- *Corresponding Author:
- Yu Han
Department of Radiology, The Third Affiliated Hospital, Sun Yat Sen University, Guangzhou, Guangdong Province 510080, China
E-mail: hany73@mail.sysu.edu.cn
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “84-89” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In addition to surgical resection, chemotherapy is needed for patients with liver metastasis of colorectal cancer. Our aim was to investigate the computed tomography manifestations of liver injury after different chemotherapy regimens. These studies retrospectively analyzed patients with liver metastasis from colorectal cancer diagnosed in our hospital between April 2015 and April 2020. The sex, age, chemotherapy regimen (FOLFOX, FOLFOXIRI, cetuximab+FOLFOXIRI, bevacizumab+FOLFOXIRI, bevacizumab+FOLFOX) and liver computerized tomography imaging findings were reviewed. The results were expressed as the frequency and percentage for classified variables or the average and standard deviation for continuous variables (age). The statistical significance was evaluated by crosstable chi-square analysis to determine the differences between groups of categorical variables. There were 170 patients with liver injury after chemotherapy, including 124 males and 46 females. The male-to-female ratio was 2.7 to 1. The imaging findings of the 170 patients were divided into diffuse, focal and small multifocal. The different chemotherapy regimens showed that 77 of the 87 cases of FOLFOX showed diffuse liver injury. Among the 45 cases of the FOLFOXIRI regimen, 43 cases showed focal liver injury. A total of 38 cases were treated with three regimens of cetuximab+FOLFOXIRI, bevacizumab+FOLFOXIRI, and bevacizumab+FOLFOX. Of these, 36 cases showed small multifocal liver injury. The chi-square test results showed that the chi-square value was 260.472 (p<0.000). The imaging changes of liver computed tomography have a certain sensitivity and specificity for liver injury after chemotherapy. Different types of liver injury after chemotherapy can be reflected by computed tomography imaging changes.
Keywords
Computerized tomography, chemotherapy, colorectal cancer, liver injury, oncology
In the past few decades, the number of chemotherapy options and combination regimens for many malignant tumors has greatly increased. However, an unfortunate consequence of this increasingly effective systemic cytotoxic therapy is their nontarget effects, which can also cause damage to normal tissues. Among these effects, hepatotoxicity is noteworthy because it affects the overall health and postoperative recovery of patients. Additionally, it also damages the regeneration ability, which is the basis of potential radical hepatectomy. The liver is a common sight of metastatic diseases. Surgeons, in particular, consider liver injuries associated with chemotherapy when planning safe operations[1].
Colorectal cancer is the 3rd most common type of cancer worldwide[2]. In addition to surgical resection, it also requires chemotherapy. The emergence of chemotherapy and the use of a variety of drug regimens increase the possibility of liver parenchyma damage, which is collectively referred to as chemotherapy-related liver injury[3-14]. Liver injury is the most serious complication after chemotherapy. It includes steatosis, dilatation of hepatic sinusoids corresponding to hepatic Sinusoid Obstruction Syndrome (SOS), Nodular Regenerative Hyperplasia (NRH) and steatohepatitis[4]. Therefore, when treating colorectal cancer, it is also necessary to consider the associated liver injury after chemotherapy.
Imaging plays an important role in the evaluation of liver injury after chemotherapy. Because the liver is the main organ responsible for drug clearance and the synthesis of many biochemical pathways, many chemotherapeutic drugs can be metabolized through the liver. These drugs may cause serious liver injury, and some changes in the liver can be observed by imaging. Additionally, these drugs have a certain sensitivity and specificity[3]. Previous studies have classified liver injury after chemotherapy according to pathophysiology, and there are also studies that classify imaging manifestations. We believe that different types of liver injury after chemotherapy can be reflected by imaging changes.
In this study, the clinical data of patients with liver injury caused by chemotherapy for colorectal cancer in our hospital were analyzed retrospectively, and the relationship between different chemotherapy regimens and liver imaging changes was observed. Combined with imaging findings, the imaging changes observed by liver Computerized Tomography (CT) were compared among different chemotherapy regimens to study the correlation between liver injury and CT findings for different chemotherapy regimens.
