- *Corresponding Author:
- Mamta Singh
Department of Pharmacy, Sardar Bhagwan Singh University, Dehradun, Uttarakhand 248161,India
E-mail: darakhshan201992@gmail.com
| Date of Received | 05 February 2022 |
| Date of Revision | 01 September 2023 |
| Date of Acceptance | 02 February 2024 |
| Indian J Pharm Sci 2024;86(1):75-86 |
This is an open access article distributed under the terms of the Creative
Commons Attribution-NonCommercial-ShareAlike 3.0 License, which
allows others to remix, tweak, and build upon the work non-commercially,
as long as the author is credited and the new creations are licensed under
the identical terms
Abstract
The fruit of Phoenix dactylifera has high nutritional value, possess several health benefits and used as traditional medicine in treating diverse ailments. The present study evaluates the effect of total flavonoid fraction and hydroalcoholic extract of dried fruits of Phoenix dactylifera on postmenopausal complications in ovotoxic female rats. 4-vinyl cyclohexene diepoxide, 80 mg/kg i.p. was injected for 15 d to induce menopause in young female rats. Menopausal rats were treated with suitably prepared total flavonoid fraction and hydroalcoholic extract of Phoenix dactylifera for 45 d. Vaginal smears were prepared and animals were evaluated for hormonal and biochemical parameters, serum mineral content, osteocalcin level and oxidative stress. Histopathology of ovaries was performed. Treatment of postmenopausal rats with total flavonoid fraction (200 mg/kg) and hydroalcoholic extract (400 mg/kg) of Phoenix dactylifera caused significant improvement (p< 0.001) in serum follicle-stimulating hormone level, luteinizing hormone levels and lipid profile with mild effect on serum estrogen level and vaginal smear pattern. The treatment normalized serum Troponin T (0.98±0.03 µg/ml, 1.35±0.05 µg/ml) and creatine kinase-MB (134.4±1.50, 164.0±0.07 IU/l) and significantly improved serum mineral content and osteocalcin level. Significant reduction (p<0.001) was observed in the level of oxidative stress in ovotoxic animals after the treatment period. Histopathological studies indicated protection of ovarian follicles in ovotoxic rats after the treatment period. Results concluded that dried fruit of Phoenix dactylifera has the potential to prevent postmenopausal complications and therefore could be used as hormone replacement therapy in postmenopausal women.
Keywords
Estrogen, flavonoids, menopause, Phoenix dactylifera, phytoestrogens
Menopause is the physiological transitional event in a women’s life at an age of early 50s that promotes changes from reproductive to non-reproductive phase, characterized by permanent cessation of menses, loss of ovarian functions and decline in the levels of estrogen[1]. It is associated with various physiological changes and many women experience sleep disorders, vasomotor symptoms (night sweats and hot flushes), mood changes and urogenital symptoms like vaginal dryness, stress incontinence as well as urinary tract infections owing to the decrease in the level of estrogen[2]. In the postmenopausal phase, reduced estrogen level increases the risk of various types of disorders such as osteoporosis, coronary heart disease, depression and cognitive impairment[3]. Postmenopausal osteoporosis is characterized by the reduction in bone mineral density and is associated with destruction of bone tissue microarchitecture resulting in increased bone fragility and fractures. Paucity of estrogen in menopausal women increases the oxidized serum Low-Density Lipoprotein (LDL), thereby raising the incidence of atherosclerosis and cardiovascular risk[4]. Hormone Replacement Therapy (HRT) is commonly used to treat postmenopausal complications and is considered as the primary treatment for relieving the menopausal complications but they are associated with complications like breast, endometrial and ovarian cancer, thromboembolism and coronary heart diseases[5].
Due to undesirable consequences associated with HRT, plant derived therapies are the first preference of many women to treat menopausal symptoms and complications. Phytoestrogens, the diphenolic compounds found in variety of foods, vegetables, fruits, soy and legumes are considered as natural alternative to estrogen and therefore used for estrogen replacement therapy[6]. Phytoestrogens comprise of lignans, isoflavones, coumestans, chalcones and possess chemical similarity with 17β-estradiol. Due to this, they bind to the estrogen receptor and exerts estrogenic effects on the target organs by regulating the target gene expression[7]. Among all the classes of isoflavones, flavonoids and lignans are the major class of phytoestrogens which show remarkable therapeutic efficacy towards menopausal symptoms. Protective effect of soy isoflavone genistein, on the menopausal symptoms and complications such as osteoporosis, cardiovascular diseases and breast cancer has been well established in the past[8].
Phoenix dactylifera (P. dactylifera) is a sweet edible fruit commonly known as date palm belonging to the family Arecaceae. It is one of the eldest monocotyledonous plants, consisting of 3000 species and more than 600 varieties has been used in diet for 6000 y in Arab countries[9]. Date fruit has a high nutritional value and offers various health benefits and pharmacological activities[10]. Previous phytochemical studies done on dates indicates that date fruits are rich in carbohydrates, fatty acids, minerals, vitamins, carotenoids, lignans, sterols, phenolics compounds and flavonoids such as flavanols, flavonoid glycosides, isoflavones and anthocyanins[11]. Various phytoestrogens such as daidzein, genistein, pinoresinol, coumestrol and lignans have been identified in the date fruit[12]. Fruit of date palm exhibits nephroprotective, antiviral, cardio protective[13], hepatoprotective[14], anticancer[15], anti-inflammatory effects, etc,[16].
Although plethora of work is done on the fruit of date palm but the effect of date fruit on the complications associated with menopause has not been studied in spite of its consumption for preventing menopausal complications. Therefore, the present work was aimed to study the effect of different doses of Total Flavonoid Fraction (TFF) and Hydroalcoholic Extract (HAE) of dried fruits of P. dactylifera on various postmenopausal complications in 4-Vinylcyclohexene Diepoxide (VCD) induced ovotoxicity in rats.
Materials and Methods
Drugs and chemicals: VCD and 17ꞵ-estradiol were purchased from Sigma-Aldrich, St. Louis, MO, USA. All the analytical grade chemicals were used for the study. Diagnostic kits of ERBA (Mumbai, India) and Enzyme-Linked Immunosorbent Assay (ELISA) kits of Cell Biolabs, Inc., USA were used for biochemical estimation.
