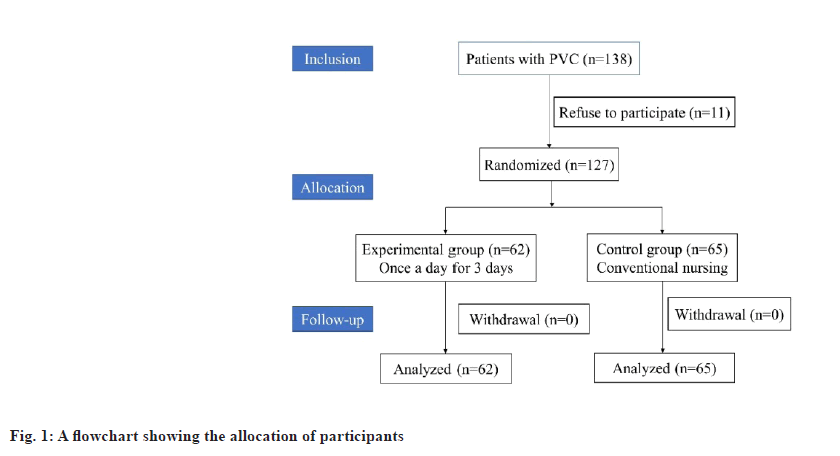
This study randomized 138 patients with peripheral venous catheters, of whom 11 declined to take part in the study. It was determined that 127 of these samples could be analyzed, 62 of which belonged to the treatment group and 65 to the control group. Follow-up was not lost as shown in fig. 1.
Table 1 shows the differences in demographic and clinical information between the experimental and control groups. 48.38 % and 50.76 % of patients were male in the experimental group and the control group. The mean age of the patients was (50.00±4.72) y and (51.33±6.43) y in the experimental group and the control group. The BMI were (21.86±3.66) and (22.42±3.91) kg/m2. The phlebitis sites were metacarpal; basilica and median cubital were nearly 40 %, 30 % and 20 % in both groups. The mean catheter dwell time was (3.56±1.17) and (3.24±1.14) d in the experimental group and the control group, respectively. No significant differences were observed in terms of the characteristics, such as age, gender, BMI, phlebitis sites, catheter dwell time, HB and WBC (p>0.05).
| Variables | Experimental group (n=62) | Control group (n=65) | p value |
|---|---|---|---|
| Age (year, mean±SD) | 50.00±4.72 | 51.33±6.43 | 0.18 |
| Gender (n, %) | 0.694 | 0.694 | 0.694 |
| Male | 30, 48.38 % | 33, 50.76 % | 0.78 |
| Female | 32, 51.62 % | 32, 49.24 % | |
| BMI (kg/m2) | 21.86±3.66 | 22.42±3.91 | 0.4 |
| Phlebitis sites (vein) (n, %) | 0.694 | 0.694 | 0.694 |
| Metacarpal | 29, 46.77 % | 31, 47.69 % | 0.92 |
| Basilica | 20, 32.25 % | 19, 29.23 % | |
| Median cubital | 13, 20.96 % | 15, 23.07 % | |
| Catheter dwell time (day) | 3.56±1.17 | 3.24±1.14 | 0.12 |
| HB (×102 g/l) | 1.41±0.31 | 1.34±0.29 | 0.19 |
| WBC (×109/l) | 5.90±1.83 | 5.91±1.67 | 0.97 |
Table 1: Demographic and Clinical Characteristics of The Patients With Phlebitis In The Experimental and Control Groups
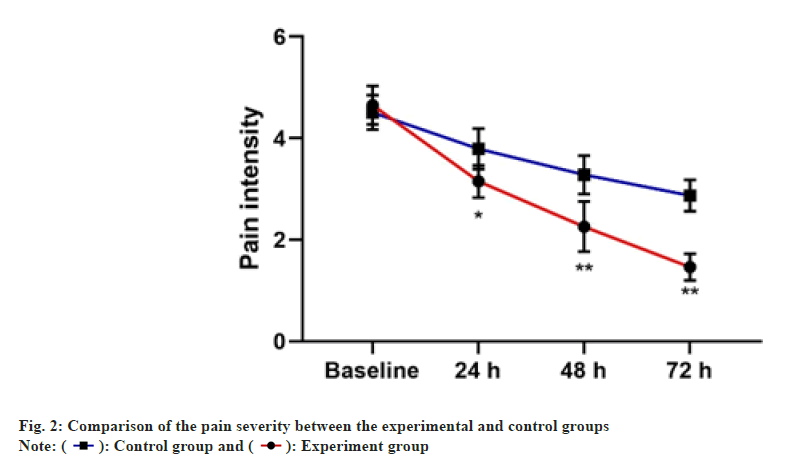
The pain severity and phlebitis score of both experimental and control groups are presented in Table 2 and fig. 2. The mean of changes in pain intensity between two groups were significantly at 24 h, 48 h and 72 h. However, the decrease in the pain severity was higher in the experimental group. Besides, compared with the control group, there was less score of phlebitis in patients in the experimental group.