Materials and Methods
Patients:
These studies retrospectively analyzed patients with liver metastasis from colorectal cancer diagnosed in our hospital between April 2015 and April 2020. The sex, age, chemotherapy regimen (Folinic acid, Fluorouracil and Oxaliplatin (FOLFOX), Folinic acid, 5-Fluorouracil, Oxaliplatin and Irinotecan (FOLFOXIRI), cetuximab+FOLFOXIRI, b e v a c i z u m a b + F O L F O X I R I , bevacizumab+FOLFOX) and liver CT imaging findings were reviewed. All patients met the following inclusion and exclusion criteria
Inclusion criteria: Colorectal adenocarcinoma confirmed by pathology; patients who were first diagnosed and untreated; plain and enhanced CT scans; re-examination of CT plain scan and enhanced scan after chemotherapy and normal laboratory results at first diagnosis, these include Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST) and Alkaline Phosphatase (ALP).
Exclusion criteria: Patients who had received chemotherapy or radiotherapy before admission; patients diagnosed with hepatitis and cirrhosis, and abnormal laboratory results at first diagnosis. Finally, 170 patients with an average age of 56.4 y were included.
Treatment and follow-up:
All patients were treated with chemotherapy and followed up by CT. The timeline of our study included CT plain scan and enhancement at the first diagnosis, laboratory examination at the beginning of treatment; these include ALT, AST, and ALP. And regular follow-up at (2-4) mo after chemotherapy until liver CT imaging abnormalities appeared. All patients did not undergo surgical treatment at the time of reexamination.
CT image acquisition:
Use Toshiba 640-Slice CT for scanning, set the tube voltage to 120 kVP, and perform enhanced CT examination with automatic tube current. All patients underwent intravenous injection of contrast agent. Obtain spiral scans of arterial and venous phases.
Image analysis:
Two radiologists in the abdominal radiology group with 5 y of experience read the film together and analyzed the results of CT images. After discussion, we decided that the distribution of lesions in more than six liver segments was diffuse type, between three to six liver segments was local type, and the distribution of less than three liver segments was small focal type. If there was a disagreement, the two doctors discussed and analyzed it.
Statistical analysis:
Statistical analyses were calculated using Statistical Package for the Social Sciences (SPSS) V.19. A descriptive analysis of the sample was conducted. The results were expressed as frequencies and percentages for categorical variables (chemotherapy regimen, image presentation of liver injury) or as the mean and standard deviation for continuous variables (e.g., age). Statistical significance was assessed through crosstab Chi-square (χ2) analysis to determine group differences in categorical variables. A two-tailed p<0.05 was considered significant for all analyses.
Results and Discussion
Among the 170 patients with liver injury after chemotherapy, there were 124 males and 46 females. The male-to-female ratio was 2.7:1. The ages ranged from 25 y to 82 y old, but the age of most patients ranged from 51 y to 60 y old as shown in Table 1.
| Age (years) | Male | Female | Total |
|---|---|---|---|
| ≤30 | 2 (1.18) | 1 (0.59) | 3 (1.76) |
| 31-40 | 9 (5.29) | 4 (2.35) | 13 (7.65) |
| 41-50 | 21 (12.35) | 9 (5.29) | 30 (17.65) |
| 51-60 | 46 (27.06) | 14 (8.24) | 60 (35.29) |
| 61-70 | 37 (21.76) | 14 (8.24) | 51 (30) |
| >71 | 9 (5.29) | 4 (2.35) | 13 (7.65) |
| Total | 124 (72.94) | 46 (27.06) | 170 (100) |
Table 1: Sex and Age Distribution of The 170 Patients
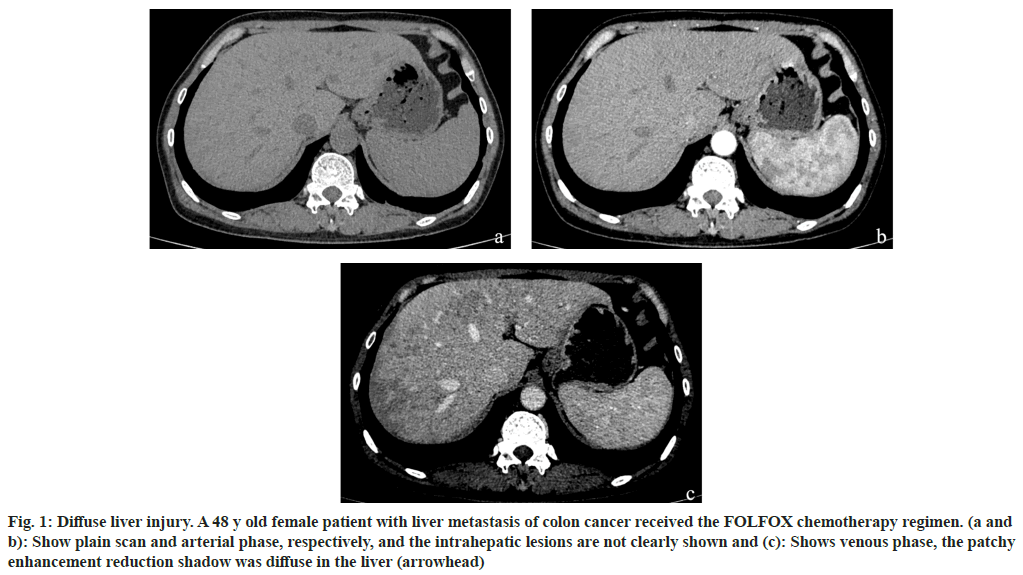
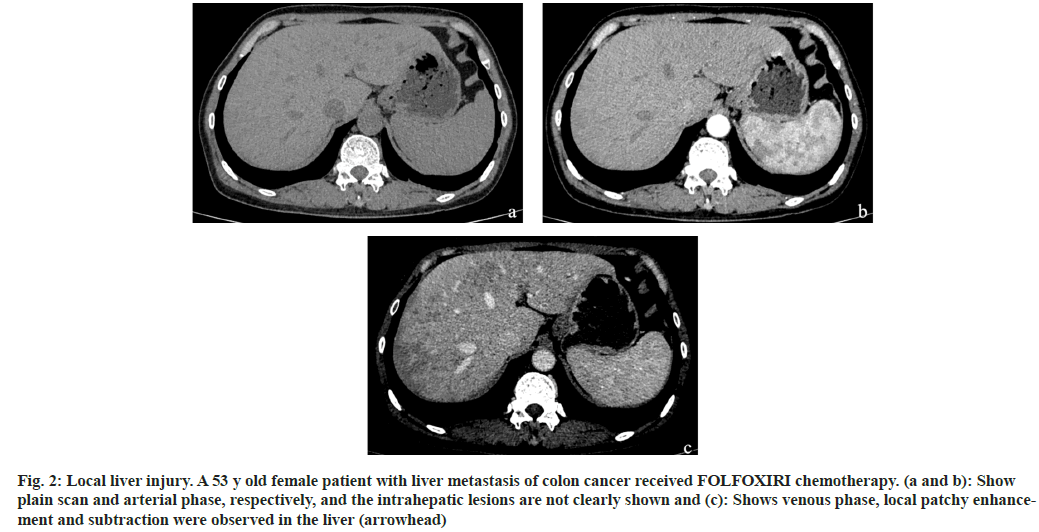
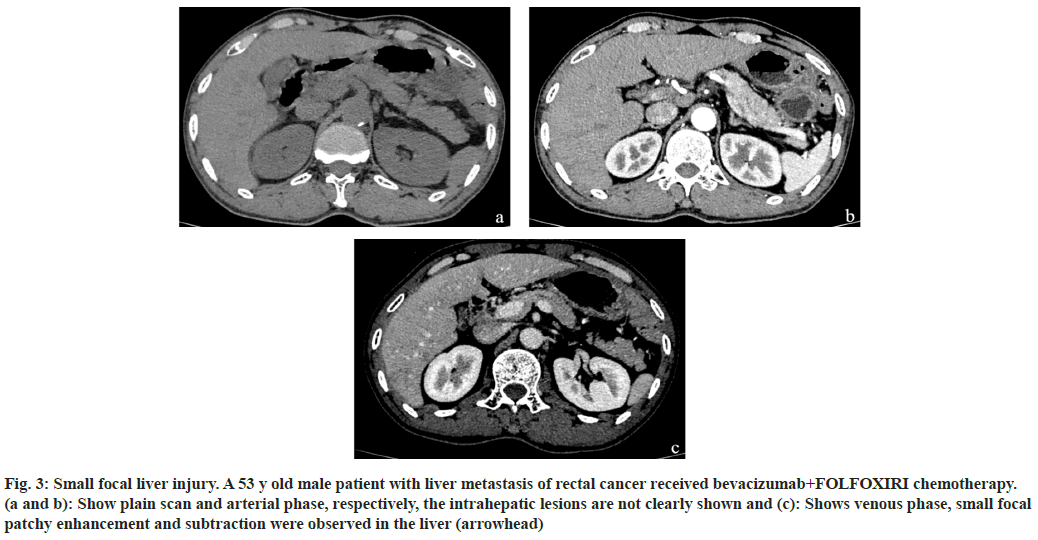
Among the 170 patients with liver injury after chemotherapy, the FOLFOX regimen accounted for the highest proportion of liver injury cases, accounting for 51 % of cases. This was followed by FOLFOXIRI, cetuximab+FOLFOXIRI, bevacizumab+FOLFOXIRI, and bevacizumab+FOLFOX, which accounted for 26 %, 9 %, 9 % and 5 %, respectively as shown in Table 2. The imaging findings of the 170 patients were divided into diffuse (fig. 1), local (fig. 2) and small focal (fig. 3). The different chemotherapy regimens showed that 77 of the 87 cases of FOLFOX showed diffuse liver injury, accounting for approximately 88.5 % of the chemotherapy regimen. Among the 45 cases of the FOLFOXIRI regimen, 43 cases showed local liver injury, accounting for approximately 95.6 % of the chemotherapy regimen. A total of 38 cases were treated with three regimens of cetuximab+FOLFOXIRI, bevacizumab+FOLFOXIRI, and bevacizumab+FOLFOX. Of these, 36 cases showed small focal liver injury, accounting for 94.7 % of the chemotherapy regimen as shown in Table 3.
| Chemotherapy regimen | Cases | Percentage |
|---|---|---|