Animals:
Female Wistar rats weighing between 150-200 g (7-8 w old) were procured from Indian Veterinary Research Institute, Bareilly, Uttar Pradesh and used in the study. The rats were housed in standard polypropylene cages under controlled room temperature and humidity. Animals were acclimatized to the laboratory conditions before experiment. Care of animals was done as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. Experimental protocol was approved by Institutional Animal Ethics Committee, SBSPGI, Dehradun with approval number of CPCSEA/IAEC/SBS/2018/001 (42 female Wistar rats were approved for the study).
Collection of plant material:
Dried fruits of P. dactylifera were purchased from the local market of Dehradun and were authenticated at Botanical Survey of India, Dehradun and granted Accession No. 429.
Preparation of HAE of fruit of P. dactylifera:
Extraction of dried fruits of P. dactylifera was performed by maceration. The edible part of dried fruits was separated from the seeds and macerated with 100 ml of water and methanol in a ratio of 60:40 (hydroalcoholic solvent) at room temperature for 24 h. The macerate was filtered using cotton cloth and evaporated to complete dryness. The percentage yield of HAE was found to be 18.8 %. The resulting HAE was stored in airtight container until used.
Determination of TFF from HAE of P. dactylifera fruit:
Colorimetric assay was used to determine the total flavonoid content. 4 ml of distilled water was added to 1 ml of the extract, followed by subsequent addition of 0.3 ml each of 5 % Sodium nitrite (NaNO2) and 10 % Aluminum chloride (AlCl3). The samples were then incubated for 5 min at room temperature. After this, 2 ml of 1 M Sodium hydroxide (NaOH) was added to the mixture, and the volume of reaction mixture was made up to 10 ml with distilled water. The mixtures were then vortexed, and the absorbance was measured at 510 nm using Ultra Violate (UV) spectrophotometer. The TFF was expressed as g of quercetin equivalent per 100 g of sample.
Acute toxicity study and lethal dose 50 determination of total flavonoid fractions:
Acute toxicity study was carried out using Organisation for Economic Co-operation and Development Guideline 423, acute toxic class method using three animals of a single sex (normally females). TFF did not cause any mortality in animals till the dose of 2000 mg/kg.
Induction of ovotoxicity and menopause:
Ovotoxicity was induced by injecting VCD at a dose of 80 mg/kg, intraperitoneally, five times per w for 15 d[17]. Vaginal smears were prepared, and blood estrogen level was checked in normal female rats before (d 0) and after injecting VCD (d 15) to confirm the induction of menopause. Female rats showing abnormality in vaginal smears and declined estrogen level on 15th d were considered ovotoxic and selected for the study.
Selection and preparation of doses:
Based on acute toxicity studies done on TFF, two doses (100 and 200 mg/kg body weight) of TFF were selected for pharmacological activity. Two doses of HAE of fruit of P. dactylifera (200 and 400 mg/kg b.w.) were selected for pharmacological activity[16]. All the treatments were given as suspension in distilled water using 1 % carboxymethylcellulose as suspending agent.
Treatment protocol:
The ovotoxic animals were divided into six groups of six animals in each and one group of normal animals was taken for the study. Animals received the following treatment for a period of 45 d.
Group 1 (normal control) where normal animals received normal saline (1 mg/kg/d p.o.); group 2 (toxicant control) where ovotoxic animals received normal saline (1 ml/kg/d, p.o.); group 3 where ovotoxic animals received lower dose (100 mg/kg/d, p.o.) of TFF of dried fruit of P. dactylifera; group 4 where ovotoxic animals received higher dose (200 mg/kg/d, p.o.) of TFF of dried fruit of P. dactylifera; group 5 where ovotoxic animals received lower dose (200 mg/kg/d, p.o.) of HAE of dried fruit of P. dactylifera; group 6 where ovotoxic animals received higher dose (400 mg/kg/d, p.o.) of HAE of dried fruit of P. dactylifera and group 7 where ovotoxic animals received standard drug estrogen (60 mg/kg/d, p.o.).
Vaginal smears were prepared and estrogen level were checked before (0 d) and after induction of ovotoxicity (d 15), and after the treatment period of 45 d. At the end of treatment period, animals were anaesthetised with ether and blood was withdrawn from the retro orbital plexus. Collected blood was centrifuged using cooling centrifuge (Remi, C24, India) and the resultant serum was used for estimation of various biochemical and hormonal parameters. After collection of blood, animals were sacrificed under euthanasia (Phenobarbitone 150 mg/kg, i.p.) and ovaries were isolated, washed in ice cold saline, weighed, homogenized in 10 % w/v tris buffer of pH 7.2 and centrifuged at 7000 ×g for 10 min at 4°. The resulting supernatant was used for the estimation of oxidative stress and antioxidant enzymes. The ovaries were kept in 10 % formalin solution and used for histopathological study.
Preparation of vaginal smear for morphological examination:
For sterile vaginal lavage, 0.9 % normal saline was prepared, autoclaved and stored in a tightly sealed container at room temperature until needed. For cytological assessment, hematoxylin/eosin was used. Every morning, between 8:00 to 9:00 am, vaginal sample was collected by inserting the tip of plastic pipette filled with normal saline (10-20 μl) into the vagina of female rat. The procedure was repeated 2-3 times to accumulate enough number of cells in a single sample. The vaginal sample was then placed on a glass slide and was left to air dry. The dried slide was initially kept under coplin jar containing haematoxylin/eosin for a min and then kept for another min in a separate coplin jar containing distilled water. The excess of distilled water from the edges of slide was removed by wiping with a tissue paper carefully without disturbing the stained vaginal smear. The resulting smear was observed under a light microscope with 10X-40X magnifications[18].
Hormonal assay:
Serum estrogen was estimated by using ELISA kit by the method of Macdonald et al.,[19]. Serum FSH and LH were estimated by Roche Follicle Stimulating Hormone kit and Roche Luteinising Hormone kit by the method of C4SA (Chemiluminescence Immunoassay (CLIA)).
Estimation of cardiovascular biomarkers:
Serum level of Triglycerides (TG), Total Cholesterol (TC), High Density Cholesterol (HDL-Cholesterol) were estimated by enzymatic colorimetric method[20], CHOD-PAP method with LCF[21] and phosphotungstic acid method[22] respectively with ERBA diagnostic kits using Erba, Chem 5X Clinical Chemistry Analyser, Mannheim, Germany.
The level of Low-Density Lipoprotein (LDL), VLDL and atherogenic index were calculated by using the following formula:
LDL=TG/5
VLDL=TC-HDL
Atherogenic Index=log10 (TG/HDL)
Assay of marker enzymes:
The estimation of creatine kinase-MB activity was done by enzymatic rate method. Troponin T was estimated by immunochromatographic assay. Lactate dehydrogenase was estimated by UV-kinetic method using diagnostic kits of ERBA.
