- *Corresponding Author:
- H. S. Prakash
Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore-570 006, India
E-mail: hasriprakash@gmail.com
| Date of Submission | 15 August 2016 |
| Date of Revision | 29 February 2017 |
| Date of Acceptance | 21 August 2017 |
| Indian J Pharm Sci 2017;79(5):844-848 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The genus Memecylon L. (Melastomataceae), an important source of traditional medicine is mainly distributed in the Western Ghats region of Karnataka. In Indian ethnomedical practices, this plant is reported to be effective for skin and viral diseases including herpes and chickenpox. The objective of the present study was to screen Memecylon species namely M. umbellatum, M. edule, M. talbotianum, M. malabaricum and M. wightii for the bioactive compounds with special reference to skin diseases. Leaf samples were dried and extracted using methanol. TLC analysis of methanol extracts of five Memecylon species showed single band with RF value 0.61 detected at 260 nm. This band was eluted and subjected to high performance liquid chromatography. Profiles of Memecylon species confirmed the presence of α-tocopherol on comparison with the standard. Among five Memecylon species, M. malabaricum (0.724%) and M. talbotianum (0.362%) showed highest α-tocopherol concentration. For the first time α-tocopherol was reported from Memecylon species. Further, the structure of α-tocopherol was confirmed by liquid chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy analysis. Presence of α-tocopherol (vitamin E) in Memecylon species validates the traditional use of Memecylon species in the treatment of skin diseases.
Keywords
Memecylon species, HPLC, α-tocopherol
Medicinal plants play a major role in the discovery of new therapeutic agents for drug development. India is a rich source of medicinal plants and market value of all therapeutically important plant species is increasing day by day [1]. Traditionally, natural products have been a source of lead molecules in drug discovery. However, natural products have been de-emphasized as high throughput screening resources in the present, because of difficulty in obtaining high quality natural products. New technologies such as mass spectrometry, nuclear magnetic resonance spectroscopy (NMR) and other spectroscopic techniques can greatly facilitate structure elucidation process for creation of high quality natural product libraries [2].
The genus Memecylon (Melastomataceae), is used for medicinal purposes in Asia-Pacific regions. It consists of more than 300 species. In India, the genus is represented by about 40 species out of which 21 are endemics. The Western Ghats is the major centre of Memecylon diversity. In Ayurveda and Siddha, several Memecylon species are reported to be used by tribals in the treatment of skin disorders, herpes, chickenpox, stomach disorders, leucorrhoea, polyuria, menorrhagia, dysentery and also in the treatment of bacterial infections and inflammation [3]. Several pharmacological activities such as, antimicrobial, antiinflammatory, antidiabetic properties including some of the phytoconstituents such as, apigenin, isorhamnetin-3-O-glycoside-7-Oglycoside etc. are reported from Memecylon species [4-6].
In the current study, methanol extracts of Memecylon species were selected as bioactive fractions. Thin layer chromatography (TLC) screening and high performance liquid chromatography (HPLC) analysis showed the active compound is highly polar, based on mobile phase in both types of chromatography in five species of Memecylon. Alpha-tocopherol was detected in aerial parts of five Memecylon species from the TLC and HPLC analysis with reference standard and relative concentration of α-tocopherol was calculated in five Memecylon species. Further the compound identification was confirmed by liquid chromatographymass spectrometry (LC-MS) and NMR analysis.
Five Memecylon species namely, M. umbellatum Burm, M. edule Roxb, M. talbotianum Brandis, M. malabaricum Clarke and M. wightii Thwaites were collected from different parts of Karnataka. Shade dried leaves were powdered in an electric blender and extracted with methanol using Soxhlet apparatus. Extracts of the species were collected and tested for different bioactive potentials namely, antioxidant, antiinflammatory, and antimicrobial potentials. Only methanol extracts showed significant activity. Therefore, methanol extracts of Memecylon species were selected for further studies.
