- *Corresponding Author:
- Netala Silvia
Department of Pharmaceutical Chemistry, Shri Vishnu College of Pharmacy, Bhimavaram, Andhra Pradesh 534202
E-mail: silvia@svcp.edu.in
| Date of Received | 16 November 2022 |
| Date of Revision | 01 February 2024 |
| Date of Acceptance | 04 March 2024 |
| Indian J Pharm Sci 2024;86(2):458-467 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Aerva sanguinolenta (L) Blume is a traditional medicinal plant possessing many medicinal values. In folklore medication, the plant was used as wound healing and anti-inflammatory agent. The plant was evaluated and found to possess hepatoprotective, wound healing, diuretic activities. Though it was found to possess many therapeutic properties the plant was not standardized in terms of its identification parameters. It is necessary to have quality control parameters to ensure the purity, safety and efficacy of medicinal plant. The present study provides the quality parameters by employing pharmacognostic and phytochemical studies. Pharmacognostic study was conducted on fresh and dried plant material as per the guidelines of World Health Organization which includes organoleptic, macroscopic, microscopic and phytochemical studies. The plant is usually herb, clambering sub-shrub with tap root and amaranth purple colored leaves and stems. Stems and leaves are covered with dense whitish-woolly hairs. Leaves are opposite, dorsiventral with anisocytic stomata. Multicellular covering trichomes are present in stem and leaf. Druses of calcium oxalate crystals are present both in the stem and leaf. The preliminary phytochemical studies showed the presence of alkaloids, flavonoids, steroids, triterpenoids and carbohydrates. The quality control parameters obtained in the study is helpful in the identification and standardization of the plant material which further supports in the preparation of monograph for the plants used in folklore medicine.
Keywords
Aerva sanguinolenta, pharmacognostic, folklore, phytochemicals
Herbal medicines have been used in medicinal practice for thousands of years and are recognized as a valuable and readily available health care resource. The World Health Organization (WHO) estimated that about 80 % of population living in the developing countries relies almost exclusively on traditional medicine for their primary health care needs. Many of the most common medicines are developed from the components of medicinal plants. Proper identification and authentication of plant material is the first step in the standardization process. It is essential for quality assurance and can be achieved by reliable pharmacognostic studies. Macroscopic, microscopic and chemical analysis of herbal materials is recommended parameter by many pharmacopeias and regulatory guidelines for quality control and standardization[1].
Aerva sanguinolenta (A. sanguinolenta) (L) Blume (Amaranthaceae) is one such traditional medicinal plant having many medicinal values. It is commonly known as climbing wool plant. In folklore medication leaf and flower of the plant were used as wound healing and anti-inflammatory agent for injuries from falls, rheumatic arthritis and pain in muscles[2]. The plant was evaluated and found to possess hepatoprotective, wound healing, diuretic and anticancer activities[3,4]. As far as the available literature is concerned, this plant has not yet been scientifically validated and there were no reports on the pharmacognostic and phytochemical parameters. The results of the study could be useful in setting quality parameters for the identification, standardization and preparation of a monograph.
Materials and Methods
Plant material:
The plant material was obtained from Bhimavaram of East Godavari District and authenticated by Dr. P. Prasanna Kumari, Department of Botany, DNR College, Bhimavaram. A specimen is preserved in the college herbarium of Shri Vishnu College of Pharmacy (Voucher number: SVCP/Cognosy/6).
Pharmacognostic evaluation-macroscopy:
Organoleptic characteristics of fresh parts of A. sanguinolenta was assessed by observing colour, odour, taste, size and shape according to WHO quality control methods for herbal medicine[5-7].
Qualitative microscopy:
Fresh parts of the plant were cut and fixed in a mixture of formalin (5 ml)+acetic acid (5 ml)+70 % ethyl alcohol (90 ml). After 24Hrs the specimens were dehydrated with graded series of Tertiary-Butyl Alcohol (TBA)[8]. Paraffin wax (melting point 58°-60°) was gradually added to the specimens. The specimens were cast into paraffin blocks. The paraffin embedded specimens were sectioned with the help of microtome. Later sections were freed from the wax. The sections and powdered samples were stained with reagents like phloroglucinol and hydrochloric acid and finally mounted in glycerin for the study of microscopical characters. Different cell components were studied according to the standard methods and their photographs were taken using photomicrography[9-11]. Staining reagents were used as per standard procedures[12-14]. The various identifying characters were studied with or without staining and recorded.