Estimation of serum mineral content:
Serum calcium, magnesium and phosphate levels were estimated using diagnostic kits from ERBA. Osteocalcin was estimated by using ELISA kit of Elba Sciences.
Thiobarbituric Acid Reactive Substance (TBARS) Assay:
Estimation of TBARS was done from tissue supernatant by the method of Slater et al.[23].
Antioxidant enzyme assay:
Reduced Glutathione (GSH) was assayed by the method of Moron et al.,[24]. SOD was estimated by the method of Sun et al.[25]. Estimation of Glutathione Reductase (GR) activity was done by the method of Mohandas et al.,[26] and Glutathione Peroxidase (GPx) was estimated by the method of Rotruck et al.,[27].
Histopathology:
The ovaries of treated female rats were cut and quickly hooked with 10 % buffered formalin for duration of a day or two. After hooking, each ovarian tissue was consistently processed and was incorporated in paraffin and its block was made. Microtone was used to cut the section of 5 μm from paraffin incorporated tissue followed by staining with haematoxylin and eosin. The sections were then observed under light microscope for histopathology.
Statistical analysis:
The statistical analysis was carried out using Graph Pad Prism 8.0 software. All the values were presented as mean±Standard Error of the mean (SEM). Multiple comparisons between different groups were performed using Analysis of Variance (ANOVA) followed by the Dunnetts Multiple Comparison Test. The difference level at p<0.05 was considered as statistically significant.
Results and Discussion
In the present study, administration of VCD, 80 mg/kg, intraperitoneally, 5 times a w for 15 d resulted in the induction of menopause in female rats, which was evident by the pattern of vaginal smears (dioestrus phase) and decrease in serum estrogen levels. Phytochemical studies revealed that the concentration of TFF in the HAE of dried fruit of P. dactylifera was 0.318 g/equivalent to quercetin.
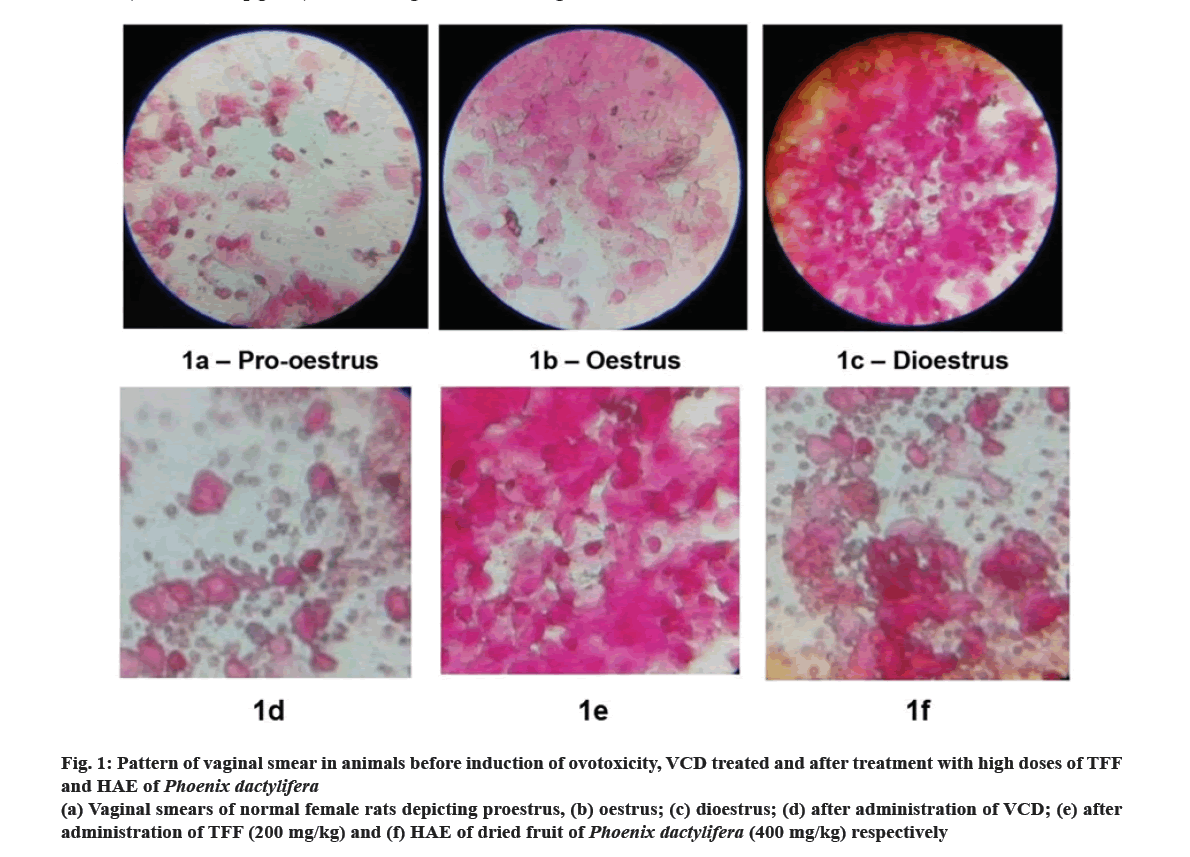
Results in fig. 1 indicates pattern of vaginal smears of normal, ovotoxic and treated rats. Fig. 1a-fig. 1c reveals presence of different stages of estrous cycle (proestrus, oestrus and dioestrus phases respectively) in normal rats. Fig. 1d illustrates vaginal smears of rats treated with VCD, revealing a pattern akin to the dioestrus phase. This observation indicates a perturbation in the oestrus cycle and the induction of a menopausal-like phase in the female rat subjects. Ovotoxic rats treated with high dose of TFF and HAE of P. dactylifera for a period of 45 d showed the presence of cornified cells and leukocytes in the vaginal smear, a pattern similar to dioestrus phase (fig. 1e and fig. 1f). TFF and HAE of P. dactylifera given to ovotoxic rats for 45 d did not have any significant changes in morphology of vaginal smears. Even after the treatment period, the smear pattern was not reversed to normal. VCD is an occupational toxicant that causes selective destruction of primary and primordial follicles in ovaries by activating apoptosis in primordial and primary follicles, increasing oxidative stress and inhibiting the phosphoinositide-3-kinase signalling pathway in oocytes that plays important role in survival and viability of oocytes and leads to ovotoxicity[28,29].