TLC of methanol extracts of Memecylon species was carried out on silica gel (TLC silica gel 60 mesh, 20×20, 0.5 mm, Merck and Co, Inc) with methanol:acetone (8.7:1.3) solvent system. The methanol extract was spotted and the solvent front was allowed to run for approximately 16 cm. The running lane was then dried thoroughly; elution of compound was detected at 260 nm. The bands corresponding to active compound by initial screening were scraped out and the compounds were washed out of silica gel with methanol. The obtained methanol solutions were filtered and subjected to HPLC analysis for further purification. A TLC bioactive fraction of Memecylon species extract was subjected to HPLC. Analytical HPLC (Waters, 10 ATVP) was performed with a reversed phase C18 column using LC-10-ATVP double unit pumps. The analytical chromatography was carried out under isocratic conditions with mobile phase of methanol and water (99:1) using a flow rate 1.0 ml/min and chromatogram recorded at wavelength of 292 nm using a SPD-10AVP multi-wavelength detector. Sample were dried and dissolved in methanol.
Concentration of α-tocopherol in different Memecylon species was calculated relative to the standard based on the comparison of peak area in HPLC profiles of samples and standard vitamin E. Bioactive HPLC fraction of M. talbotianum was injected to LC-MS (Waters Acquity, Milford, MA) Series UPLC/SYNAPT G2 HDMS with electrospray ionization) connected with a waters C18 column (particle diameter 5 μm, 150×4.6 mm i.d.). Mobile phase: acetonitrile:water (75:25) containing 0.1% formic acid with a flow rate of 1.1 ml/min.
The NMR spectrum of the purified compound of M. talbotianum was recorded on a Bruker Avance-III 400 Hz spectrometer equipped with BBFO probe and connected with VT Unit (temp. range from −80°+130°). 1H NMR was recorded in deuterated dimethyl sulfoxide (DMSO) and 2D data were acquired in CD3 OD at a temperature of 300 K.
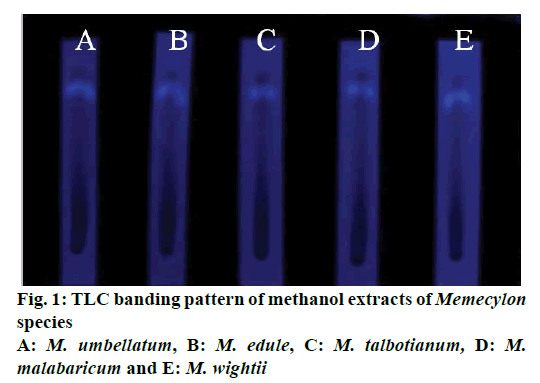
The TLC analysis of methanol extracts of five Memecylon species showed single band with RF value 0.61 detected at 260 nm (Figure 1). This band was filtered and subjected to HPLC analysis for further purification.