| Variables | Experimental group (n=62) | Control group (n=65) | p value |
|---|---|---|---|
| Phlebitis | |||
| Baseline | 4.02±0.91 | 3.80±0.71 | 0.13 |
| 24 h | 2.53±0.55 | 3.15±0.53 | <0.001 |
| 48 h | 2.14±0.59 | 2.74±0.44 | <0.001 |
| 72 h | 1.75±0.34 | 2.52±0.33 | <0.001 |
| Pain intensity | |||
| Decreased at 24 h | -1.50±0.08 | -0.72±0.08 | <0.001 |
| Decreased at 48 h | -2.39±0.13 | -1.23±0.06 | <0.001 |
| Decreased at 72 h | -3.19±0.14 | -1.64±0.08 | <0.001 |
Table 2: Comparison of The Pain Severity and Phlebitis Between The Experimental and Control Groups
During the intervention, 5 Adverse Events (AEs) were recorded. There was no severe AE. The comparison of AEs in the 2 groups had no statistical significance (2 vs. 3, p=0.68). Of all AEs, all of which were skin irritations or allergies. Three cases led to ceasing the intervention, while the other 2 were merely mild skin reactions. The incidence of intervention related AEs was 3.9 % (3.22 % vs. 4.61 %).
In the present study, we explored the clinical effects of Qingluo San on patients with phlebitis and pain intensity. The main findings of the study suggested that Qingluo San provided a better effective clinical treatment on pain intensity and phlebitis.
Nursing professionals are responsible for preventing, diagnosing and treating phlebitis as part of their health care duties[12,13]. Peripheral Venous Catheter (PVC) related phlebitis can be treated in several ways in clinical practice, including pharmacological interventions (anticoagulants, anti-inflammatory and vasodilators), phytotherapeutic products (Chamomilla recutita, notoginseng, aloe vera) and physical measures [13]. It is more common to apply topical therapies than it is to administer systemic drugs[3].
Traditionally, TCM has been used in China to prevent and treat inflammation[11]. It is possible that the effects of TCM on ulcers can be attributed primarily to the improvement of local blood circulation and acceleration of tissue regeneration despite the fact that there are a variety of prescription formulations in TCM[14]. Adding fructus forsythiae, rhubarb, corydalis rhizoma and scorpion can have medicinal effects such as removing heat and dehumidifying, removing poison and dispersing knots. These TCM penetrate directly into the target joints through the skin. They promote blood circulation in the area affected[15]. Chinese herbal medicine is based on the theory of "transdermal" absorption, in which the medicine is applied externally to the skin and then absorbed into the circulatory system through the stratum corneum by the capillaries, thereby exerting therapeutic effects on the body[13].
In addition to being safe, simple and easy to apply, Chinese herbal medicine can be applied externally to the affected area. In addition, this method can prevent the occurrence of the "first pass effect," which may occur during oral administration, as well as the degradation of the drugs in the gastrointestinal tract, which may occur during oral administration. It should be noted that this eliminates the stimulating effect of drugs on the gastrointestinal tract and this also eliminates the gastrointestinal factors that could affect the absorption of drugs. Additionally, this is an easy-to-use device, which can be interrupted at any time in the course of drug administration. For the treatment of phlebitis, Chinese herbal medicine is applied externally with the aim of "clearing heat and dispelling dampness"[13]. Several studies have demonstrated that Chinese herbal medicine formulas, such as the external application of TCM, can reduce phlebitis severity and pain intensity. Several studies have demonstrated that Chahuang ointment provides effective prevention and protection against phlebitis following peripherally inserted central catheter insertion[13]. Phlebitis caused by vincristine can be treated with Xianchen. The drug is particularly effective in the treatment of edema and a remarkable dose-response relationship has been observed[16]. In general, Qingluo San contains the following ingredients; S. flavescens radix, rhubarb, corydalis rhizoma, fructus forsythiae, Huang boon, green wind vine, rhizoma Dioscoreae tokoro, etc. S. flavescens radix was the monarch herbal. In addition to being bitter and cold in nature, S. flavescens radix has the function of clearing heat and detoxifying, dispelling wind and drying dampness and serving as a diuretic[17]. Among these substances, picrasidine and oxymatrine picrasidine have been shown to exert anti-inflammatory and immunosuppressive effects due to their capacity to inhibit the proliferation of T cells[18].
A study was conducted to examine the effects of Qingluo San dressing on phlebitis. Our study included patients who had peripheral venous catheters. The treated group received Qingluo San dressings, whereas the control group received routine nursing care. All patients received nursing interventions in addition to the pharmacotherapy. In our study, Qingluo San was shown to reduce the severity and intensity of pain associated with phlebitis when applied externally.
The study has several limitations. Firstly in the future, a multi-center Randomized Controlled Trial (RCT) will be conducted since this research was conducted at one hospital. Secondly, in order to collect and evaluate objective data, researchers should be blinded to the allocation of groups.
In patients with peripheral venous catheters, Qingluo San external treatment significantly reduced pain severity and phlebitis. It is recommended that future studies be conducted to determine the safety and feasibility of external Qingluo San treatment among patients with a variety of phlebitis, and to compare the analgesic effects of this treatment with other chemical medications or placebos over the long term, taking gender into account as a primary confounding variable. It is also suggested that further studies be conducted to determine Qingluo San’s biological and pharmacological properties as an analgesic agent, particularly for phlebitis.