Quantitative microscopy:
Quantitative determinations like stomatal number, stomatal index of the abaxial and adaxial surfaces of the leaf, vein islet and vein termination number of the leaf were determined. Stomatal number is the average number of stomata per square mm of epidermis. The percentage proportion of the ultimate divisions of the epidermis of a leaf which can be converted into stomata is termed as stomatal index. Stomatal index can be calculated by using following equation; I=S/(E+S)×100, where, I=stomatal index, S=number of stomata per mm2 and E=number of epidermal cells per mm2.
A piece of leaf was cleaned and the upper and lower epidermis was peeled out separately. It was kept on slide and mounted in glycerin water. Camera lucida was attached and drawing board was placed for drawing the cells. A square of 1 mm was drawn by means of stage micrometer on it. The slide with cleared leaf was placed on the stage and the epidermal cells and stomata were traced. The number of stomata and the number of epidermal cells in each field were counted. The number of stomata was calculated as stomatal number and the stomatal index calculated by using the above formula separately for upper and lower surface.
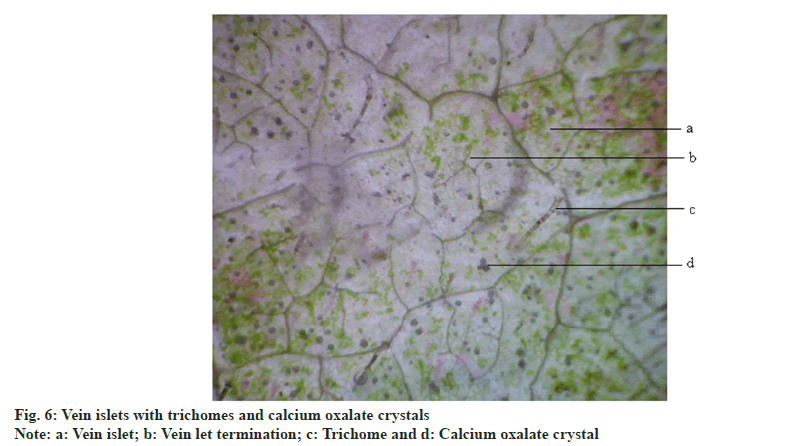
Vein islet is the minute area of photosynthetic tissue encircled by the ultimate division of the conducting strands. Vein termination number is the number of veinlet terminations per square mm of leaf surface. A piece of the leaf was cleared by boiling in chloral hydrate solution and camera lucida and drawing board were arranged and 1 sqare millimeter area was drawn with help of stage mm. A square was constructed on this line in the center of the field. The slide was placed on the stage. The veins included within the square were traced off, completing the outline of those islets which overlap two adjustment side of the square. The average number of vein islet from the four adjoining square, to get the value for one square mm was calculated. The number of veinlet termination present within the square was counted and the average number of veinlet termination number from the four adjoining square to get the value for 1 square mm was found and known as vein termination number [15].Powder microscopy:
The dried and powdered material was studied as per the method mentioned by Khandelwal [5].
Physicochemical parameters such as ash values and extractive values were determined according to the well-established official method and procedure[16,17]. Preliminary phytochemical screening was carried out using the standard procedure described by Khandelwal[18].
Fluorescence analysis:
When the sample is exposed to Ultraviolet (UV) radiation many crude drugs exhibit the fluorescence. Evaluation of crude drugs based on fluorescence in daylight is not much used, as it is usually unreliable due to the weakness of the fluorescence effect. Fluorescence lamps eliminate visible radiation from the lamp as they are fitted with suitable filters and transmit UV radiation of definite wavelength. If the substances themselves are not fluorescent, they may often be converted into fluorescent derivatives by applying different reagents hence some crude drugs are often assessed qualitatively in this way and it is an important parameter of pharmacognostical evaluation. The changes in appearance and colour were observed and recorded. Powdered plant material was treated with various chemical reagents and exposed to visible and UV light to study their fluorescence behavior[19].
Results and Discussion
Kingdom: Plantae, Subkingdom: Viridiplantae, Phylum: Tracheophyta, Subphylum: Euphyllophytina, Class: Spermatopsida, Subclass: Caryophyllidae, Superorder: Caryophyllanae, Order: Caryophyllales, Suborder: Chenopodiineae, Family: Amaranthaceae, Subfamily: Amaranthoideae, Tribe: Amarantheae, Genus: Aerva, Specific epithet: Sanguinolenta Blume; Botanical name: A. sanguinolenta (L) Blume. Synonyms: A. sanguinolenta L., Achyranthes scandens Roxb., Aerva scandens (Roxb.) Moq., Aerva timorensis Moq., Aerva velutina Moq[20,21].