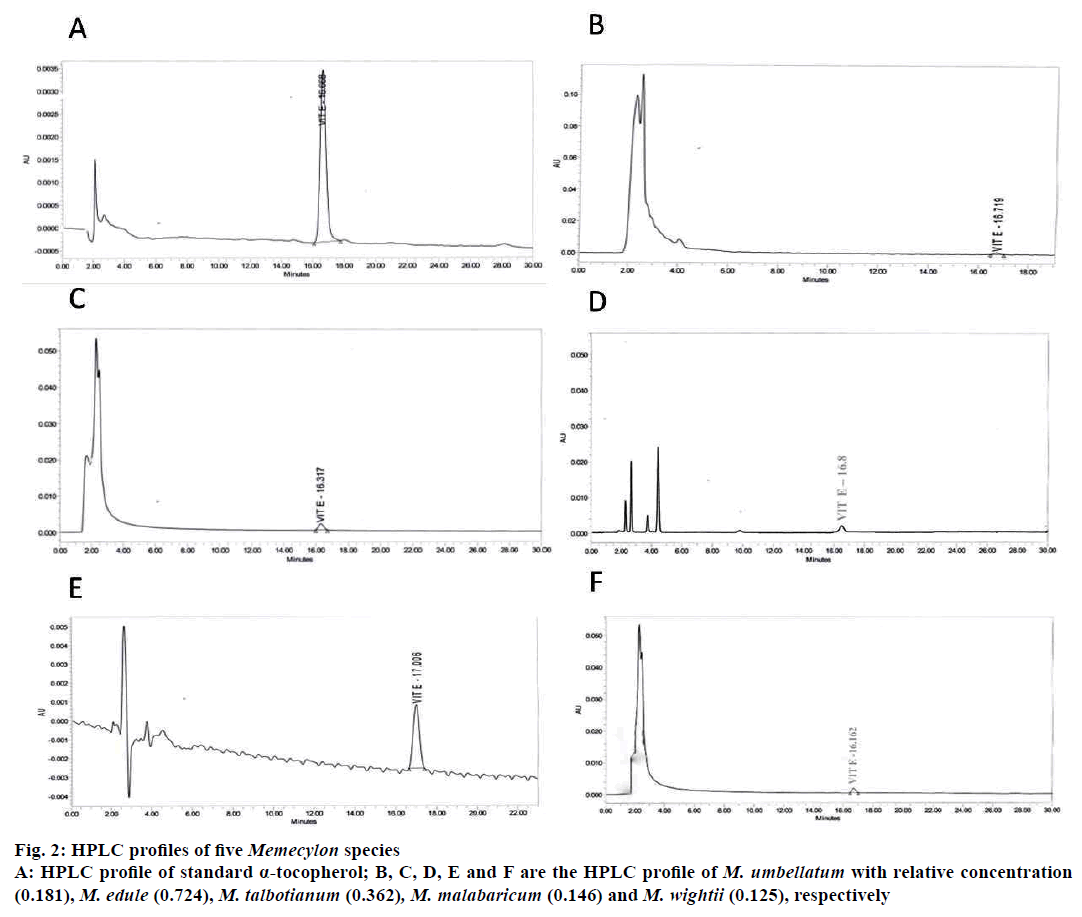
HPLC profiles of α-tocopherol (standard) and α-tocopherol of Memecylon species were given in Figure 2. Standard α-tocopherol showed the retention time (Rt) at 16.668 min. Rt of α-tocopherol in M. umbellatum, M. edule, M. talbotianum, M. malabaricum and M. wightii, were 16.719, 16.162, 16.317, 17.006, and 16.8 min, respectively. M. malabaricum and M. talbotianum showed highest α-tocopherol concentration (0.724 and 0.362%) compared to other Memecylon species. In M. umbellatum, M. edule and M. wightii α-tocopherol was present in minute concentrations of 0.181, 0.125 and 0.146% (Table 1).
| Memecylonspecies | Relative concentration of a-tocopherol (%) |
|---|---|
| M. umbellatum | 0.181 |
| M. malabaricum | 0.724 |
| M. talbotianum | 0.362 |
| M. edule | 0.146 |
| M. wightii | 0.125 |
Table 1: Relative concentration of α-tocopherol in memecylon species
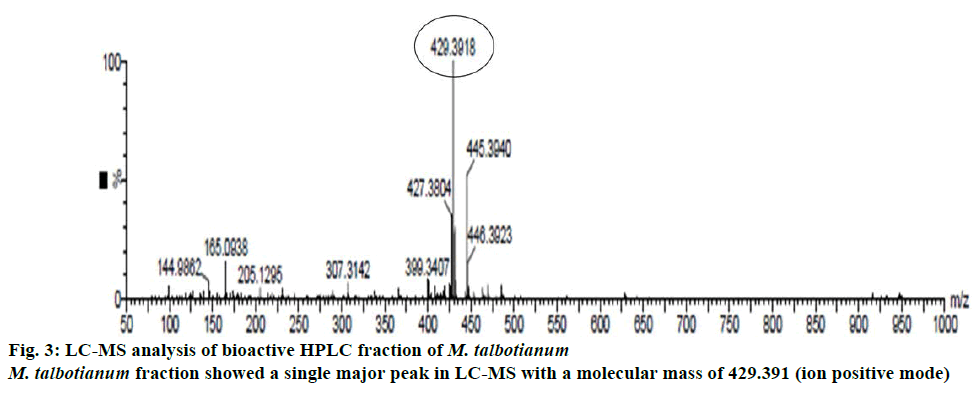
Among five Memecylon species, M. malabaricum and M. talbotianum showed highest concentration of α-tocopherol. Hence the bioactive fractions of these two plant species were purified using preparative HPLC and then subjected to LC-MS for the detection and confirmation of bioactive molecule. Among these two fractions, M. talbotianum fraction showed a single major peak in LC-MS with a molecular mass of 429.391 (ion positive mode, Figure 3). Hence this fraction was subjected to NMR spectra analysis for confirming the structure of the molecule.
