- *Corresponding Author:
- Kun Cheng
Department of Pancreatic Surgery, Digestive and Vascular Surgical Center, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang 830000, China
E-mail: 18199324449@163.com
| Date of Received | 15 January 2023 |
| Date of Revision | 24 August 2023 |
| Date of Acceptance | 04 March 2024 |
| Indian J Pharm Sci 2024;86(2):442-447 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Recent studies have shown that calycosin possess multiple pharmacological properties, containing anti-cancer activity. Hence, this project explored the effect and possible mechanism of calycosin in pancreatic cancer. After being cultured, SW1990 cells were exposed to different concentrations of calycosin. 3-(4,5-dimethylthiazol 2-yl)-2,5 diphenyl tetrazolium bromide, plate colony formation, and transwell experiments assessed cell proliferation, colony formation, migration, and invasion. Circ_0000285 expression was assessed using quantitative reverse transcription-polymerase chain reaction experiment. E-cadherin and N-cadherin protein levels were examined using Western blot. After calycosin treatment, SW1990 cell proliferation, migration, invasion, circ_0000285 expression, N-cadherin were repressed, and E-cadherin was increased in dose-dependent way. Circ_0000285 deficiency might hinder cell proliferation, migration, and invasion. Furthermore, the upregulation of circ_0000285 might partly antagonize calycosin-mediated SW1990 cell proliferation, migration, and invasion inhibition. Calycosin treatment might block pancreatic cancer cell development through down-regulating circ_0000285.
Keywords
Pancreatic cancer, calycosin, circ_0000285, cell proliferation, migration, invasion
As a highly fatal malignancy of the digestive system, pancreatic cancer is often diagnosed at an advance stage or even metastasis, owning to its highly aggressive nature and atypical early symptoms[1,2]. Significant advances in conventional treatments, containing surgical resection, radiotherapy, and chemotherapy, have recently obtained some benefits, but the long-term survival is still poor[3]. During recent years, some Traditional Chinese Medicines (TMCs) possessed anti-oxidative stress and anti-tumor effects for alleviating the progression and development in different human cancers, including pancreatic cancer[4,5]. As a functional phytoestrogen isoflavone isolated from TCM radix Astragalus, Calycosin (CC) is evidenced with diverse pharmacological properties, such as cytoprotection, anti-oxidative, and hypoglycemic effects[6]. Beneficially, CC has demonstrated favorable anti-tumor properties in a variety of malignancies via different signal pathways[7,8]. However, the influence of CC on the biological behavior of pancreatic cancer cells is unclear. Interestingly, some laboratory work have suggested the repression of CC on human cancer cell growth and metastasis by regulating noncoding Ribonucleic Acid (RNA), such as lncRNA HOTAIR and microRNA (miR)-375[9,10]. Unlike other non-coding RNAs, circular RNAs (circRNAs) have covalently closed loop structure produced by the back-spliced mechanism of pre-messenger RNA (mRNA), which are abnormally expressed in numerous tumors and partake in the process of tumorigenesis and development[11]. Previous studies have indicated that circ_0000285 acted as a well-known carcinogenic factor in various human cancers[12,13], but its role in pancreatic cancer has not been reported. Accordingly, this project focused on whether CC might control pancreatic cancer progression via modulating circ_0000285.
Materials and Methods
Reagents:
CC (purity >98 %; Biopurify, Chengdu, China) was dissolved in Dimethyl Sulfoxide (DMSO). Human pancreatic cancer cells (SW1990 cells; American Type Culture Collection (ATCC), Manassas, Virginia, United States of America (USA)) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Beyotime, Shanghai, China) containing 10 % Fetal Bovine Serum (FBS). Trizol reagent and Lipofectamine™ 3000 transfection reagent were provided by Invitrogen (Carlsbad, California, USA). Tiangen (Beijing, China) supplied reverse transcription and fluorescent quantitative Polymerase Chain Reaction (qPCR) reagents RiboBio (Guangzhou, China) and Genomeditech (Shanghai, China) respectively offered si-circ_0000285 and control (si-NC), and plasmid cloning Deoxyribonucleic Acid (pcDNA)-circ_0000285 and its control (pcDNA). Transwell chamber and Matrigel® were supplied by Solarbio (Beijing, China). Rabbit antihuman E-cadherin, N-cadherin, internal reference Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), and secondary antibodies were acquired from Cell Signaling Technology (CST) (Danvers, Massachusetts, USA).