Vernacular names in India[22]: Common name: Climbing wool plant, Gujarati: gorakhganjo; Hindi: nuriya, sufed phulia; Kannada: Nela Hindi soppu; Telugu: Pedda pindikura, puriti-tige; in other countries: Nepal: aitinbot; Indonesia: ki sambang (Sundanese), sambang colok, gondang kasih (Javanese); Laos: do: k khaix ped (Luang Prabang); Thailand: khruea khaao tok (northern), yaa dok khaao (central), phan nguu yai (Saraburi); Vietnam: Phu Khanh, Rau chua, Mao.
Folklore medicinal uses of the plant were as follows. The whole plant was used as diuretic and demulcent[23], tender shoot of the plant used as decoction form for galactogue to nursing mother[24] and decoction of whole plant was taken twice a day to expel intestinal worms[25]. Leaves and root of the plant have been used traditionally for body pain and the paste of leaf and root is applied to affected area[26].
Plant is distributed throughout tropical India[27]. This species is widespread in the parts of Bhutan, Cambodia, Laos, Myanmar, Nepal, Sikkim and Vietnam. Foothill and sub-Himalayan zone in Pakistan, India, Indo-China, Southern China and Taiwan to Thailand and the Malaysian region (Java, the Lesser Sunda Islands, Sulawesi, The Moluccas and Philippines)[28,29]. In India, plant is common throughout in Maharashtra; some parts of Karnataka including Chikmagalur, Dharwar, Kanara; some parts of Kerala includes Kottayam, Kozhikode, Thrissur and some parts of Tamil Nadu includes Coimbatore, Salem, Tiruchchirappalli, Tiruvannamalai.
Climbing wool-plant is a perennial herb, frequently woody below, prostrate to erect or frequently scrambling, 0.4-1-(2) m, densely tomentose or canescent with whitish or yellowish, appressed or patent hairs (the lowest internodes sometimes glabrescent). Root is usually a tap-root, laterally branched, cylindrical, up to 0.8 cm in thickness and about 25 cm long pieces, externally light brown and rough but cut surface white and smooth; fibrous fracture and hard. Stems are nearly cylindrical, branching alternate, external surface shows slight ridges and furrows, hairy and purple in colour; cut surface white; granular fracture. Stems and leaves are amaranth purple in colour. Stems are branched from the base and usually also above, upper branches commonly long and slender. Stem and branches are round, channeled, densely velvety with whitish or yellowish hairs. Leaves and branches mostly alternate, the lower not rarely and all occasionally opposite; leaves are broadly to narrowly elliptic or elliptic-lanceolate or elliptic-ovate, narrowed to flat at the base, acute to acuminate at the apex, densely whitishwoolly on both surfaces (rarely green and only thinly hairy). Those of the main stem below the inflorescence are 1.5×0.8-18×6 cm, petiole up to 2 cm long, branch and inflorescence leaves gradually reduce upwards. Flowers are small, actinomorphic, bisexual or unisexual, or sterile and reduced, bracteate and bracteolate, solitary or aggregated in cymes. Flowers are minute cluster as axillary spike; greenish-white; with 5 perianths, bracteolate; actinomorphic, bisexual; with 5 stamen, opposite to perianth, anthers bi lobed; stigma bifid, superior ovary, unilocular with campylotropous ovule. Flowers arise in stalkless spikes, (or pedunculate by reduction of branches), forming a lax raceme or terminal panicle, the lower axillary but the upper without subtending leaves,0.5×0.4-8×0.6 cm, cylindrical (conical when young), silky, white to pale pink or pale brown. Bracts are 1-1.5 mm, deltoid-ovate, membranous with an excurrent yellowish midrib, thinly pilose and persistent. Bracteoles similar to bracts but slightly smaller are also persistent. Flowers are hermaphrodite or hermaphrodite and female. Outer 2 tepals hyaline, elliptic-oblong, tapering above with a distinct mucro formed by the excurrent midrib, 1.75-2.25 mm without the mucro; inner slightly shorter and narrower, acute, hyaline with a narrow central green vita along the midrib, the vita bordered by two fine lateral nerves and extending for about two-thirds of the length of each tepal. All tepals are densely woolly dorsally. Stamens are delicate, at anthesis attaining about half the length of the style. Style and two short, divergent stigmas subequalling the ovary in length at anthesis. Capsule 1 mm in size, rotund, compressed. Seed 0.8-1 mm, reniform, black, shining, the testa shallowly but distinctly reticulate. Flowering and fruiting occurs during September-May (fig. 1). Fruit is greenish, round, compressed membranous, utricle or circumscissile capsule with a coriaceous upper part or lid and containing a single seed. Capsule is 1 mm rotund, compressed. Seed 0.8-1 mm, reniform, black, shining, the testa shallowly but distinctly reticulate. Seed-minute, 0.5 to 0.7 cm in dia, black, polished and kidney shaped; pungent taste[30,31].