Fig 1: Pattern of vaginal smear in animals before induction of ovotoxicity, VCD treated and after treatment with high doses of TFF and HAE of Phoenix dactylifera
(a) Vaginal smears of normal female rats depicting proestrus, (b) oestrus; (c) dioestrus; (d) after administration of VCD; (e) after administration of TFF (200 mg/kg) and (f) HAE of dried fruit of Phoenix dactylifera (400 mg/kg) respectively
VCD administration in female rats for a period of 15 d caused significant (p<0.001) decrease (15.03±0.13pg/ml) in estrogens level as compared to d 1 (52.5±0.12 pg/ml) indicating severe damage of ovarian follicles and induction of menopause (Table 1). HAE of P. dactylifera at a dose of 400 mg/kg and TFF at a dose of 200 mg/kg administered for a period of 45 d caused mild increase (p<0.05) in serum estrogen level in ovotoxic animals as compared to the 1st d (15 d of study) of the treatment (Table 1). Standard drug estrogen (60 mg/kg), lower doses of TFF (100 mg/kg) and HAE (200 mg/kg) did not show any increase in estrogens level in ovotoxic rats. Mild increase in serum estrogen level after administration of higher doses of TFF and HAE could be due to presence of phytoconstituents that repair only few left-over damaged follicles in the ovaries and release small amount of estrogen. It is well known that the date fruit contains large amounts of flavonoids, isoflavones and lignans which acts as phytoestrogens and exerts estrogenic effects on various target organs. These, however, do not increase the secretion of estrogen in the ovaries of postmenopausal women[30].
| Groups/treatment | Estrogen (pg/ml) | FSH (mIU/ml) | LH (mIU/ml) | ||||
|---|---|---|---|---|---|---|---|
| 0 d | 15th d | 60th d | 15th d | 60th d | 15th d | 60th d | |
| Normal animal (normal saline 1 ml/kg) | 57.5±0.2 | 54.3±0.17 | 53.0±0.60 | 2.5±0.20 | 2.4±0.71 | 1.8±0.05 | 1.9±0.54 |
| VCD (80 mg/kg i.p.) | 52.5±0.12 | 15.03±0.13a | 14.5±0.15 | 6.7±0.14b | 6.0±1.20 | 4.5±0.32b | 4.8±0.17 |
| VCD+TFF of dried fruit of P. dactylifera (100 mg/kg) | 45.0±0.41 | 13.0±0.20a | 15.8±0.41 | 5.3±0.52 | 4.2±0.53* | 4.2±0.14 | 3.7±0.65* |
| VCD+TFF of dried fruit of P. dactylifera (200 mg/kg) | 50.7±0.05 | 12.7±0.90a | 30.0±0.32* | 5.6±1.30 | 3.3±0.61*** | 4.8±0.70 | 2.5±0.50*** |
| VCD+HAE of dried fruit of P. dactylifera (200 mg/kg) | 47.6±0.06 | 14.8±0.23a | 15.2±0.51 | 4.9±0.71 | 4.5±0.53* | 4.3±0.32 | 3.6±0.42* |
| VCD+HAE of dried fruit of P. dactylifera (400 mg/kg) | 48.0±0.80 | 15.0±0.14a | 29±0.63* | 5.1±0.23 | 3.5±0.82*** | 4.5±0.90 | 2.7±0.61*** |
| VCD+Estrogen (60 mg/kg) | 51.5±0.53 | 15.6±0.06a | 16.2±0.23 | 4.0±0.13 | 3.1±0.24*** | 4.4±0.54 | 3.9±0.75* |
Note: All values are expressed as mean±SEM; n=6 in each group; p values: *p<0.05; *p<0.01; ***p<0.001 when results of 60th d were compared with results of 15th d for each group; ap<0.001 when results of 15th d were compared with 0 d of the study and bp<0.001 when results of toxicant control are compared with normal control
Table 1: Effect of TFF and HAE of Dried Fruit of P. DACTYLIFERA on Serum Estrogen, FSH and LH Level in Ovotoxic Female Rats
In the present study, level of FSH and LH were significantly (p<0.001) increased after administration of VCD (on d 15) in all the group of animals as compared to normal control (Table 1). Administration of higher dose of TFF (200 mg/kg) and HAE (400 mg/kg) of P. dactylifera for a period of 45 d caused significant decrease (p<0.001) in the level of FSH and LH in ovotoxic rats as compared to 15th d of the study (Table 1). TFF and HAE of P. dactylifera at low doses were mildly effective (p<0.05) in decreasing the level of FSH and LH in ovotoxic animals. Estrogen administered to the ovotoxic animals caused significant decrease (p<0.001) in the level of FSH but slightly (p<0.05) inhibited the secretion of LH from the pituitary gland. Above results indicate that VCD caused increase in serum FSH and LH levels, a characteristic feature of menopause. TFF and HAE of P. dactylifera caused dose dependent inhibition of FSH and LH secretions in ovotoxic rats. Rich flavonoids and phytoestrogen content of the dried fruit could be responsible for this effect which was in accordance with previously reported studies[31].
