- *Corresponding Author:
- Y. D. Sun
Department of Otolaryngology, Hospital of Traditional Chinese Medicine Affiliated to Southwest Medical University, Luzhou, Sichuan 646000, China
E-mail: sunyongd@126.com
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “41-46” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Laryngeal cancer is a malignant tumor of the head and neck with clinical features of high recurrence and metastasis and a poor prognosis. The pathogenesis of laryngeal cancer is not clearly understood, but it is undoubtedly a complex developmental process involving multiple pathways and genes. Finding new ways to treat laryngeal cancer is particularly important as it is the second most common cancer of the head and neck, with many post-operative complications, poor quality of life and poor outcomes for patients with mid to latestage laryngeal cancer. The pathogenesis of laryngeal cancer is currently a hot topic of research. p38 mitogenactivated protein kinase/prostaglandin-endoperoxide synthase 2-related targets and signalling pathways have repeatedly been shown to play an important role in the development of tumours, including laryngeal cancer. With the continuous development of immunology, biochemistry and experimental techniques, it has become a current research trend to investigate the pathogenesis of laryngeal cancer at the molecular level. p38 mitogenactivated protein kinase and prostaglandin-endoperoxide synthase 2 have repeatedly been shown to play an important role in tumorigenesis and are also closely associated with the development of laryngeal cancer. This paper explores the potential association of p38 mitogen-activated protein kinase/prostaglandin-endoperoxide synthase 2 related targets and pathways with the development of laryngeal cancer in the context of the mechanisms of action of these pathways in a variety of tumors, with the aim of predicting possible targets for drug treatment of laryngeal cancer.
Keywords
Laryngeal cancer, p38 mitogen-activated protein kinase, prostaglandin-endoperoxide synthase 2, matrix metalloproteinase
Head and neck tumors are the sixth most common malignant tumors in the world, in which Squamous Cell Carcinoma (SCC)[1] predominantly, accounting for approximately 90 % of cases. Laryngeal cancer ranks second among head and neck malignancies[2] and is also predominantly SCC, with an age of onset among the middle-aged and older men, due to many causative factors, including smoking, alcohol consumption, laryngopharyngeal reflux, radiation, HPV infection in the oropharynx and hormonal disorders. The treatment of laryngeal cancer focuses on surgery, including radiotherapy and chemotherapy, etc. Although the 5 y survival rate for patients with early-stage laryngeal cancer is high, reaching about 80 % to 90 %, patients with mid to late-stage laryngeal cancer have a poor survival prognosis and a high recurrence rate[3-5]. Overall, patients with laryngeal cancer have a wide range of fluctuating 5 y survival rates[6] and common complications such as hoarseness and dysphagia[7], which seriously affect their quality of life. In recent years, the incidence of laryngeal cancer has been increasing year by year[8] and a more in depth exploration of the pathogenesis of laryngeal cancer is to find more optimal treatment options to improve the prognosis of laryngeal cancer patients has become a key issue in the current treatment. The study of p38 Mitogen-Activated Protein Kinase (p38 MAPK)/Prostaglandin- Endoperoxide Synthase 2 (PTGS2) related targets and signaling pathways can help to elucidate and refine the biological characteristics of laryngeal cancer, and provide guidance for the diagnosis and treatment of laryngeal cancer and the development of laryngeal cancer drugs.
p38 MAPK/PTGS2 Signaling Pathway:
p38 MAPK:
MAPK is an intracellular serine-threonine protein kinase that is activated by a variety of factors, including cytokines, neurotransmitters, cellular stress and cell adhesion. The MAPK signaling pathway is a three-step enzymatic cascade signaling pathway, in the order MAP Kinase Kinase Kinase (MEKK or MKKKK), MAP Kinase Kinase (MEK or MKK) and MAP Kinases (MAPKs). The MAPK signaling pathway is one of the major pathways in the integrated cell signaling network and is widely involved in the proliferation, differentiation and apoptosis of tumor cells. p38 kinase is one of the four classical signaling pathways of the MAPK pathway. The other three signaling pathways, c-Jun N-terminal Kinase (JNK), Extracellular Signal Regulated Protein Kinase (ERK) and large MAPK like Big MAP Kinase 1 (BMK1)/ERK5), can also be involved in the activation of p38 kinase.