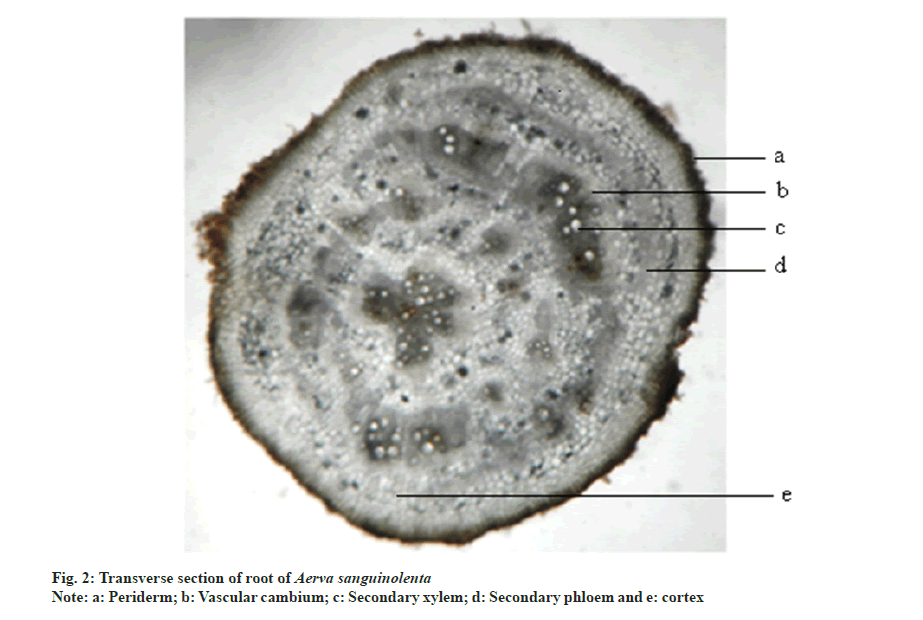
Transverse section of root is circular in outline. It consists of periderm, cortex and secondary xylem regions. Periderm is 1-5 layered and brown to black in colour. Cortex is composed of rectangular parenchymatous cells. Vascular cambium cuts off secondary phloem towards outer surface and secondary xylem towards inner side. Major portion of the root is occupied by secondary xylem which is in the form of radiating bands. The cells in between them are the medullary rays. Remaining ground tissue is composed of rounded polygonal parenchymatous cells. Pith is nearly absent (fig. 2).
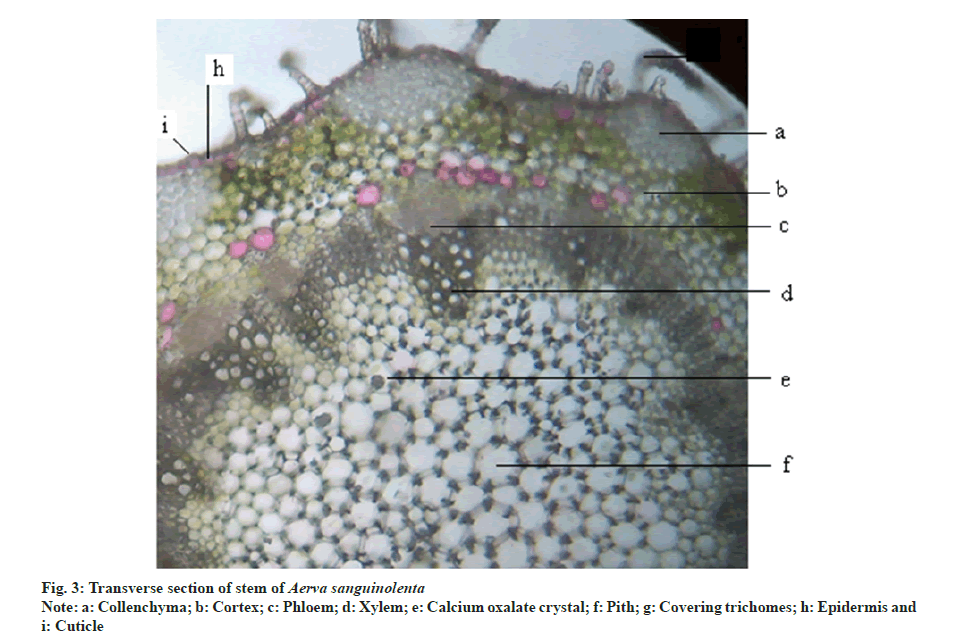
Transverse section of stem is rounded polygonal in outline. It consists of following regions epidermis, cortex, stelar tissue and pith. Epidermis is covered with cuticle. Epidermal cells are rounded rectangular and uniseriate with multicellular covering trichomes. It is followed by cortex which is differentiated into outer collenchyma and inner parenchymatous regions. Groups of collenchyma are present in the angular regions which gives the rounded polygonal appearance to the stem. Inner cortex is 5-6 layered. The stelar region is composed of collateral vascular bundles with phloem towards outer region and endarc xylem towards inner region. Major portion of the stem was occupied parenchymatous pith consisting of druses of calcium oxalate crystals (fig. 3).