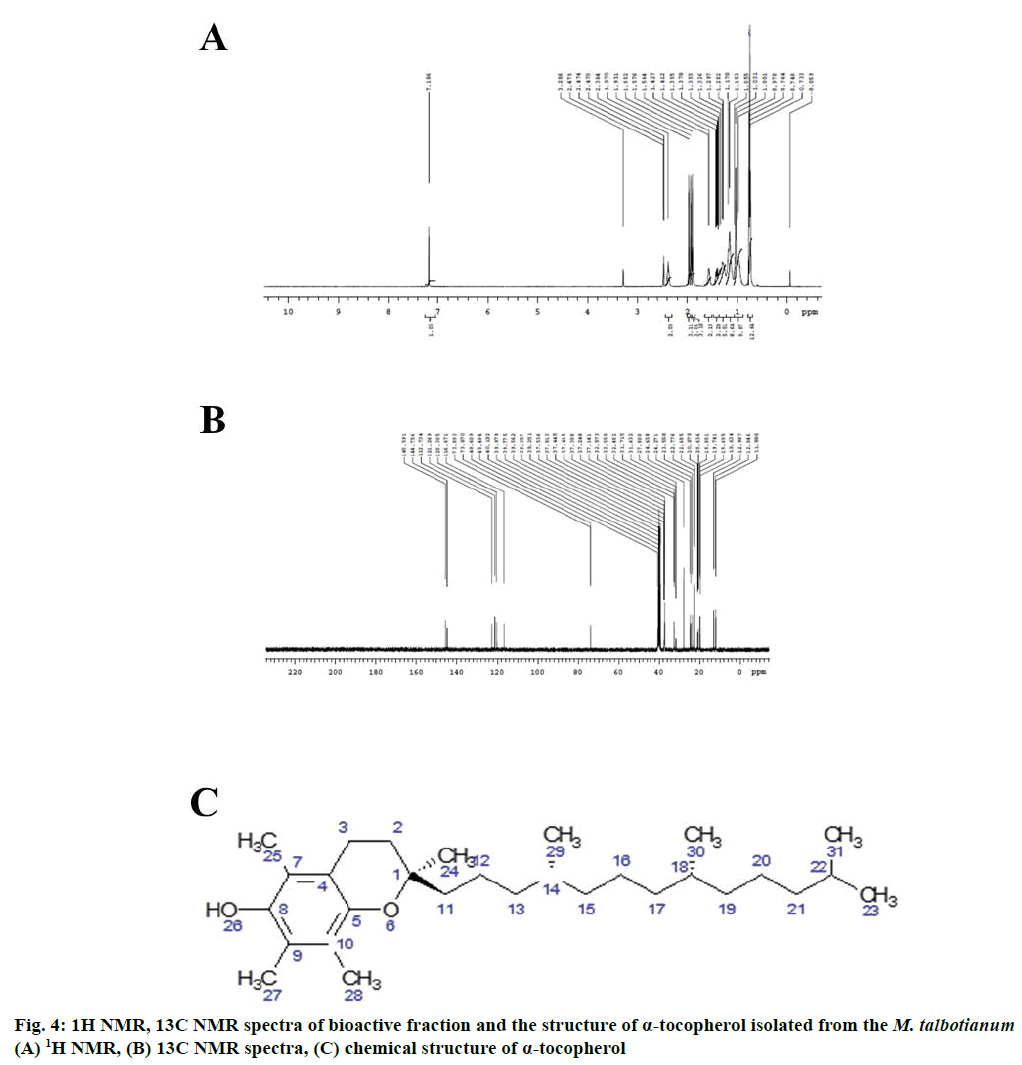
The NMR spectral analysis of LC-MS fraction of mass 429.391 from M. talbotianum confirms that the compound is α-tocopherol. This is the first report for α-tocopherol in M. talbotianum. The 1H-NMR chromatogram of M. talbotianum was reported in Figure 4. Further, to confirm the structural arrangements of the M. talbotianum, 1H-NMR and 13C-NMR data revealed 50 protons and 29 carbons as confirmed by deuterium exchange (Figure 4A and 4B). The 1H-NMR and 13C-NMR data are expressed as follows.
1H-NMR (400MHz, DMSO-d6): δ 7.18 (s, 1H, H-26), 2.38 (t, 2H, H-3), 1.97(s, 3H, H-28), 1.93 (s, 3H, H-25), 1.90 (s, 3H, H-27), 1.57 (q, 2H, H-2), 1.4 (m, 2H, H-11) 1.29-1.37 (m, 4H, H-12, 14, 18), 1.17 (m, 8H, H-13, 15, 16, 20), 1.103 (s, 3H, H-12), 0.97 (s, 7H, H-17, 19, 21, 22), 0.73-0.76 (t, 12H, H-29, 30, 31, 23) (Figure 4A).
13C-NMR (100MHz, DMSO-d6): δ 145.59 (C-8), 144.45 (C-5), 122.75 (C-4), 121.73 (C-10), 120.30 (C-9), 116.67 (C-7), 73.8 (C-1), 37.1-37.5 (C-11, 13, 15, 17, 19, 21), 32.5 (c-14), 31.7 (C-18), 27.8 (C-2), 24.6 (C-22), 24.2 (C-20), 22.7 (C-22,23), 22.6 (C-29, 30), 20.8 (C-3), 19.65 (C-25, 27, 28) (Figure 4B).
Traditional knowledge about the therapeutic potential of plants is necessary to isolate and identify biologically active products from plants [7]. Therefore, isolation and identification of bioactive compounds present in a crude extract serve as the building block for the development of new type of therapeutics with new mechanisms of action [8]. Traditionally, natural products have been a source of lead molecules in drug discovery. However, natural products have been de-emphasized as high