The p38 MAPK was first discovered in 1993[9] and is a 38 kDa protein consisting of 360 amino acids in a specific order. p38 MAPK can be activated by hormones, cytokines and cellular stressors and plays a major role in inflammation, immune response and tumorigenesis[10]. There are four isoforms of p38 MAPK namely, p38 alpha (α) (MAPK14), p38 beta (β) (MAPK11), p38 gamma (γ) (MAPK12) and p38 delta (δ) (MAPK13). Among the four isoforms, p38α and p38β are widely distributed and significantly expressed in vivo, while p38δ is mainly located in tissues such as testis, pancreas and kidney, and p38γ is mainly expressed in muscle[11,12]. Of these, p38α is the best characterized and most studied. It is considered as a potential therapeutic target for the treatment of inflammation and cancer[13]. When cells are stimulated by external factors, Intracellular Apoptosis Signal- Regulating Kinase 1 (ASK1) is activated, which in turn activates MKK3 and MKK6. p38 MAPK can be further phosphorylated by upstream activated MKK3 and MKK6 kinases, thereby activating the p38 MAPK pathway[14,15]. Sometimes p38 can also be activated by MKK4, the activator of JNK. Activated p38 MAPK is translocated from the cytoplasm to the nucleus to regulate transcription and messenger Ribonucleic Acid (mRNA) expression of genes such as ETS like-1 protein (Elk-1), p53, Activating Transcription Factor-2 (ATF2) and other factors in the nucleus[16].
The p38 MAPK pathway can receive extracellular stimuli and translate them into cellular responses such as apoptosis, proliferation and differentiation. The effects of p38 MAPK phosphorylation status on some tumor cells are shown in Table 1 [17-21]. For example, rhodopsin and huperzine can induce apoptosis in hepatoma cells by activating the p38 MAPK pathway[17]; total alkaloids from Phyllostachys spp. can activate the JNK/p38 MAPK pathway leading to apoptosis in Adenocarcinomic Human Alveolar Basal Epithelial (A549) cells[18]; aloe rhodopsin can inhibit tumor angiogenesis, cell migration and invasion by activating the p38 MAPK pathway[19]. Meanwhile, it has also been shown that the p38 MAPK pathway can also be inhibited to play a therapeutic role in the disease, as per the study explained by Yan et al.[20], which concluded that the level of p38 MAPK phosphorylation in gastric cancer cells was significantly reduced and the motor growth of gastric cancer cells was inhibited after treatment with citicoline; Zhang[16], who concluded that the activation of the p38 MAPK/ Nuclear Factor kappa B (NF-κB) pathway could be inhibited and the levels of inflammatory factors could be downregulated to ameliorate radiation lung injury; and researcher Liao, who found that Heme Oxygenase-1 (HMOX1) knockdown decreased phosphorylated p38 (p-p38) MAPK expression and caused increased apoptosis in glioma neutrophils[21]. In conclusion, p38 MAPK has completely opposite effects on tumors in terms of promotion or inhibition, the exertion of which depends on the type and stage of cancer development[22], and plays an important role in the regulation of apoptosis, growth inhibition and differentiation, and cell cycle arrest.