Cardiovascular and coronary heart diseases are the major health problems in postmenopausal women after breast cancer. Before menopause, estrogen regulates lipid metabolism, increase HDL and TG and decrease LDL, cholesterol and lipoprotein levels. Due to the antioxidant activity, estrogen produces atheroprotective RATSeffects and inhibits the migration and adhesion of inflammatory molecules. In the postmenopausal women, deficient estrogen level leads to decrease in HDL level, increase in atherogenic index and abnormalities in the lipid profile which further results in cardiovascular complications[32]. In the present study, VCD administration for 15 d caused significant increase (p<0.001) in serum total cholesterol, triglyceride, LDL, VLDL levels and atherogenic index, and significant decrease (p<0.001) in serum HDL level as compared to the normal animals (Table 2). Treatment of ovotoxic rats with TFF (200 mg/kg) of P. dactylifera for 45 d caused significant (p<0.001) decrease in the level of serum cholesterol, TG, LDL and VLDL and increase in HDL level in ovotoxic rats as compared to the toxicant control group (Table 2). HAE (400 mg/kg) of P. dactylifera for 45 d caused significant (p<0.001) decrease in the level of serum total cholesterol while moderate (p<0.01) decrease was observed in the level of TG, LDL and VLDL and moderate (p<0.01) increase in HDL level (45.6±1.80 mg/dl) in ovotoxic rats as compared to the toxicant control group (23.5±0.08 mg/dl). However, lower doses of TFF (100 mg/kg) and HAE (200 mg/kg) caused mild (p<0.05) improvement in the level of lipid profile in ovotoxic rats. Estrogen supplement for 45 d caused significant (p<0.001) improvement in the lipid profile. Higher doses of TFF given for a period of 45 d caused complete restoration of the level of Artificial Intelligence (AI) in ovotoxic animals while higher dose of HAE of has shown moderate decrease (3.41±1.4) in the level of AI as compared to toxicant control (6.20±0.05) (Table 2). VCD administration also caused significant increase (p<0.001) in the level of serum LDH, a marker of tissue damage, CK-MB (228.03±0.80 IU/l) and troponin T (2.54±0.06 µg/ml) as compared to the normal animals indicating cardiac dysfunction in ovotoxic rats (Table 3). Treatment of ovotoxic animals with TFF (200 mg/kg) of P. dactylifera and estrogen (60 mg/kg) for 45 d caused significant (p<0.001) decrease in serum LDH, CK-MB and troponin T levels as compared to the toxicant control group (Table 3). The HAE at a dose of 200 mg/kg and 400 mg/kg caused mild (p<0.05) to moderately significant (p<0.01) decrease in serum LDH, CK-MB and troponin T levels in ovotoxic rats. In this study, improved lipid profile and atherogenic index in ovotoxic rats treated with higher doses of TFF and HAE, and estrogen supplementation indicates their atheroprotective effects. Decrease in the level of LDH and improvement in cardiac biomarker enzymes (CK-MB and troponin T by TFF and HAE reflected their cardioprotective effect in ovotoxic rats. High content of flavonoids, carotenoids and phytoestrogens (isoflavones and lignans) present in date fruit may attribute to this effect. A study performed on aqueous extract of date fruit also reported its significant cardioprotective effect owing to rich content of flavonoids and phenolic compounds in it[33]. The mechanism hypothesized are in accordance with some previous studies which documented significant antiatherosclerotic, antihyperlipidemic and cardioprotective effects of flavonoids[34]. Literature survey also reports that phytoestrogens inhibit LDL oxidation and increases clearance of LDL and VLDL by hepatocytes, thus contributes to lipid lowering effect.
| Groups/Treatments | HDL (mg/dL) | Total Cholesterol (mg/dL) | Triglyceride (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) | AI |
|---|---|---|---|---|---|---|
| Normal animal (normal saline 1 ml/kg) | 55.4±0.03 | 105.4±0.08 | 85.0±0.13 | 41.2±0.8 | 18.7±0.16 | 2.64 ±0.04 |
| VCD (80 mg/kg) | 23.5±0.08a | 179.2±2.0a | 181.3±0.07a | 119.3±1.02a | 35.6±0.09a | 6.20±0.05a |
| VCD+TFF of dried fruit of P. dactylifera (100 mg/kg) | 32.5±1.72* | 146.5±0.50* | 149.6±0.14* | 99.7±0.82* | 28.6±1.04* | 3.81±0.04* |
| VCD+TFF of dried fruit of P. dactylifera (200 mg/kg) | 50.6±1.17*** | 108.7±0.15*** | 98.3±0.54*** | 50.0±0.08*** | 19.2±0.06*** | 2.69±0.05*** |
| VCD+HAE of dried fruit of P. dactylifera (200 mg/kg) | 30.29±0.06* | 144.2±0.48* | 146.5±0.41* | 100.7±0.25* | 26.8±0.03* | 3.85±0.32* |
| VCD+HAE of dried fruit of P. dactylifera (400 mg/kg) | 45.6±1.80** | 110.5±0.04*** | 101.5±0.7** | 69.0±0.16** | 21.5±1.02** | 3.41±1.4** |
| VCD+Estrogen (60 mg/kg) | 52.5±1.03*** | 104.4±0.06*** | 98. 7±0.09*** | 46.6±0.02*** | 19.5±0.34*** | 2.4±0.65*** |
Note: All values are expressed as mean±SEM; n=6 in each group; p values: *p<0.05; **p<0.01; ***p<0.001 when results of the treated groups were compared with the results of toxicant control; ap<0.001 when results of toxicant control were compared with normal animals
Table 2: Effect of TFF and HAE of Dried Fruit of P. DACTYLIFERA on Lipid Profile and Atherogenic Index in Ovotoxic Female Rats
| Groups/treatments | LDH (IU/L) | CK-MB (IU/l) | Troponin T(µg/ml) |
|---|---|---|---|
| Normal animal (normal saline 1 ml/kg) | 139.5±0.34 | 110±0.12 | 0.72±0.45 |
| VCD (80 mg/kg) | 296.7±1.25a | 228.03±0.80a | 2.54±0.06a |
| VCD+TFF of dried fruit of P. dactylifera (100 mg/kg) | 237.43±0.20* | 192.1±0.12* | 1.87±0.62* |
| VCD+TFF of dried fruit of P. dactylifera (200 mg/kg) | 158.6±0.05*** | 134.4±1.50*** | 0.98±0.03*** |
| VCD+HAE of dried fruit of P. dactylifera (200 mg/kg) | 244.05±0.04* | 195.0±1.40* | 1.97±0.06* |
| VCD+HAE of dried fruit of P. dactylifera (400 mg/kg) | 185.23±0.54** | 164.0±0.07** | 1.35±0.05** |
| VCD+Estrogen (60 mg/kg) | 154.0±0.61*** | 131.1±0.51*** | 0.97±0.21*** |
Note: All values are expressed as mean±SEM; n=6 in each group; p values: *p<0.05; **p<0.01; ***p<0.001 when results of the treated groups were compared with the results of toxicant control; ap<0.001 when results of toxicant control were compared with normal animals
Table 3: Effect of TFF and HAE of Dried Fruit of P. DACTYLIFERA on the Level of Cardiac Biomarkers in Ovotoxic Female Rats
Menopause in women is linked with changes in bone health and mineral metabolism, along with hormonal disturbances. Low level of ovarian hormones in menopause induces synthesis of interleukin-6 and other cytokines by osteoblasts and T cells, increases osteoclast formation and bone resorption. Further increased levels of Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) enhance formation of osteoclast cells, that increases the risk of osteoporosis and other deformities in bones[35]. In menopausal women, calcium is released and gets deposited again on the bones, leading to the loss of bone matrix, increased porosity, and bone resorption.