throughput screening resources in the present, because of difficulty in obtaining high quality natural products. New technologies such as mass spectrometry, NMR and other spectroscopic technique can greatly facilitate structure elucidation process for creation of high quality natural product libraries. In the current study, methanol extracts of Memecylon species were selected as bioactive fractions. TLC and HPLC analysis showed the active compound is α-tocopherol. α-tocopherol is also identified in several other plants such as Cyamopsis tetragonoloba, Moringa oleifera, Stevia rebaudiana, Millingtonia hortensis and Jasminum sambac through GC-MS analysis [9-13].
For the first time α-tocopherol was reported in five Memecylon species in the present study. Higher concentration of α-tocopherol was detected in M. malabaricum, M. talbotianum. The higher α-tocopherol concentration might be one of the reasons for the superior bioactive property of M. malabaricum and M. talbotianum than other Memecylon species. HPLC method is easy, precise and showed better resolution of α-tocopherol in different Memecylon species. Further the compound identification was confirmed by LC-MS and NMR analysis. Since α-tocopherol is preferentially absorbed and accumulated in humans it is widely used as an inexpensive antioxidant in cosmetics and foods, vitamin E is good for the skin and is a common ingredient of skin creams and lotions that encourages skin healing and reduces scarring after injuries such as burns [14,15]. Hence presence of α-tocopherol (vitamin E) in Memecylon species validates the traditional use of Memecylon species in the treatment of skin diseases.