| p38 MAPK phosphorylation status | Effects on tumor cells | Reference |
|---|---|---|
| Activation | Promoting apoptosis of liver cancer cells | [17] |
| Promoting apoptosis of lung cancer cell A549 | [18] | |
| Inhibiting of angiogenesis, migration and invasion of colorectal cancer cells | [19] | |
| Inhibition | Inhibiting the growth and movement of gastric cancer cells | [20] |
| Promoting apoptosis of neutrophils in glioma | [21] |
Table 1: Effect of p38 MAPK Phosphoryylation Status on Tumor Cells
PTGS2:
PTGS, also known as Cyclooxygenase (COX), is divided into two isozymes, PTGS1 and PTGS2, the former structurally expressed and the latter inducibly expressed, which are widely distributed in the body and play a key role in prostaglandin biosynthesis. Inducible expression of PTGS2 (also known as COX2), a membrane-bound, rate-limiting, short-lived enzyme whose expression is mainly induced by inflammation[23], is often considered as a target for the treatment of inflammation and pain[24]. PTGS2 is expressed at extremely low and almost negligible levels in normal tissues, but is stimulated by cytokines, inflammatory mediators, hormones and growth factors, and is overexpressed in a variety of tumors and cancers including adenocarcinoma, SCC, metastatic cell carcinoma, Hepatocellular Carcinoma (HCC), Cholangiocarcinoma (CCA) and Endometrial Cancer (EC)[25-29]. Studies have shown that prostaglandin, the major product of PTGS2, has proproliferative, angiogenic, anti-apoptotic and inhibitory effects on immune surveillance[30].
One study found high expression of PTGS2 in oesophageal adenocarcinoma and it was shown to be an independent predictor of poor prognosis[31]. In endometrial cancer cells, PTGS2 is overexpressed and confers cellular resistance to apoptosis[32]; targeted knockdown of PTGS2 promotes apoptosis against hepatic astrocytes[33], among others. Currently, several studies[34-37] have demonstrated that COX2 is highly expressed in Laryngeal Squamous Cell Carcinoma (LSCC) tissues and its expression is closely related to and inversely correlated with the prognosis, pathological stage, and lymph node metastasis of LSCC.
p38 MAPK/PTGS2 Signaling Pathway and Laryngeal Cancer:
The p38 MAPK signaling pathway plays an important role in promoting proliferation, invasion and metastasis of head and neck tumors and is highly expressed[38,39]. Members of the MAPK family induce PTGS2 expression and p38 MAPK is an upstream signaling molecule of COX2[40,41]. Our group also found that the MAPK signaling pathway in the preliminary network pharmacological analysis is one of the main pathways in the regulation of Head and Neck Squamous Cell Carcinoma (HNSCC) by the tonic formula, and PTGS2 is one of the top five key target proteins in the pharmacological mechanism of the network regulation of HNSCC by the tonic formula[42]. Matrix Metalloproteinase 9 (MMP-9) is a member of the MMP family involved in angiogenesis.
Kang reported that Hepatocyte Growth Factor (HGF) can upregulate COX-2 expression through the p38 MAPK pathway, which leads to increased MMP-9 expression and induces breast cancer cell invasion[43]. Yang et al. found that microRNA (miR) may be an upstream regulatory molecule of the p38 MAPK/COX2 pathway in cardiovascular diseases[44]. COX2 overexpression in tumor cells increases prostaglandin production, induces high downstream B-cell lymphoma 2 (Bcl-2) expression and low Transforming Growth Factor (TGF)-beta (β) type II Receptor (TβRII) expression, and ultimately leads to enhanced tumor aggressiveness. COX2 promotes tumor cell proliferation, metastasis, increases cancer recurrence rate and even correlates with cancer cell resistance to radiotherapy drugs[45], which is highly significant in the development of tumor cancer. In recent years, clinical Immune Checkpoint Inhibitors (ICI) using Programmed Death-1 (PD-1) antibodies has shown breakthrough improvements in the treatment of a variety of cancers, but most patients with HNSCC have had little success. Research into the pathogenesis of LSCC, the discovery of new target genes and the development of targeted drugs for HNSCC has become urgent issues.