Method:
Cell treatment: In this research, SW1990 cells in 6-well plates were respectively treated with 5 μmol/l, 10 μmol/l, and 20 μmol/l CC for 24 h[14], marked CC-Low (L), CC-Medium (M), and CC-High (H) group. At the same time, normal cultured SW1990 cells acted as control group. Based on Lipofectamine method, we knockdowned circ_0000285 by transfecting si-NC or si-circ_0000285 into SW1990 cells. In addition, pcDNA or pcDNA-circ_0000285 was transfected into SW1990 cells, followed by incubation with 20 μmol/l CC for 24 h, recorded as CC+pcDNA and CC+pcDNA-circ_0000285 group.
3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2- H-Tetrazolium Bromide (MTT) assay: In 96-well plates, collected SW1990 cells (2×103) were mixed with 20 μl MTT solution at 37°. After continuing to incubate for 4 h, discard the supernatant, each well were incubated with 150 μl DMSO, followed by culture for 5 min avoiding light. Lastly, an enzyme labeling instrument at 490 nm was implemented for Absorbance value (A value).
Clone formation assay: In 6-well plates, 1000 SW1990 cells were cultured for about 2 w. After Phosphate Buffer Solution (PBS) washing, cells were subjected to 4 % paraformaldehyde fixture for 15 min and crystal violet staining for 5 min. Finally, the number of cell clone formation was assessed using a microscope (≥50 cells were regarded as a colony).
Transwell assay: For migration experiment, 5×104 SW1990 cells in serum-free medium were added to upper chamber, and bottom counterpart was charged with 600 μl medium with 10 % FBS. Then, the cells were continued for 24 h. After staining for 10 min, a microscope was applied for the analysis of migrated cell number. For invasion, 2×105 cells were added to upper chamber, which was pre-spread with Matrigel® matrix gel dilution (40 μl/well). Subsequent steps were similar to the cell migration experiment.
qRT-PCR assay: After 1 ml Trizol reagent treatment, total RNAs from SW1990 cells were prepared, followed by synthesizing complementary DNA (cDNA) using reverse transcription kit. Subsequently, qRT-PCR amplification reaction was carried out using the template cDNA. Finally, the data from each group was analyzed using Roche LightCycler® 480 fluorescence quantitative PCR.
Western blot assay: According to 400 μl Radioimmunoprecipitation Assay (RIPA) lysate, total SW1990 cell proteins were extracted. After Bicinchoninic Acid Assay (BCA) method determination, protein samples were taken for Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Following shifted onto membranes, primary antibodies; E-cadherin (1:1000), N-cadherin (1:1000), and GAPDH (1:2000) were labeled at 4° for 24 h. After soaking in the secondary antibody (1:5000) dilution for 2 h, each band was analyzed.
Statistical analysis:
In this research, the data was analyzed using Statistical Package for the Social Sciences (SPSS) 21.0 and displayed as (x̄ ±s). Data comparison was made by Student’s t-test or one-way Analysis of Variance (ANOVA). Difference was deemed statistically significant at p<0.05.
Results and Discussion
As shown in Table 1, CC treatment might improve cell proliferation inhibition rates in a dosedependent way, and reduced the number of colony formation, migration, and invasion. Based on the results presented in Table 2, circ_0000285 expression was gradually decreased in SW1990 cells with increasing concentrations of CC.
| Group | Inhibition rate % | Cell number | ||
|---|---|---|---|---|
| Colony | Migration | Invasion | ||
| Control | 0.00±0.00 | 120.89±7.58 | 188.56±6.57 | 148.11±7.56 |
| CC-L | 13.53±0.85* | 95.33±4.16* | 153.44±4.95* | 114.22±6.11* |
| CC-M | 25.60±2.04*# | 75.44±2.87*# | 116.67±5.62*# | 84.89±2.96*# |
| CC-H | 43.11±1.20*#& | 48.67±3.77*#& | 84.89±2.88*#& | 58.78±4.29*#& |
| F | 1908.961 | 346.382 | 675.342 | 437.497 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. control, #p<0.05 vs. CC-L, and &p<0.05 vs. CC-M
Table 1: Influences Of CC on SW1990 Cell Development (x̄±s, n=9)
| Group | circ_0000285 |
|---|---|
| Control | 1.00±0.00 |
| CC-L | 0.77±0.05* |
| CC-M | 0.41±0.03*# |
| CC-H | 0.14±0.01*#& |
| F | 491.003 |
| p | 0.000 |
Note: *p<0.05 vs. control, #p<0.05 vs. CC-L, and &p<0.05 vs. CC-M
Table 2: Effects of CC on circ_0000285 Content in SW1990 Cells
Data from Table 3 displayed that circ_0000285 expression clearly declined after si-circ_0000285 introduction, and circ_0000285 level was significantly enhanced by pcDNA circ_0000285 transfection.