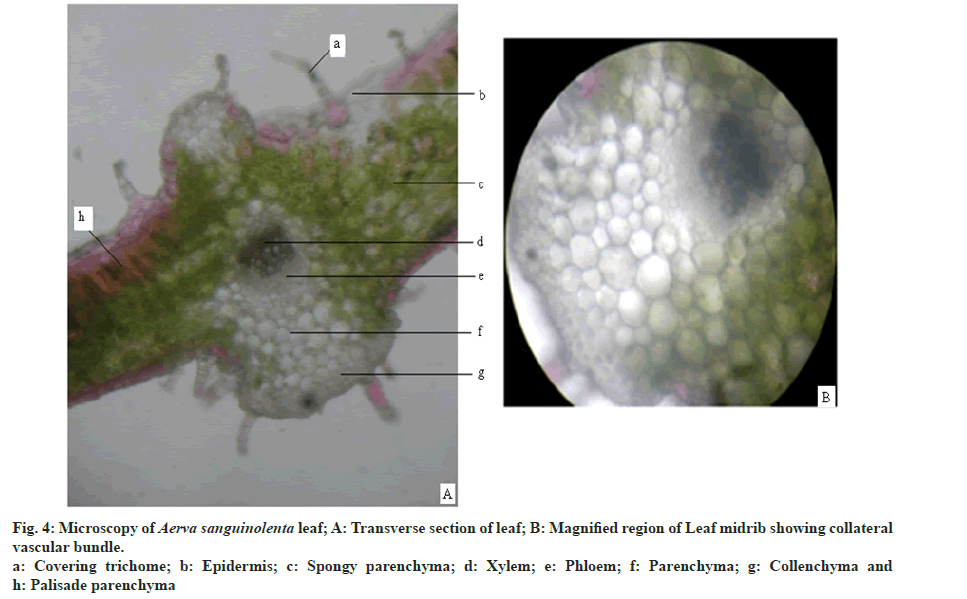
Leaf is dorsiventral and shows bifacial arrangement (fig. 4A). Lamina region is composed of epidermis, palisade and spongy parenchyma layers. Epidermis is composed of rectangular cells covered with thick cuticle. Epidermis is single layered and consists of multicellular covering trichomes and anisocytic stomata. Number of stomata is more on the abaxial surface. Number of trichomes is more on the abaxial surface and in the midrib region. Mesophyll is differentiated into uniseriate palisade parenchyma and multiseriate spongy parenchyma. Palisade tissue is single layered. Spongy parenchyma consists of druses of calcium oxalate crystals. Mid rib region is composed of single layered epidermis with covering trichomes followed by 3-5 layers of collenchyma on both the sides. At the center collateral vascular bundle is present with xylem towards adaxial surface and phloem towards abaxial surface (fig. 4B). Ground tissue is made up of thin walled parenchyma with abundant druses of calcium oxalate crystals.