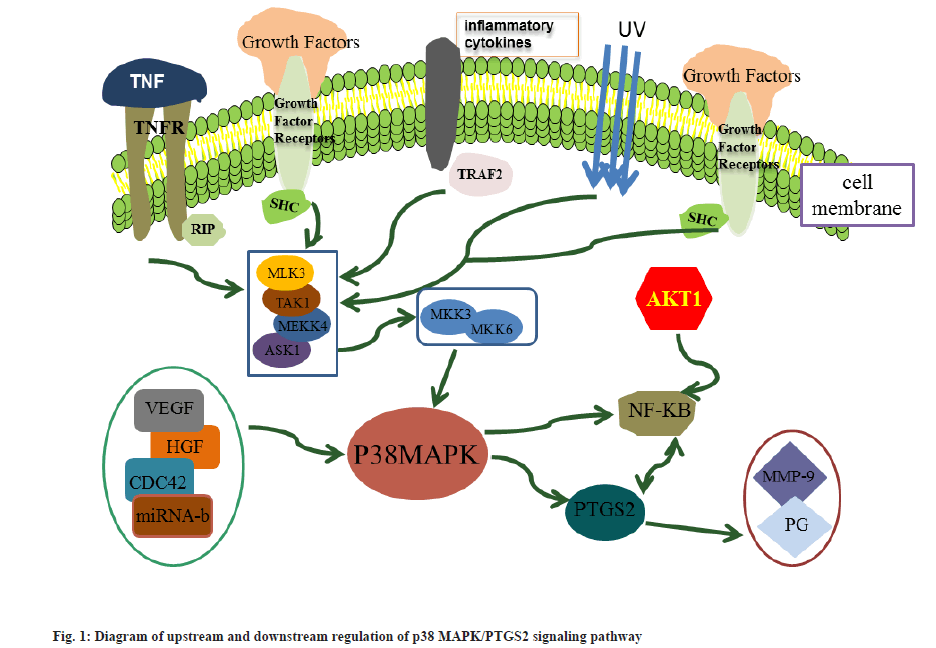
Intercellular signaling is cross-coupled and complex, and AKT Serine/Threonine Kinase 1 (AKT1) can phosphorylate Inhibitor of NF-κB kinase subunit beta (IκB) and release the pro-inflammatory transcription factor NF-κB, leading to NF-κB activation. The p38 MAPK can act both directly on PTGS2 and indirectly by activating downstream NF-κB, thereby activating PTGS2[46,47]. At the same time, NF-κB can bind to the NF-κB consensus element in the upstream promoter region of PTGS2 to counteract PTGS2 and promote its transcription[48]. The upstream and downstream regulatory relationships of the p38 MAPK/PTGS2 signaling pathway is shown in fig. 1.
The interaction between p38 MAPK and PTGS2 not only facilitates angiogenesis and promotes invasiveness of cancer cells[49], but is also associated with the resistance of cancer cells to apoptosis[50]. According to this study, the p38 MAPK/PTGS2 signaling pathway plays a direct role in the proliferation, invasion and apoptosis of laryngeal cancer cells. Although its mechanism of action in the development of laryngeal cancer has not been fully elucidated, studies such as its upstream and downstream regulatory relationships in other cancers can still provide ideas and directions for the study of laryngeal cancer in this pathway.
Conclusion
Modern medicine focuses not only on efficacy, but also on humanistic care for patients, putting people first and respecting life. Therefore, it has become a major challenge for otolaryngologists to improve the clinical outcome of patients with mid to latestage laryngeal cancer, to reduce the complications associated with surgery in laryngeal cancer patients and to improve their quality of life. p38 MAPK and PTGS2 are highly expressed in laryngeal cancer cells and are closely associated with proliferation, invasion and apoptosis of laryngeal cancer cells. This study has initially described the potential associations between the p38 MAPK/PTGS2 pathway and the development of laryngeal cancer, but experimental validation is still lacking. Further studies will be carried out in the future with the aim of understanding the pathogenesis of laryngeal cancer at the molecular level, which will provide a basis for overcoming the difficulties in developing targeted drugs for laryngeal cancer and finding targeted drugs for laryngeal cancer as soon as possible.
Conflict of interests:
The authors declared no conflict of interest.