Acknowledgements
The authors acknowledge the recognition of University of Mysore as an Institution of Excellence and financial support from the Ministry of Human Resource Development, Govt. of India through UGC under UOM/IOE/RESEARCH/1/2010-11, dated: 22-04- 2010 project and UGC fellowship scheme (Or. No. DV9/192/NON-NETFS/2013-14 dated: 11-11-2013).
Financial assistance
Nill.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tshibangu JN, Chifundera K, Kaminsky R, Wright AD, König GM. Screening of African medicinal plants for antimicrobial and enzyme inhibitory activity. J Ethnopharmacol 2002;80(1):25-35.
- Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep 2009;10:194-200.
- Bharathi TR, Madhusudan MC, Pradeep Kumar PM, Chandranayaka S, Prakash HS. Antimicrobial Potential of Memecylon L. species from Western Ghats against clinical isolates of pathogenic bacteria. Res J Pharm Biol Chem Sci 2015;6:1280-7.
- Bharathi TR, Nadafi R, Prakash HS. In vitro antioxidant and anti-inflammatory properties of different Solvent Extracts of Memecylon talbotianum Brandis. Int J Phytopharm 2014;4:148-52.
- Bharathi TR, Sampathkumara KK, Prakash HS. Memecylonspecies: A review of Traditional information and taxonomic description. Int J Pharm Pharm Sci 2016a;8:1-9.
- Bharathi TR, Shailasree S, Sampath Kumara KK, Madhusudan MC, Prakash HS. Metabolite profiling by UPLC-PDA-ESI/HDMS and antibacterial activity of Memecylon talbotianum Brandis. Phcog Commn 2016b;6:225-31.
- Sampietro DA, Catalan CA, Vattuone MA. Isolation, identification and characterization of allelochemicals/natural products. Boca Raton, Florida: CRC Press; 2009.
- Lee MJ, Prabhu S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem 2000;279:164-9.
- Surendran S, Vijayalakshmi K. GC-MS analysis of phytochemicals in Cyamopsis tetragonoloba fruit and Cyperus rotundus rhizome. Int J Pharmacognosy Phytochem Res 2011;3:102-6.
- Inbathamizh L and Padmini E. Gas Chromatography-Mass Spectrometric analyses of methanol extract of Moringa oleifera flowers. Int J Chem Anal Sci2012;3:1394-7.
- Verma RN, Batra A. Isolation and analytic characterization of rebaudioside A and GC-MS analysis of methanolic leaves extract of Stevia rebaudiana Bert. Ann Phytomed 2013;2:108-14.
- Kumar A, Iyer K, Shanthi V, Ramanathan K. Extraction of bioactive compounds from Millingtonia hortensis for the treatment of dapsone resistance in leprosy. J Microb Biochem Technol 2014;R1:006.
- Tomar K, Rijhwani S. Evaluation of antibacterial activity of phytoconstituents isolated from Jasminum sambac L. and their identification through GC-MS. Int J Eng Technol Manag Appl Sci 2015;3:451-9.
- Palmieri B, Gozzi G, Palmieri G. Vitamin E added silicone gel sheets for treatment of hypertrophic scars and keloids. Int J Dermatol 1995; 34:506-9.
- Ramasetty BT, Bajpe Sk, Sampath Kumara KK, Saini RK, Shashibhushan NB, Kini KR, et al. Identification and Genetic Diversity analysis of Memecylon species using ISSR, RAPD and Gene-Based DNA barcoding tools. Elect J Biotech 2016;24:1-8.