| Group | circ_0000285 |
|---|---|
| si-NC | 1.00±0.00 |
| si-circ_0000285 | 0.36±0.03* |
| t | 64.000 |
| p | 0.000 |
| pcDNA | 1.00±0.00 |
| pcDNA-circ_0000285 | 3.22±0.14# |
| t | 47.571 |
| p | 0.000 |
Note: *p<0.05 vs. si-NC and #p<0.05 vs. pcDNA
Table 3: Detection Of circ_0000285 Expression
As exhibited in Table 4, circ_0000285 absence might augment cell proliferation inhibition rates, and constrain cell colony formation, migration, and invasion.
| Group | Inhibition rate % | Cell number | ||
|---|---|---|---|---|
| Colony | Migration | Invasion | ||
| si-NC | 0.00±0.00 | 124.44±7.63 | 182.22±7.39 | 145.44±12.14 |
| si-circ_0000285 | 35.61±1.73* | 61.00±3.74* | 98.67±5.06* | 70.78±4.05* |
| t | 61.751 | 22.398 | 27.986 | 17.502 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. si-NC group
Table 4: Effects of circ_0000285 Silencing on SW1990 Cell Growth and Metastasis
Relative to the CC+pcDNA group, proliferation inhibition rates were suppressed in the CC+pcDNAcirc_ 0000285 group, and the numbers of colony formation, migration, invasion were increased as shown in Table 5.
| Group | Inhibition rate % | Cell number | ||
|---|---|---|---|---|
| Colony | Migration | Invasion | ||
| CC+pcDNA | 42.90±2.69 | 49.67±2.83 | 85.22±3.85 | 57.78±4.08 |
| CC+pcDNA-circ_0000285 | 15.29±0.84* | 87.89±4.58* | 136.44±6.09* | 97.56±5.68* |
| t | 29.392 | 21.297 | 21.327 | 17.064 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. si-NC group
Table 5: circ_0000285 Might Abolish the Repression of CC on Cell Malignant Behaviors
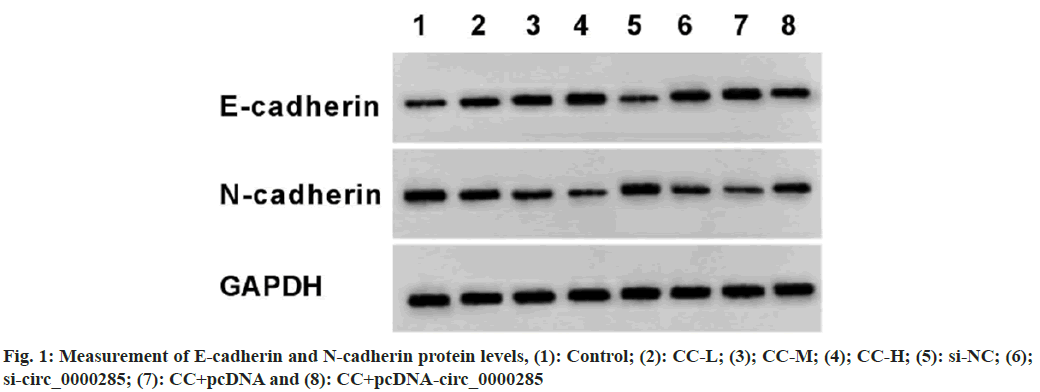
According to the results displayed in fig. 1 and Table 6, CC exposure might enhance E-cadherin and reduce N-cadherin expression in a dosedependent manner. Circ_0000285 silencing might elevate E-cadherin expression and decrease N-cadherin level. In addition, the overexpression of circ_0000285 might restrain E-cadherin content and increase N-cadherin expression in CC-treated SW1990 cells.