References
- Badr H, Sobrero M, Chen J, Kotz T, Genden E, Sikora AG, et al. Associations between pre-, post and peri-operative variables and health resource use following surgery for head and neck cancer. Oral Oncol 2019;90:102-8.
[Crossref] [Google Scholar] [PubMed]
- Chu EA, Kim YJ. Laryngeal cancer: Diagnosis and preoperative work-up. Otolaryngol Clin North Am 2008;41(4):673-95.
[Crossref] [Google Scholar] [PubMed]
- Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2018;36(11):1143-69.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Zhu L, Luo W, Wen K, Wu DJ. Comparison of the clinical effect of surgery and surgery combined with radiotherapy and chemotherapy for 70 patients with laryngeal cancer. Pract J Cancer 2018;33(2):236-9.
- Peng YH, Yang WF, Lu SW. Mechanism research progress of miRNA in laryngeal carcinoma. J Clin Otolaryngol Head Neck Surg 2017;31(14):1134-9.
- Ko HC, Harari PM, Chen S, Wieland AM, Yu M, Baschnagel AM, et al. Survival outcomes for patients with T3N0M0 squamous cell carcinoma of the glottic larynx. JAMA Otolaryngol Head Neck Surg 2017;143(11):1126-33.
[Crossref] [Google Scholar] [PubMed]
- Okamoto I, Tsukahara K, Shimizu A, Sato H. Post-operative complications of salvage total laryngectomy forpost-radiotherapy recurrent laryngeal cancer using pectoralis major myocutaneous flaps. Acta Otolaryngol 2019;139(2):167-71.
[Crossref] [Google Scholar] [PubMed]
- Sher DJ, Pham NL, Shah JL, Sen N, Williams KA, Subramaniam RM, et al. Prospective phase 2 study of radiation therapy dose and volume de-escalation for elective neck treatment of oropharyngeal and laryngeal cancer. Int J Radiat Oncol Biol Phys 2021;109(4):932-40.
[Crossref] [Google Scholar] [PubMed]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science 1993;259(5102):1760-3.
[Crossref] [Google Scholar] [PubMed]
- Sanz-Ezquerro JJ, Cuenda A. p38 signalling pathway. Int J Mol Sci 2021;22(3):1003.
[Crossref] [Google Scholar] [PubMed]
- Lee SH, Park J, Che Y, Han PL, Lee JK. Constitutive activity and differential localization of p38α and p38β MAPKs in adult mouse brain. J Neurosci Res 2000;60(5):623-31.
[Crossref] [Google Scholar] [PubMed]
- Corre I, Paris F, Huot J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017;8(33):55684.
[Crossref] [Google Scholar] [PubMed]
- Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov 2009;8(6):480-99.
[Crossref] [Google Scholar] [PubMed]
- Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer letters. 2014;344(2):174-9.
[Crossref] [Google Scholar] [PubMed]
- Obsilova V, Honzejkova K, Obsil T. Structural insights support targeting ASK1 kinase for therapeutic interventions. Int J Mol Sci 2021;22(24):13395.
[Crossref] [Google Scholar] [PubMed]
- Zhang GH. Clinical and animal studies exploring the p38 MAPK/NF-κB-based pathway in the treatment of RILI with Salvia miltiorrhiza soup. Chin Acad Tradit Chin Med; 2022.
- Tan ZB, Xu YC, Liu Q. Activation of p38MAPK by rhubarb extract promotes apoptosis in HepG2 hepatocellular carcinoma cells. Mod Gastroenterol Interv Med 2022;27:970-3.
- Lin S, Qin HZ, Li ZY, Xu LB, Deng LY, Luo J, et al. Effects of total alkaloids of Phyllanthus pseudostellatus on apoptosis and PI3K/Akt and JNK/p38 MAPK signaling pathways in human lung cancer A549 cells. Chin J Exp Tradit Med Formulae 2023;4:69-76.
[Crossref]
- Chen Q, Li KT, Tian S, Yu TH, Yu LH, Lin HD, et al. Photodynamic therapy mediated by aloe-emodin inhibited angiogenesis and cell metastasis through activating MAPK signaling pathway on HUVECs. Technol Cancer Res Treat 2018;17.