| FOLFOX | 87 | 51.18 |
| FOLFOXIRI | 45 | 26.47 |
| Cetuximab+FOLFOXIRI | 15 | 8.82 |
| Bevacizumab+FOLFOXIRI | 15 | 8.82 |
| Bevacizumab+FOLFOX | 8 | 4.71 |
| Total | 170 | 100 |
Table 2: Distribution of Chemotherapy Regimens for The 170 Patients
| Chemotherapy regimen | Total | |||||
|---|---|---|---|---|---|---|
| Combination | FOLFOX | FOLFOXIRI | ||||
| Image classification | Diffuse | Count | 0 | 77 | 0 | 77 |
| Expected count | 17.2 | 39.4 | 20.4 | 77 | ||
| Adjusted residual | -6.4 | 11.6 | -7.1 | |||
| Local | Count | 2 | 8 | 43 | 53 | |
| Expected count | 11.8 | 27.1 | 14 | 53 | ||
| Adjusted residual | -3.9 | -6.3 | 10.9 | |||
| Small focal | Count | 36 | 2 | 2 | 40 | |
| Expected count | 8.9 | 20.5 | 10.6 | 40 | ||
| Adjusted residual | 11.7 | -6.7 | -3.5 | |||
| Total | Count | 38 | 87 | 45 | 170 | |
| Expected count | 38 | 87 | 45 | 170 | ||
Table 3: Image Classification and Cross Table of the Chemotherapy Regimens
Fig. 1: Diffuse liver injury. A 48 y old female patient with liver metastasis of colon cancer received the FOLFOX chemotherapy regimen. (a and b): Show plain scan and arterial phase, respectively, and the intrahepatic lesions are not clearly shown and (c): Shows venous phase, the patchy enhancement reduction shadow was diffuse in the liver (arrowhead)
Fig. 2: Local liver injury. A 53 y old female patient with liver metastasis of colon cancer received FOLFOXIRI chemotherapy. (a and b): Show plain scan and arterial phase, respectively, and the intrahepatic lesions are not clearly shown and (c): Shows venous phase, local patchy enhancement and subtraction were observed in the liver (arrowhead)
Fig. 3: Small focal liver injury. A 53 y old male patient with liver metastasis of rectal cancer received bevacizumab+FOLFOXIRI chemotherapy. (a and b): Show plain scan and arterial phase, respectively, the intrahepatic lesions are not clearly shown and (c): Shows venous phase, small focal patchy enhancement and subtraction were observed in the liver (arrowhead)
The results of this study showed that among the 170 patients with liver injury after chemotherapy. Male patients are more common and older. This reflects that there are older patients with liver injury after chemotherapy. This may be due to the decrease in blood flow, drug clearance, basal metabolic rate, albumin levels and drug release with increasing age, which leads to an increase in susceptibility risk[5,6].