In the present study, administration of VCD for a period of 15 d resulted in significant (p<0.001) increase in the serum level of calcium, phosphate and magnesium indicating resorption of bones (Table 4). VCD treated animals showed significantly increased level of osteocalcin (11.4±0.50 ng/ml) as compared to the normal animals (7.84±0.17 ng/ml) indicating menopausal osteoporosis. Treatment of ovotoxic rats with 200 mg/kg of TFF and estrogen for 45 d caused most significant (p<0.001) decrease in serum calcium, phosphate, magnesium and osteocalcin levels while HAE of P. dactylifera at a dose of 400 mg/kg caused moderately significant decrease (p<0.01) in the level of above-mentioned bone markers as compared to the toxicant control animals (Table 4). Results indicate that higher doses of TFF and HAE of P. dactylifera , and estrogen replacement significantly restored the serum level of calcium, magnesium, phosphate and osteocalcin in ovotoxic rats. Previous studies also suggests that plant extracts containing phytoestrogens improve menopausal bone deformities by activating the formation of osteoblast cells, increasing bone formation and inhibiting bone resorption[36]. Hence, it can be assumed that flavonoids and phytoestrogens present in the TFF and HAE of date fruit are responsible for their favourable effects on bone health.
| Groups | Calcium (mg/dl) | Magnesium (mg/dl) | Phosphate (mg/dl) | Osteocalcin (ng/ml) |
|---|---|---|---|---|
| Normal animal (normal saline 1 ml/kg) | 6.99 ± 0.03 | 2.02±0.03 | 3.84±0.04 | 7.84±0.17 |
| VCD (80 mg/kg) | 14.65 ± 0.12a | 5.68±0.02a | 8.10±0.23a | 11.4±0.50a |
| VCD+TFF of dried fruit of P. dactylifera (100 mg/kg) | 11.09±0.02* | 4.06±0.13* | 7.17±0.07* | 10.07±0.08* |
| VCD+TFF of dried fruit of P. dactylifera (200 mg/kg) | 8.48±0.71*** | 2.72±0.04*** | 4.07±0.64*** | 8.26±2.30*** |
| VCD+HAE of dried fruit of P. dactylifera (200 mg/kg) | 11.85±0.03* | 4.40±0.52* | 7.58±1.60* | 10.21±0.05* |
| VCD+HAE of dried fruit of P. dactylifera (400 mg/kg) | 9.53±0.24** | 3.19±0.43** | 5.10±0.06** | 9.44±0.73** |
| VCD+Estrogen (60 mg/kg, p.o.) | 7.05±1.09*** | 2.50±0.08*** | 3.92±0.45*** | 8.03±0.42*** |
Note: All values are expressed as mean±SEM; n=6 in each group; p values: *p<0.05; **p<0.01; ***p<0.001 when results of the treated groups were compared with the results of toxicant control; ap<0.001 when results of toxicant control were compared with normal animals
Table 4: Effect of TFF and HAE of P. DACTYLIFERA on Serum Calcium, Magnesium, Phosphate and Osteocalcin Level in Ovotoxic Female Rats
In young women, generation of free radicals and the antioxidant defence mechanisms are in balanced state, due to the antioxidant activity of estrogen. But in postmenopausal women, a pro-oxidant state exists in the body, due to lack of estrogen. This increased oxidative stress in postmenopausal women is responsible for the premature ovarian failure and complications such as atherosclerosis, osteoporosis, hot flushes, vasomotor disturbances, breast cancer and endometriosis[4]. In the present study, VCD caused significant increase (p<0.001) in the level of MDA; the product of lipid peroxidation and decrease (p<0.001) in GSH and the levels of all antioxidant enzymes (SOD, GPx and GR) in the ovaries of female rats (Table 5). Ovotoxic animals treated with TFF and HAE of P. dactylifera at a dose of 200 mg/kg and 400 mg/kg respectively, and estrogen caused significant decrease (p<0.001) in the level of MDA in ovotoxic animals. Treatment also restored the level of GSH and antioxidant enzymes (SOD, GPx and GR) significantly (p<0.001) as compared to the toxicant control group. Lower doses of TFF and HAE caused mild improvement (p<0.05) in MDA, GSH and antioxidant enzymes level in the ovaries of ovotoxic animals. On the basis of above findings, it can be concluded that TFF and HAE of P. dactylifera significantly reduced the level oxidative stress in the ovaries of VCD treated rats indicating their antioxidant activity. This effect could be attributed to phenolic compounds present in TFF and HAE of P. dactylifera . Our results concord with a previous study conducted to evaluate the effect of aqueous extract of date fruit on isoproterenol induced cardiotoxicity, which showed a similar effect on lipid peroxidation and the level of antioxidant enzymes[13]. Phytochemical study by Al-Alawi et al.[37] revealed the presence of high amounts of phenolic acids like ferulic acid, gallic acid, flavonoids like luteolin, quercetin, rutin, kaempferol, isoflavones, lignans and vitamins in date fruit which might contribute to its antioxidant activity[37]. Plethora of studies in past also demonstrated the usefulness of antioxidant activity of flavonoids and isoflavones in treating variety of diseases[38]. Inhibition of the formation of reactive oxygen species due to chelation with trace elements or inhibition of enzymes involved in the generation of the free radicals, upregulation of antioxidant enzymes and scavenging of free radicals are some of the suggested mechanisms responsible for the antioxidant activity of flavonoids and phytoestrogens.