[Crossref] [Google Scholar] [PubMed]
- Yan E, Fan Z, Shen B, Zhang Yi. Effects of taxifolin on growth, motility, oxidative stress and p38 MAPK signaling pathway in SGC-7901 gastric cancer cells. J Huaihai Med 2022;4:340-5.
[Crossref]
- Liao Q. The antioxidant gene HMOX1 regulates the role of neutrophils in the glioma microenvironment through the p38 MAPK signaling pathway. J Chongqing Med Univ 2022.
[Crossref]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 2009;9(8):537-49.
[Crossref] [Google Scholar] [PubMed]
- Kunzmann AT, Murray LJ, Cardwell CR, McShane CM, McMenamin ÚC, Cantwell MM. PTGS2 (Cyclooxygenase-2) expression and survival among colorectal cancer patients: A systematic review. Cancer Epidemiol Biomarkers Prev 2013;22(9):1490-7.
[Crossref] [Google Scholar] [PubMed]
- Qiu HY, Wang PF, Li Z, Ma JT, Wang XM, Yang YH, et al. Synthesis of dihydropyrazole sulphonamide derivatives that act as anti-cancer agents through COX-2 inhibition. Pharmacol Res 2016;104:86-96.
[Crossref] [Google Scholar] [PubMed]
- Chi NJ, Liu MY, Feng J, Shi XP, Wang JW. Effects of Ruyi Jinhuang Patch on TGFβ1, PTGS2 mRNA and related proteins in rats with benign prostatic hyperplasia. Chin J Gerontol 2022;42:4831-4.
- Gurram B, Zhang S, Li M, Li H, Xie Y, Cui H, et al. Celecoxib conjugated fluorescent probe for identification and discrimination of cyclooxygenase-2 enzyme in cancer cells. Anal Chem 2018;90(8):5187-93.
[Crossref] [Google Scholar] [PubMed]
- Raj V, Bhadauria AS, Singh AK, Kumar U, Rai A, Keshari AK, et al. Novel 1, 3, 4-thiadiazoles inhibit colorectal cancer via blockade of IL-6/COX-2 mediated JAK2/STAT3 signals as evidenced through data-based mathematical modeling. Cytokine 2019;118:144-59.
[Crossref] [Google Scholar] [PubMed]
- Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: Pharmacologic targets for chemoprevention. J Clin Oncol 2005;23(2):254-66.
[Crossref] [Google Scholar] [PubMed]
- Mortezaee K. Human hepatocellular carcinoma: Protection by melatonin. J Cell Physiol 2018;233(10):6486-508.
[Crossref] [Google Scholar] [PubMed]
- Popovics P, Awadallah WN, Kohrt SE, Case TC, Miller NL, Ricke EA, et al. Prostatic osteopontin expression is associated with symptomatic benign prostatic hyperplasia. Prostate 2020;80(10):731-41.
[Crossref] [Google Scholar] [PubMed]
- Spence AD, Trainor J, McMenamin Ú, Turkington RC, McQuaid S, Bingham V, et al. High PTGS2 expression in post?neoadjuvant chemotherapy?treated oesophageal adenocarcinoma is associated with improved survival: A population?based cohort study. Histopathology 2019;74(4):587-96.
[Crossref] [Google Scholar] [PubMed]
- Sexton É, van Themsche C, Leblanc K, Parent S, Lemoine P, Asselin E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol Cancer 2006;5:1-3.
[Crossref] [Google Scholar] [PubMed]
- Wang YZ. Effect of CRISPR/Cas9 knockdown of PTGS2 on apoptosis in hepatic stellate cells. Hunan Norm Univ; 2019.
- Li F, Yang GF. Correlation of cyclooxygenase family protein expression levels with clinical staging of primary squamous laryngeal carcinoma. J Clin Exp Med 2017;16(6):549-52.