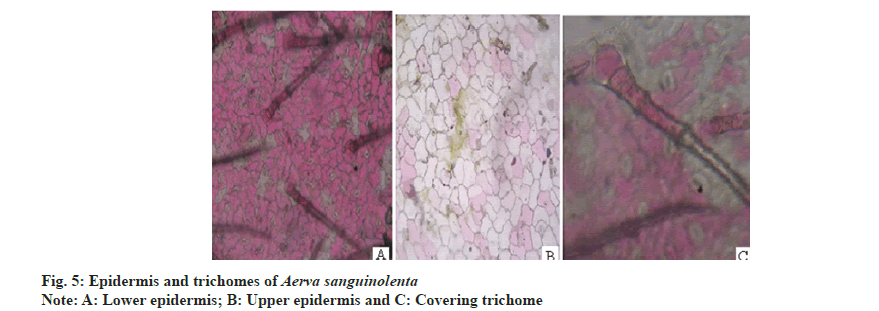
Anisocytic stomata were present on both the surfaces of leaves. Number of stomata and epidermal cells were found to be more on the abaxial surface (fig. 5A and fig. 5B). The stomatal number of upper surface and lower surface was found as 370.37 and 370.37, respectively. Stomatal index of upper surface and lower surface were 31.825 and 20.15, respectively. The vein islet number and vein termination number were 103.85 and 123.5 respectively (fig. 6).The palisade ratio was 5.02.
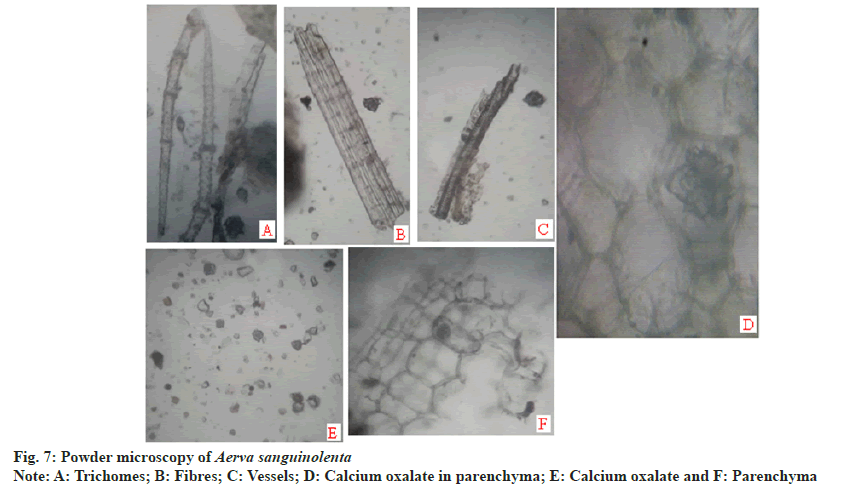
Powder microscopy revealed the presence of anisocytic stomata (fig. 5A and fig. 5B), multicellular covering trichomes (fig. 5C), calcium oxalate crystals, xylem vessels and parenchyma (fig. 7).
Ash values of the drug gives an idea of earthy matter or the inorganic composition and other impurities present along with drug. The ash values are important indices to illustrate the quality as well as purity of herbal medicine. The results of physiochemical parameters were summarized in Table 1 and Table 2. The evaluated physicochemical parameters will be helpful in assessing the quality of the raw material.
| S no | Physicochemical constants | Leaves (% w/w) | Stems (% w/w) | Roots (% w/w) |
|---|---|---|---|---|
| 1 | Total ash | 17 | 10.5 | 12.16 |
| 2 | Acid insoluble ash | 7 | 6.5 | 3.5 |
| 3 | Acid soluble ash | 9 | 4.5 | 8.5 |
| 4 | Water insoluble ash | 6.6 | 3.83 | 3.5 |
| 5 | Water soluble ash | 8 | 5.5 | 8.83 |
Table 1: Summary of Physicochemical Analysis
| S no | Physicochemical constants | (% w/w) |
|---|---|---|
| 1 | Foreign organic matter | 1.23 |
| 2 | Moisture content (not more than) | 8.67 |
| 3 | Extractive Value (Methanol) | 4.12 |
Table 2: Summary of Physicochemical Analysis
| S no | Phytoconstituent | Leaves | Stems | Roots |
|---|---|---|---|---|
| 1 | Carbohydrates | + | + | + |
| 2 | Proteins | - | - | - |
| 3 | Lipids | - | - | - |
| 4 | Alkaloids | + | + | + |
| 5 | Glycosides | - | - | - |
| 6 | Flavonoids | - | - | - |
| 7 | Tannins | - | - | - |
| 8 | Saponins | - | - | - |
| 9 | Steroids/triterpenoids | + | + | + |
| 10 | Coumarins | - | - | - |
| 11 | Phenolic compounds | - | - | - |
| 12 | Suberine | + | + | + |
| 13 | Acidic compounds | + | + | + |
Note: (+): Present and (-): Absent
Table 3: Summary of Phytochemical Analysis
The medicinal properties of A. sanguinolenta are due to the presence of numerous secondary metabolites. It was found to contain alkaloids, glycosides, saponins, terpenoids, tannins, flavonoids, carbohydrates, protein and minerals. The results are summarized in Table 3. Fluorescence analysis results are summarized in Table 4.