| Group | E-cadherin | N-cadherin |
|---|---|---|
| Control | 0.21±0.02 | 0.60±0.05 |
| CC-L | 0.37±0.03* | 0.45±0.04* |
| CC-M | 0.53±0.05*# | 0.33±0.03*# |
| CC-H | 0.75±0.05*#& | 0.18±0.02*#& |
| F | 303.810 | 212.000 |
| p | 0.000 | 0.000 |
| si-NC | 0.20±0.02 | 0.62±0.05 |
| si-circ_0000285 | 0.58±0.06△ | 0.23±0.02△ |
| t | 18.025 | 21.726 |
| p | 0.000 | 0.000 |
| CC+pcDNA | 0.73±0.05 | 0.19±0.03 |
| CC+pcDNA-circ_0000285 | 0.39±0.05□ | 0.41±0.03□ |
| t | 14.425 | 15.556 |
| p | 0.000 | 0.000 |
Note: *p<0.05 vs. control, #p<0.05 vs. CC-L, &p<0.05 vs. CC-M, △p<0.05 vs. si-NC, and □p<0.05, vs. CC+pcDNA
Table 6: Western Blot Analysis of E-Cadherin and N-Cadherin Expression
Currently, increasing attention has been paid to the active ingredients extracted from TCM that possess anti-tumor effects on pancreatic cancer via regulating the differential gene expression[15]. Of note, convincing evidence have indicated that dysregulation of circRNA might participate in the process of pancreatic cancer via sponging miRNAs[16,17]. Nevertheless, it has not been elucidated whether circRNA can be a potential target for pancreatic cancer treatment with TCM. In recent years, numerous literatures presented the many pharmacological properties of CC, in particular the significant antitumor effects on various types of cancer cells. For example, CC treatment might diminish breast cancer cell invasion via reducing Epithelial-Mesenchymal Transition (EMT)[18]. Apart from that, CC addition might hinder cervical cancer cell proliferation and induce apoptosis through increasing miR-375[10]. Yet, the research related to CC treatment and pancreatic cancer has not been reported. In this work, SW1990 cell proliferation inhibition rate was improved and colony formation number was reduced after CC treatment, implying the repression of CC on cell proliferative ability. The absence of the epithelial cell marker E-cadherin and the upregulation of the expression of the mesenchymal cell marker N-cadherin are considered to be characteristic of EMT, which exert a key impact in the early stages of cancer cell metastasis[19]. Herein, the present work verified that CC exposure might decrease migration and invasion numbers, reduced N-cadherin protein expression, and reinforce E-cadherin content, suggesting that CC treatment might repress tumor cell migration and invasion via inhibition of EMT transformation.
Circ_0000285 was highly expressed in osteosarcoma cells and could promote osteosarcoma cell growth via regulating miR-409-3p/IGFBP3 expression[20]. Furthermore, circ_0000285 promotes laryngeal cancer cell growth and invasion via activating the Wnt/β-catenin pathway[21]. Consistent with these reports, our data confirmed that circ_0000285 knockdown might impede SW1990 cell proliferation, migration, and invasion. Of interest, our data displayed that circ_0000285 content was clearly reduced in CC-induced SW1990 cells, and its overexpression might partly abolish the suppression of CC on SW1990 cell malignant behaviors. This observation indicated that applying CC was able to dampen SW1990 cell growth and metastasis by decreasing circ_0000285 expression. In summary, CC treatment repress pancreatic cancer cell malignant behaviors through modulating circ_0000285. These results suggested that circ_0000285 may be a potential target for tumor treatment with CC, laying an experimental foundation for further revealing the molecular mechanism of CC against pancreatic cancer. However, its specific mechanism of action still needs to be further investigated.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett 2021;520:1-11.
[Crossref] [Google Scholar] [PubMed]
- Halbrook CJ, Lyssiotis CA, di Magliano MP, Maitra A. Pancreatic cancer: Advances and challenges. Cell 2023;186(8):1729-54.
[Crossref] [Google Scholar] [PubMed]
- Kolbeinsson HM, Chandana S, Wright GP, Chung M. Pancreatic cancer: A review of current treatment and novel therapies. J Invest Surg 2023;36(1):2129884.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Xu H, Li Y, Sun Y, Peng X. Advances in the treatment of pancreatic cancer with traditional Chinese medicine. Front Pharmacol 2023;14:1089245.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Chen S, Sun J. Traditional Chinese medicine may be further explored as candidate drugs for pancreatic cancer: A review. Phytother Res 2021;35(2):603-28.