- Sun X, Ge R, Dong Q, Zheng C. The expression of cyclooxygenase-2 in laryngeal squamous cell carcinoma and its relationship with MVD. J Clin Otorhinolaryngol 2005;19(21):967-70.
[Google Scholar] [PubMed]
- Lu H, Gong S. Expression of cyclooxygenase-2 and p53 and their correlation in carcinoma of larynx. J Clin Otorhinolaryngol 2004;18(7):421-3.
[Google Scholar] [PubMed]
- Zhuang LP. Expression of COX2, CDX2, NDRG1 and nm23 in laryngeal squamous carcinoma and their relationship with the prognosis of laryngeal squamous cell carcinoma. Acad J Kunming Med Coll; 2009.
- Zhong Y, Wang L, Chen XM. Urokinase-type fibrinogen activator and p38 mitogen-activated protein kinase signaling pathways and head and neck tumor invasion. Int J Stomatol 2011;38:367-9.
- Junttila MR, Ala-Aho R, Jokilehto T, Peltonen J, Kallajoki M, Grenman R, Jaakkola P, et al. p38α and p38δ mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 2007;26(36):5267-79.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Reinmuth N, Stoeltzing O, Parikh AA, Tellez C, Williams S, et al. Cyclooxygenase-2 is up-regulated by interleukin-1β in human colorectal cancer cells via multiple signaling pathways. Cancer Res 2003;63(13):3632-6.
[Google Scholar] [PubMed]
- Li YB, Li WM, Han JY. Role of the p38 signaling pathway in the expression of cyclooxygenase-2 by human umbilical vein endothelial cells. Grad Med J 2005;6(3):490-2.
[Crossref]
- Sun YD, Liu Q, Zhu WY, Zeng L, Li F, Wang HJ, et al. Network pharmacological analysis based on pharmacodynamics group multi-target multi-pathway of buzhong yiqi formula in head and neck squamous cell carcinoma. J Hubei Minzu Univ 2022;39(1):1-5.
[Crossref]
- Kang WB. HGF induces invasion of breast cancer cells by upregulating COX2 through PI3K/Akt and p38 MAPK signaling pathways. Chongqing Med Univ; 2012.
- Yang M, Lu L, Wu F. Effect of miR-146b on COX2 expression in human monocyte line THP-1 cells and possible mechanisms. Chin J Atheroscler 2017;25:890-4.
- Li J, Zhou Y, Wang H, Gao Y, Li L, Hwang SH, et al. COX-2/sEH dual inhibitor PTUPB suppresses glioblastoma growth by targeting epidermal growth factor receptor and hyaluronan mediated motility receptor. Oncotarget 2017;8(50):87353.
[Crossref] [Google Scholar] [PubMed]
- Wang LZ. Exploring the effects of discharged extracorporeal shock wave on chronic non-bacterial prostatitis in rats based on the p38MAPK/NF-ΚB signaling pathway. Shanghai Inst Phys Educ; 2021.
- Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-κB and pp38 mitogen-activated protein kinase. J Biol Chem 2004 ;279(45):46384-92.
[Crossref] [Google Scholar] [PubMed]
- Li B, Lu Y, Yu L, Han X, Wang H, Mao J, et al. miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem Biol Interact 2017;277:33-42.
[Crossref] [Google Scholar] [PubMed]
- Hu H, Han T, Zhuo M, Wu LL, Yuan C, Wu L, et al. Elevated COX-2 expression promotes angiogenesis through EGFR/p38-MAPK/Sp1-dependent signalling in pancreatic cancer. Sci Rep 2017;7(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Semaan J, Pinon A, Rioux B, Hassan L, Limami Y, Pouget C, et al. Resistance to 3?HTMC?induced apoptosis through activation of PI3K/Akt, MEK/ERK and p38/COX?2/PGE2 pathways in human HT?29 and HCT116 colorectal cancer cells. J Cell Biochem 2016;117(12):2875-85.
[Crossref] [Google Scholar] [PubMed]