The cross table of imaging classifications and chemotherapy schemes (Table 3) shows the distribution of various chemotherapy schemes for the different imaging classifications of liver injury. The table shows that any expected count in this study is >5, so the difference of each group can be judged according to the chi-square test table. The chisquare test results showed that the chi-square value was 260.472 (p<000). This indicates that the types of chemotherapy were different for different image types, and there was a correlation between the image types and the types of chemotherapy regimens (Table 4). The intensity of the correlation is shown in the symmetry measurement (Table 5). The Cramer's V (value range 0-1; the larger the value is, the stronger the correlation) was 0.875. The results showed that there was a strong correlation between imaging classifications and chemotherapy regimens. The adjusted standardized residual was obtained by using post-hoc testing. Because multiple comparisons are involved, we choose the absolute value of the adjusted standardized residual to be bounded by 3. The table shows that the absolute value of the adjusted standardized residual is >3, indicating that there is a statistically significant difference between the observed frequency and the expected frequency. Among the associations, the absolute value of the standardized residual of the diffuse type and FOLFOX chemotherapy regimens was the largest. This was followed by local type and FOLFOXIRI chemotherapy, which was followed by small focal type and mixed chemotherapy. The values for these associations were 11.6, 10.9 and 11.7, respectively. This shows that the diffuse manifestation was more prominent in the FOLFOX chemotherapy regimen cases, the local type was more prominent in the FOLFOXIRI chemotherapy regimen cases, and the small focal type was mostly observed in the mixed chemotherapy regimen cases. In this study, plain and enhanced CT scans were performed for all patients. It was found that the imaging changes of liver injury after chemotherapy were related to the chemotherapy regimen.
| Value | Freedom | Progressive significance (bilateral) | |
|---|---|---|---|
| Pearson chi square | 260.472a | 4 | 0 |
| Likelihood ratio | 257.151 | 4 | 0 |
| Number of valid cases | 170 |
Note: (a) Expected count of 0 cells (0 %) is <5. The minimum expected count is 8.94
Table 4: Chi-Square Test
| Value | Progressive significance | |
|---|---|---|
| Phi | 1.238 | 0 |
| Clem V | 0.875 | 0 |
| Number of valid cases | 170 |
Table 5: Symmetrical Measurement
The FOLFOX regimen showed diffuse liver injury, which may be related to the use of oxaliplatin in the FOLFOX regimen. Oxaliplatin has been reported to be the main cause of SOS. It can cause morphological changes in the micro vessels of the liver, leading to skin lesions and sinus congestion. It has been reported that 78 % of patients treated with oxaliplatin have significant changes in sinus congestion, which is followed by SOS[9]. This change in sinus congestion showed patchy reduced lesions in the liver under the background of normal hepatic parenchyma on enhanced CT scans[12].
The FOLFOXIRI regimen showed local liver injury. The use of oxaliplatin caused morphological changes in liver micro vessels, resulting in skin lesions and sinus congestion. Irinotecan is associated with steatosis of the liver. Vauthee et al. have shown that the use of irinotecan greatly increases the incidence of steatosis in the liver. Its use results in the accumulation of fat globules in the liver, which can develop into steatohepatitis. The distribution of fatty liver can range from diffuse infiltrative steatosis to localized steatosis. Fatty liver shows diffuse or a localized decrease in density of the liver parenchyma on CT plain scan. The combination of oxaliplatin results in hepatic sinusoidal obstruction syndrome, which shows patchy enhancement on the CT enhanced scan.