[Crossref] [Google Scholar] [PubMed]
- Deng M, Chen H, Long J, Song J, Xie L, Li X. Calycosin: A review of its pharmacological effects and application prospects. Expert Rev Anti Infect Ther 2021;19(7):911-25.
[Crossref] [Google Scholar] [PubMed]
- Zhu L, Liu S, Liao YF, Sheng YM, He JC, Cai ZX, et al. Calycosin suppresses colorectal cancer progression by targeting ERβ, upregulating PTEN, and inhibiting PI3K/Akt signal pathway. Cell Biol Int 2022;46(9):1367-77.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhang JQ, Zhang T, Xue H, Zuo WB, Li YN, et al. Calycosin induces gastric cancer cell apoptosis via the ROS-mediated MAPK/STAT3/NF-κB pathway. Onco Targets Ther 2021;14:2505-17.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Miao H, Wei W, Tian J, Chen J. Inhibitory effect of calycosin on breast cancer cell progression through downregulating lncRNA HOTAIR and downstream targets: HuR and IGF2BP1. Acta Biochim Biophys Sin 2022;55(2):225-36.
[Crossref] [Google Scholar] [PubMed]
- Zhang D, Sun G, Peng L. Calycosin inhibits viability, induces apoptosis, and suppresses invasion of cervical cancer cells by upregulating tumor suppressor miR-375. Arch Biochem Biophys 2020;691:108478.
[Crossref] [Google Scholar] [PubMed]
- Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol 2022;19(3):188-206.
[Crossref] [Google Scholar] [PubMed]
- Zhang B, Li Q, Song Z, Ren L, Gu Y, Feng C, et al. Hsa_circ_0000285 facilitates thyroid cancer progression by regulating miR-127-5p/CDH2. J Clin Lab Anal 2022;36(7):e24421.
[Crossref] [Google Scholar] [PubMed]
- Zeng Q, Ji X, Li X, Tian Y. Circ_0000285 regulates nasopharyngeal carcinoma progression through miR-1278/FNDC3B axis. Hum Exp Toxicol 2023;42:9603271221141689.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Zhang LY, Zhao ZL. Study on the mechanism of calycosin inhibiting glioma cell proliferation. J Shenyang Pharm Univ 2020;37(8):725-9.
- Zhang Y, Zhang XX, Yuan RY, Ren T, Shao ZY, Wang HF, et al. Cordycepin induces apoptosis in human pancreatic cancer cells via the mitochondrial-mediated intrinsic pathway and suppresses tumor growth in vivo. Onco Targets Ther 2018;11(1):4479-90.
[Crossref] [Google Scholar] [PubMed]
- Li L, Wang N, Wang J, Li J. Hsa_circRNA_001859 regulates pancreatic cancer progression and epithelial-mesenchymal transition through the miR-21-5p/SLC38A2 pathway. Cancer Biomark 2023;37(1):39-52.
[Crossref] [Google Scholar] [PubMed]
- Hou YS, Li X. Circ_0005273 induces the aggravation of pancreatic cancer by targeting KLF12. Eur Rev Med Pharmacol Sci 2020;24(22):11578-86.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Lin M, Wang J, Yang F, Yang P, Liu Y, et al. Calycosin inhibits breast cancer cell migration and invasion by suppressing EMT via BATF/TGF-beta1. Aging (Albany NY) 2021;13(12):16009-23.
[Crossref] [Google Scholar] [PubMed]
- de Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13(2):97-110.
[Crossref] [Google Scholar] [PubMed]
- Long Z, Gong F, Li Y, Fan Z, Li J. Circ_0000285 regulates proliferation, migration, invasion and apoptosis of osteosarcoma by miR-409-3p/IGFBP3 axis. Cancer Cell Int 2020;20(1):481-91.
[Crossref] [Google Scholar] [PubMed]
- Qin JB, Chang W, Yuan GH, Huang L, Qiu ZF. Circular RNA hsa_circ_0000285 acts as an oncogene in laryngocarcinoma by inducing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci 2020;24(19):9773-83.
[Crossref] [Google Scholar] [PubMed]