| Groups/ Treatments | MDA (nmol/mg) | SOD (EU/mg protein) | GSH (µg/mg protein) | GPx (U/mg protein) | GR (U/mg protein) |
|---|---|---|---|---|---|
| Normal animal (Normal saline 1 ml/kg) | 4.51±0.70 | 37.4±0.45 | 68.04±1.25 | 10.12±0.33 | 218.0±0.71 |
| VCD (80 mg/kg) | 11.0±0.30a | 15.6±0.04a | 37.0±0.09a | 6.12±1.50a | 96.4±0.04a |
| VCD+TFF of dried fruit of P. dactylifera (100 mg/kg) | 8.31±0.12* | 21.6±1.40* | 51.2±0.68* | 7.17±0.42* | 132.7±1.26* |
| VCD+TFF of dried fruit of P. dactylifera (200 mg/kg) | 4.93±0.20*** | 34.5±0.80*** | 64.03±0.05*** | 9.61±0.61*** | 195.7±0.21*** |
| VCD+HAE of dried fruit of P. dactylifera (200 mg/kg) | 8.92±0.40* | 22.1±0.75* | 52.6±0.56* | 7.38±0.06* | 143.0±0.70* |
| VCD+HAE of dried fruit of P. dactylifera (400 mg/kg) | 4.79±0.45*** | 35.6±0.06*** | 65.6±0.17*** | 9.46±0.36*** | 190.6±0.43*** |
| VCD+Estrogen (60 mg/kg) | 4.63±0.05*** | 36.0±0.12*** | 66.12±0.4*** | 9.96±0.80*** | 202.5±0.30*** |
Note: All values are expressed as mean±SEM; n=6 in each group; p values *p<0.05; **p<0.01; ***p<0.001 when results of the treated groups were compared with the results of toxicant control; ap<0.001 when results of toxicant control were compared with normal animals
Table 5: Effect of TFF and HAE of Dried Fruit of P. DACTYLIFERA on MDA, SOD, GSH, GPX and GR Levels in Ovotoxic Female Rats
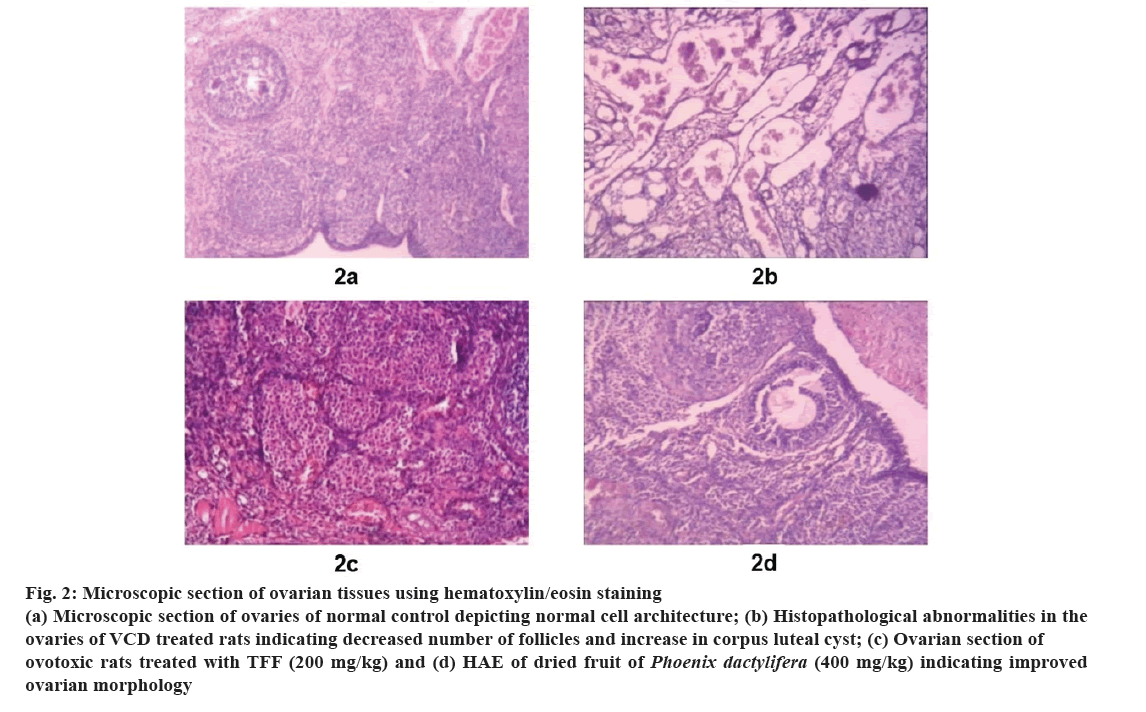
Histological studies of ovaries reveal the normal architecture of ovarian tissues with no sign of corpus luteum, unremarkable ovarian tissues and appropriate number of primordial follicles (fig. 2a). The ovarian tissues of rats treated with VCD showed marked oedema in ovarian tissue with few numbers of primordial and primary ovarian follicles and large corpus luteal cysts were noted in the oedematous ovarian stroma (fig. 2b). The ovaries of animals treated with higher doses of TFF and HAE of P. dactylifera showed marked increase in the number of primary and primordial follicles, reduction in corpus luteal cyst and oedematous ovarian stroma (fig. 2c and fig. 2d).
Fig 2: Microscopic section of ovarian tissues using hematoxylin/eosin staining
(a) Microscopic section of ovaries of normal control depicting normal cell architecture; (b) Histopathological abnormalities in the ovaries of VCD treated rats indicating decreased number of follicles and increase in corpus luteal cyst; (c) Ovarian section ofovotoxic rats treated with TFF (200 mg/kg) and (d) HAE of dried fruit of Phoenix dactylifera (400 mg/kg) indicating improved ovarian morphology
Intake of TFF and HAE of P. dactylifera at a dose of 200 mg/kg and 400 mg/kg respectively were effective in improving the postmenopausal complications in VCD induced ovotoxicity in rats. TFF as well as HAE were effective in improving cardiovascular complications, hormonal level, osteoporosis, morphology of ovaries and exhibited significantly high antioxidant activity. However, TFF of HAE of P. dactylifera was more effective than HAE in normalizing certain parameters. High content of flavonoids and phytoestrogens present in TFF may contribute for this effect. Our study concludes that daily intake of date palm fruit by women can help to prevent the postmenopausal complications and protect them from the harmful effects of HRT. In future, the effect of date fruit extract can be explored on various parameters of osteoporosis, anxiety, and cognition in the postmenopausal rats. Clinical studies can be performed to explore its effect in postmenopausal women.
Acknowledgement:
The authors are immensely thankful to the management SBS University, Balawala, Dehradun for providing all the facilities.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zeng LN, Yang Y, Feng Y, Cui X, Wang R, Hall BJ, et al. The prevalence of depression in menopausal women in China: A meta-analysis of observational studies. J Affect Disord 2019;256:337-43.
[Crossref] [Google Scholar] [PubMed]
- Lee HW, Jun JH, Choi J, Choi TY, Lee JA, Ang L, et al. Herbal prescription for managing menopausal disorders: A practice survey in Korean medicine doctors. Complement Ther Clin Pract 2020;38:101073.
[Crossref] [Google Scholar] [PubMed]
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin 2015;44(3):497-515.
[Crossref] [Google Scholar] [PubMed]
- Doshi SB, Agarwal A. The role of oxidative stress in menopause. J Mid-Life Health 2013;4(3):140.
[Crossref] [Google Scholar] [PubMed]
- Ameye L, Antoine C, Paesmans M, de Azambuja E, Rozenberg S. Menopausal hormone therapy use in 17 European countries during the last decade. Maturitas 2014;79(3):287-91.