Conflict of interests:
The authors declared no conflict of interests.
References
- Corley A, Ullman AJ, Mihala G, Ray-Barruel G, Alexandrou E, Rickard CM. Peripheral intravenous catheter dressing and securement practice is associated with site complications and suboptimal dressing integrity: A secondary analysis of 40 637 catheters. Int J Nurs Studies 2019;100:103409.
[Crossref] [Google Scholar] [PubMed]
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: Peripheral IV catheter failure. J Infus Nurs 2015;38(3):189-203.
[Crossref] [Google Scholar] [PubMed]
- Takahashi T, Murayama R, Abe-Doi M, Miyahara-Kaneko M, Kanno C, Nakamura M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep 2020;10(1):1550.
[Crossref] [Google Scholar] [PubMed]
- Marsh N, Webster J, Larsen E, Cooke M, Mihala G, Rickard CM. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: A multivariable analysis of peripheral intravenous catheter failure. J Hospital Med 2018;13(2):83-9.
[Crossref] [Google Scholar] [PubMed]
- Wallis MC, McGrail M, Webster J, Marsh N, Gowardman J, Playford EG, et al. Risk factors for peripheral intravenous catheter failure: A multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol 2014;35(1):63-8.
[Crossref] [Google Scholar] [PubMed]
- Simin D, Milutinovic D, Turkulov V, Brkic S. Incidence, severity and risk factors of peripheral intravenous cannula-induced complications: An observational prospective study. J Clin Nurs 2019;28(9-10):1585-99.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Yao Q, Yang P, Zhao D, Yang R, Bai H, et al. Multi-omics approaches for in-depth understanding of therapeutic mechanism for Traditional Chinese Medicine. Front Pharmacol 2022;13:1031051.
[Crossref] [Google Scholar] [PubMed]
- Sha S, Liu W, Cheng L, Ge J. Review of traditional Chinese medicine external applications to treat chemistry phlebitis. Zhongguo Zhong Yao Za Zhi 2011;36(18):2592-4.
- Wang X, Lv X, Zhang J, Wang Y. Effect of Chahuang ointment on prevention of phlebitis from peripherally inserted central catheter: Randomized clinical trial. Rev Esc Enferm USP 2021;55:e3680.
[Crossref] [Google Scholar] [PubMed]
- Zheng GH, Yang L, Chen HY, Chu JF, Mei L. Aloe vera for prevention and treatment of infusion phlebitis. Cochrane Database Syst Rev 2014;2014(6):CD009162.
[Crossref] [Google Scholar] [PubMed]
- Li S, Lu AP, Wang YY, Li YD. Suppressive effects of a Chinese herbal medicine Qing-Luo-Yin extract on the angiogenesis of collagen-induced arthritis in rats. Am J Chin Med 2003;31(5):713-20.
[Crossref] [Google Scholar] [PubMed]
- di Nisio M, Peinemann F, Porreca E, Rutjes AW. Treatment for superficial infusion thrombophlebitis of the upper extremity. Cochrane Database Syst Rev 2015;2015(11):D11015.
[Crossref] [Google Scholar] [PubMed]
- Wu F, Wang Y, Mei Q, Chen Q, Sun C, Lv X, et al. UGTs-mediated metabolic interactions contribute to enhanced anti-inflammation activity of Jinhongtang. J Ethnopharmacol 2023;304:116016.
[Crossref] [Google Scholar] [PubMed]
- Shu C, Yang F, Zhu F, Hua D. Effect of external use of Qingluo San on clinical efficacy in patients with acute gouty arthritis. Eur J Med Res 2022;27(1):245.
[Crossref] [Google Scholar] [PubMed]
- Zhou HM, Chen SQ, Sun BG, Yin LR, Ye XY, Chen ZX. A clinical research of Jiawei simiaosan oral administration combined with the external application of sihuangshuimi in the treatment of acute gouty arthritis. Zhong Yao Cai 2007;30(9):1196-8.
[Google Scholar] [PubMed]
- Zhang J, Shen J, Yin W, Wei X, Wu L, Liu H. The intervention research on treatment by Xianchen to rabbits model of chemotherapeutic phlebitis. Acta Cir Bras 2016;31:549-56.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Dong Z, Jin L, Zhang K, Zhao X, Fu J, et al. Arsenic trioxide-induced hERG K+ channel deficiency can be rescued by matrine and oxymatrine through up-regulating transcription factor Sp1 expression. Biochem Pharmacol 2013;85(1):59-68.
[Crossref] [Google Scholar] [PubMed]
- Shen T, Tao Y, Liu B, Kong D, Zhang R, Xiao W. Machine learning assisted discovery of novel p38a inhibitors from natural 2 products 3. Comb Chem High Throughput Screen 2023;26(6):1214-23.
[Crossref] [Google Scholar] [PubMed]
 ): Control group and (
): Control group and ( ): Experiment group
): Experiment group