| Reagent | Normal light | Under UV Light | |
|---|---|---|---|
| 254 nm | 365 nm | ||
| Powder as such | Pale Green | Colourless | Colourless |
| Powder+Conc. HCl | Colourless | Colourless | Colourless |
| Powder+dil. HCl | Colourless | Colourless | Colourless |
| Powder+Conc. H2SO4 | Brown | Brown | Brown |
| Powder+dil. H2SO4 | Colourless | Colourless | Colourless |
| Powder+Conc. HNO3 | Reddish brown | Brown | Colourless |
| Powder+dil. HNO3 | Red | Colourless | Colourless |
| Powder+Iodine | Reddish brown | Yellow | Colourless |
| Powder+FeCl3 | Yellowish green | Pale green | Colourless |
| Powder+dil. NH3 | Pale green | Colourless | Colourless |
| Powder+Bromine water | Orange | Yellow | Colourless |
| Powder+NaOH | Pale green | Colourless | Colourless |
Note: Conc: Concentrated, dil: dilute, HCl: Hydrochloric acid, H2SO4: Sulphuric acid, HNO3: Nitric acid, FeCl3: Ferric chloride, NaOH: Sodium hydroxide and NH3: Ammonia
Table 4: Summary of Fluorescence Studies
To establish identity, purity, safety and quality for herbal drugs standardization is necessary. In order to standardize a drug macroscopic, microscopic, fluorescence analysis are done. One of the cheapest and simplest methods used for establishing the correct identification of the source material is microscopic method[31]. Morphological and microscopical studies are useful to identify the crude drug and for detecting adulteration. The quantitative determinations like stomatal number, stomatal index, vein islet and vein termination values are useful for setting standards for crude drugs. These values help in the evaluation of purity of drugs[33,34]. The information obtained from preliminary phytochemical screening will be useful in finding out the quality of the drug. These studies can also help the herbal manufacturers for selection and identification of the raw material.
Conclusion
In conclusion, the parameters which are reported here can be considered as distinctive enough to identify and decide the authenticity of this drug in herbal industry/trade and this can be included as microscopic standards in Indian herbal pharmacopeia. As far as the available literature is concerned, this plant has not yet been scientifically validated. There is a lot of scope for the possible isolation of newer compounds which may act as lead compounds for various pharmacological activities as it has many folklore uses. These findings might be useful to supplement information with regard to its identification parameters, which are assumed significant in the way of acceptability of herbal drugs.
Now a days the there is an increasing demand for herbal medicinal products. Folklore medicinal plants have to be supported by scientific data as they were used as an alternative and/or a complementary medicine worldwide. There is lot of scope for research on this plant as it was investigated very little for its medicinal value. The safety and efficacy of the herbal formulation depends on the raw material. Correct identification of the plant species is the first step in the authentication of the plant material. The present study provides few parameters as supportive scientific data for the evaluation of herbal materials in modern lines. As there were no available reports on the morphoanatomical features, the findings of the current study enable to authenticate the folklore medicinal plant, A. sanguinolenta (L) Blume. Morphological and microscopical characteristics, essential in confirming the identity, are useful in constructing the monograph. Phytochemical and physicochemical findings help in the isolation and purification of therapeutically active constituents. Future Phytopharmacological investigations on this plant may result in discovery of new bioactive components for the treatment of various diseases.
Conflict of interest:
The authors declare that there are no conflicts of interest.
References
- Sridharan S, Gounder SC. Pharmacognostic standardization and physicochemical analysis of the leaves of Barleria montana Wight and Nees. Asian Pacif J Trop Dis 2016;6(3):232-4.
- Li S, Long C, Liu F, Lee S, Guo Q, Li R, et al. Herbs for medicinal baths among the traditional Yao communities of China. J Ethnopharmacol 2006;108(1):59-67.
[Crossref] [Google Scholar] [PubMed]
- Lalee A, Bhattacharaya B, Das M, Mitra D, Kaity S, Bera S, et al. Hepatoprotective activity of ethanolic extract of Aerva sanguinolenta (Amaranthaceae) against paracetamol induced liver toxicity on Wistar Rats. NSHM J Pharm Healthcare Manag 2012;3:57-65.
- Srinivas RK, Rajeev RE, Ganapaty S. Diuretic and anti-inflammatory activity of aqueous extract of Aerva sanguinolenta (L.) Blume. Int Res J Pharm 2011;2(7):65-7.