[Crossref] [Google Scholar] [PubMed]
- Rietjens IM, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol 2017;174(11):1263-80.
[Crossref] [Google Scholar] [PubMed]
- Kulasari S, Singh MF, Bhandari S. Polyphenols: Phytochemistry and health benefits. J Pharmacog Phytochem 2019;8(4):3344-58.
- Thangavel P, Puga-Olguín A, Rodríguez-Landa JF, Zepeda RC. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 2019;24(21):3892.
[Crossref] [Google Scholar] [PubMed]
- Ahmed A, Bano N, Tayyab M. Phytochemical and therapeutic evaluation of date (Phoenix dactylifera). A review. J Pharm Altern Med 2016;9:11-7.
- Qadir A, Shakeel F, Ali A, Faiyazuddin M. Phytotherapeutic potential and pharmaceutical impact of Phoenix dactylifera (date palm): Current research and future prospects. J Food Sci Technol 2020;57:1191-204.
[Crossref] [Google Scholar] [PubMed]
- Martin-Sanchez AM, Cherif S, Ben-Abda J, Barber-Valles X, Perez-Alvarez JA, Sayas-Barbera E. Phytochemicals in date co-products and their antioxidant activity. Food Chem 2014;158:513-20.
[Crossref] [Google Scholar] [PubMed]
- Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer 2006;54(2):184-201.
[Crossref] [Google Scholar] [PubMed]
- Alhaider IA, Mohamed ME, Ahmed KK, Kumar AH. Date palm (Phoenix dactylifera) fruits as a potential cardioprotective agent: The role of circulating progenitor cells. Front Pharmacol 2017;8:592.
[Crossref] [Google Scholar] [PubMed]
- Saafi EB, Louedi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, et al. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol 2011;63(5):433-41.
[Crossref] [Google Scholar] [PubMed]
- Elberry AA, Mufti ST, Al-Maghrabi JA, Abdel-Sattar EA, Ashour OM, Ghareib SA, et al. Anti-inflammatory and antiproliferative activities of date palm pollen (Phoenix dactylifera) on experimentally-induced atypical prostatic hyperplasia in rats. J Inflamm 2011;8:1-3.
[Crossref] [Google Scholar] [PubMed]
- Arzi A, Azarbani S, Aghel N, Nazari Z, Rezaei M. The preventive effect of date palm (Phoenix dactylifera) seed and fruit hydroalcoholic extracts on carrageenan-induced inflammation in male rat’s hind paw. Jundishapur Sci Med J 2014;13(5):495-502.
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 1996;139(2):394-401.
[Crossref] [Google Scholar] [PubMed]
- Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 2015;43(6):776-93.
[Crossref] [Google Scholar] [PubMed]
- Macdonald PC, Madden JD, Brenner PF, Wilson JD, Siiteri PK. Origin of estrogen in normal men and in women with testicular feminization. J Clin Endocrinol Metab 1979;49(6):905-16.
[Crossref] [Google Scholar] [PubMed]
- Rust MB, Faulhaber J, Budack MK, Pfeffer C, Maritzen T, Didie M, et al. Neurogenic mechanisms contribute to hypertension in mice with disruption of the K-Cl cotransporter KCC3. Circul Res 2006;98(4):549-56.
[Crossref] [Google Scholar] [PubMed]
- Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977;55(5):767-72.
[Crossref] [Google Scholar] [PubMed]
- Miller NE, Thelle DS, Forde OH, Mjos OD. The tromsøheart-study: High-density lipoprotein and coronary heart-disease: A prospective case-control study. Lancet 1977;309(8019):965-8.
[Crossref] [Google Scholar] [PubMed]
- Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J 1971;123(5):805-14.
[Crossref] [Google Scholar] [PubMed]
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta Gen 1979;582(1):67-78.
[Crossref] [Google Scholar] [PubMed]
- Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 1978;90(1):81-9.
[Crossref] [Google Scholar] [PubMed]
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res 1984;44(11):5086-91.
[Google Scholar] [PubMed]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973;179(4073):588-90.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-xL protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod 2001;65(5):1489-95.
[Crossref] [Google Scholar] [PubMed]
- Keating AF, Fernandez SM, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Inhibition of PIK3 signaling pathway members by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol Reprod 2011;84(4):743-51.
[Crossref] [Google Scholar] [PubMed]
- Kuhnle GG, Dell’Aquila C, Aspinall SM, Runswick SA, Joosen AM, Mulligan AA, et al. Phytoestrogen content of fruits and vegetables commonly consumed in the UK based on LC–MS and 13C-labelled standards. Food Chem 2009;116(2):542-54.
- Malaivijitnond S, Kiatthaipipat P, Cherdshewasart W, Watanabe G, Taya K. Different effects of Pueraria mirifica, a herb containing phytoestrogens, on LH and FSH secretion in gonadectomized female and male rats. J Pharmacol Sci 2004;96(4):428-35.
[Crossref] [Google Scholar] [PubMed]
- Ko SH, Kim HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020;12(1):202.
[Crossref] [Google Scholar] [PubMed]
- Al-Yahya M, Raish M, AlSaid MS, Ahmad A, Mothana RA, Al-Sohaibani M, et al. ‘Ajwa’dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 2016;23(11):1240-8.
[Crossref] [Google Scholar] [PubMed]
- Ciumarnean L, Milaciu MV, Runcan O, Vesa SC, Rachisan AL, Negrean V, et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020;25(18):4320.
[Crossref] [Google Scholar] [PubMed]
- Qureshi HJ, Hussain G, Jafary ZA, Bashir MU, Latif N, Riaz Z. Calcium status in premenopausal and postmenopausal women. J Ayub Med Coll Abbottabad 2010;22(2):143-5.
[Google Scholar] [PubMed]
- Nikander E, Metsä-Heikkilä M, Ylikorkala O, Tiitinen A. Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. J Clin Endocrinol Metab 2004;89(3):1207-12.
[Crossref] [Google Scholar] [PubMed]
- Al-Alawi RA, Al-Mashiqri JH, Al-Nadabi JS, Al-Shihi BI, Baqi Y. Date palm tree (Phoenix dactylifera L.): natural products and therapeutic options. Front Plant Sci 2017;8:845.
[Crossref] [Google Scholar] [PubMed]
- Petrine JC, Del Bianco‐Borges B. The influence of phytoestrogens on different physiological and pathological processes: An overview. Phytother Res 2021;35(1):180-97.
[Crossref] [Google Scholar] [PubMed]