The two images influenced each other, and CT showed local patchy enhancement and reduction. The three regimens of cetuximab+FOLFOXIRI, bevacizumab+FOLFOXIRI, and bevacizumab +FOLFOX showed small focal liver injury, and the three regimens caused small focal liver injury. This may be because bevacizumab and cetuximab are anti-vascular endothelial growth factors and have protective effects on the development of vascular lesions. Additionally, they can reduce the severity of SOS (hepatic sinus obstruction syndrome). This reduces the incidence of central lobular fibrosis and lobular hypertrophy[11]. Contrast-enhanced CT showed a small patchy enhancement and a reduction in focus in the background of the liver parenchyma.
Our study also has some limitations. First, this is a single-center retrospective study with a small sample size. Second, there was no comparison of pathological samples in this study. Third, the Magnetic Resonance Imaging (MRI) images of patients were not included in this study for evaluation. MRI is more sensitive to changes in hepatic steatosis, which may be more helpful for the quantitative evaluation of steatosis in drug-induced liver injury. In the next step, we can include the MRI images of patients with drug-induced liver injury for analysis, especially for patients with liver steatosis, to compare steatosis with drug dose. In short, different chemotherapy regimens for colorectal cancer patients with liver metastasis will produce different hepatotoxic effects and lead to different degrees of liver injury. Imaging plays an important role in the evaluation of liver injury after chemotherapy. The observation of liver imaging changes has a certain sensitivity and specificity, especially the venous phase of enhanced scan. Different types of liver injury after chemotherapy can be reflected by imaging changes, which is a more intuitive method that has a certain reference value for the clinical diagnosis of liver injury after chemotherapy.
Authors’ contributions:
Shuai Ye and Kaikai Wei have contributed equally to this work.
Conflicts of interests:
The authors declare no conflict of interests.
References
- White MA, Fong Y, Singh G. Chemotherapy-associated hepatotoxicities. Surg Clin North Am 2016;96(2):207-17.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Houshyar R, Bhosale P, Choi JI, Gulati R, Lall C. Chemotherapy induced liver abnormalities: An imaging perspective. Clin Mol Hepatol 2014;20(3):317-26.
[Crossref] [Google Scholar] [PubMed]
- Vigano L, de Rosa G, Toso C, Andres A, Ferrero A, Roth A, et al. Reversibility of chemotherapy-related liver injury. J Hepatol 2017;67(1):84-91.
[Crossref] [Google Scholar] [PubMed]
- Diehl AM. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am J Physiol Gastrointest Liver Physiol 2002;282(1):G1-5.
[Crossref] [Google Scholar] [PubMed]
- Veteläinen R, van Vliet AK, van Gulik TM. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Ann Surg 2007;245(1):44-50.
[Crossref] [Google Scholar] [PubMed]
- Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: Impact on hepatic histology and postoperative outcome. J Gastrointest Surg 2007;11(7):860-8.
[Crossref] [Google Scholar] [PubMed]
- Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol 2006;24(31):4983-90.
[Crossref] [Google Scholar] [PubMed]
- Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15(3):460-6.
[Crossref] [Google Scholar] [PubMed]
- Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24(13):2065-72.
[Crossref] [Google Scholar] [PubMed]
- Rubbia‐Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, X Zhu A, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin‐associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology 2010;56(4):430-9.
[Crossref] [Google Scholar] [PubMed]
- Zhou H, Wang YX, Lou HY, Xu XJ, Zhang MM. Hepatic sinusoidal obstruction syndrome caused by herbal medicine: CT and MRI features. Korean J Radiol 2014;15(2):218-25.
[Crossref] [Google Scholar] [PubMed]
- Shaunak S, Munro JM, Weinbren K, Walport MJ, Cox TM. Cyclophosphamide-induced liver necrosis: A possible interaction with azathioprine. Q J Med 1988;67(1):309-17.
[Crossref] [Google Scholar] [PubMed]
- Zhang D, Hart J, Ding X, Zhang X, Feely M, Yassan L, et al. Histologic patterns of liver injury induced by anti-PD-1 therapy. Gastroenterol Rep 2020;8(1):50-5.
[Crossref] [Google Scholar] [PubMed]