- Khandelwal KR. Practical Pharmacognosy Techniques and Experiments. 19th ed. New Delhi: Nirali Prakashan; 2002.
- Anonymous. Quality control methods for medicinal plant materials. Geneva: Office of the Publications, World Health Organization; 1998.
- Kokate CK. Practical Pharmacognosy. 1st ed. New Delhi: Vallabh Prakashan; 2005.
- Sass JE. Elements of botanical micro technique. New York: Mc Graw Hill Book; 1940.
- Johanson DA. Plant Micro Technique. New York: Mc Graw Hill Book; 1940.
- Brain KR, Turner TD. The practical evaluation of phytopharmaceuticals; 1975.p. 190-91.
- Wallis TE. Text book of pharmacognosy. 15th ed. London: TA. Churchill; 1985.
- Pandya DJ, Desai TR, Nadpara NP, Mehta HA, Modi AM. Pharmacognostic study and establishment of quality parameters of leaves of Bombax insigne Linn. Int J Pharmacogn Phytochem Res 2010;2(3):1-5.
- Kokate CK. Practical pharmacognosy. New Delhi: Vallabh Prakashan; 2005.
- Ali M. Text book of pharmacognosy. New Delhi: CBS Publishers and Distributers; 2008.
- Kumar BS, Prabhakarn V, Lakshman K, Nandeesh R, Subramanyam P, Khan S, et al. Pharmacognostical studies of Portulaca oleracea Linn. Rev Brasileira Farmacogn 2008;18(4):527-31.
- Kokate CK, Gokhale SB. Practical Pharmacognosy. 12th ed. Pune: Nirali Prakashan; 2008.
- William Charles Evans. Trease and Evan’s Pharmacognosy. 16th ed. USA: Elsevier Health Sciences; 2009.
- Khandelwal KR. Practical Pharmacognosy. 19th ed. Pune: Nirali Publication; 2008.
- Edwin S, Joshi SB, Jain DC. Comparative pharmacognostic studies on root powder of Plumbago zeylanica and Plumbago rosea. Indian J Nat Prod 2008;2:27-9.
- Aerva sanguinolenta (L.) Blume. India Biodiversity Portal 1826;547.
- Stewart RR. Stewart RR. An annotated catalogue of the vascular plants of West Pakistan and Kashmir. Flora of West Pakistan (eds. Nasir E & Ali SI). Karachi: Fakhri Press; 1972.
- Fischer CE. A survey of the flora of the anamalai hills in the Coimbatore district, madras presidency Rec. Bot Surv India 1921;9(1):128.
- Pardeshi S, Dungriyal NS. Aerva sanguinolenta. Flowers of India; 2019.
- Adhikari BS, Babu MM, Saklani PL, Rawat GS. Medicinal plants diversity and their conservation status in Wildlife Institute of India (WII) campus, Dehradun. Ethnobotanical Leaflets 2010;2010(1):6.
- Buragohain J. Folk medicinal plants used in gynecological disorders in Tinsukia district, Assam, India. Fitoterapia 2008;79(5):388-92.
[Crossref] [Google Scholar] [PubMed]
- Kosalge SB, Fursule RA. Investigation of ethnomedicinal claims of some plants used by tribals of Satpuda Hills in India. J Ethnopharmacol 2009;121(3):456-61.
[Crossref] [Google Scholar] [PubMed]
- Rahmatullah M, Mollik AH, Ali M, Abbas FB, Khatun RJA, Seraj S et al. An ethnomedicinal survey of vitbilia village in sujanagar sub-district of Pabna District, Bangladesh. Am Euras J Agric Environ Sci 2011;10:106-11.
- Guha Baksi DN. Flora of Murshidabad District, West Bengal, India. Jodhpur: Scientific publishers; 1984.
- Antony. Aerva sanguinolenta (L.) Blume. Syst Stud Fl Kottayam Dist 1989.
- Sasidh. Aerva sanguinolenta (L.) Blume. Bot Stud Med Pl Kerala1996.
- Manilal S. Aerva sanguinolenta (L.) Blume Fl Calicut 1982;244.
- Bojian Bao, Thomas Borsch and Steven E. Clemants “Amaranthaceae" in Flora of China. 5th ed. Beijing: Science press and Missouri botanical garden press; 2013.
- Singh S, Machawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacogn Phytother 2010;2(5):71-5.
- Kumar S, Kumar V, Prakash OM. Microscopic evaluation and physiochemical analysis of Dillenia indica leaf. Asian Pac J Trop Biomed 2011;1(5):337-40.
[Crossref] [Google Scholar] [PubMed]