- *Corresponding Author:
- Qiyuan Sun
Department of Orthopedics, Suzhou Ninth Hospital Affiliated to Soochow University, Suzhou, Jiangsu 215200, China
E-mail: sunqiyuan0321@163.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “10-19” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Puerarin can promote the proliferation of osteoblasts and it can also be used as a scaffold material, but its mechanism is unknown. In this study, we explored the primary molecular mechanism of puerarin in diabetic osteoporosis treatment. We used traditional Chinese medicine systems pharmacology database and analysis platform, PubChem, web-based gene set analysis toolkit, cytoscape and so on to screen the drug targets of puerarin and the disease targets of diabetic osteoporosis. We analyzed the puerarin mechanism based on the protein-protein interaction co-expression network, gene ontology, Kyoto encyclopedia of genes and genomes enrichment, etc., and molecular docking by AutoDock. The main enriched signaling pathways in the Kyoto encyclopedia of genes and genomes were non-small cell lung cancer, endocrine resistance and so on. The main enriched biological processes were positive regulation of cell migration, cytokine-mediated signaling pathway and so on. The main enriched cell components of the cellular component were the integrin complex, platelet alpha granule membrane and so on. The main cellular functions of the molecular function were C-X3-C chemokine binding, nitric oxide synthase regulator activity and so on. The tumor necrosis factor, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha and prostaglandin-endoperoxide synthase 2 with puerarin docking binding energy were all less than -5 kcal/mol-1. In conclusion puerarin may act through multiple targets and some pathways in diabetic osteoporosis.
Keywords
Network pharmacology, puerarin, diabetic osteoporosis, protein-protein interactions
Diabetes Mellitus (DM) is a group of metabolic syndromes typically characterized by chronic hyperglycemia. Experts predict that in 2035, there will be 592 million DM patients in the world and the prevalence rate of DM is 10.4 % in China alone. About 33 % of DM patients will have Diabetic Osteoporosis (DOP) due to abnormal bone metabolism[1,2]. As a metabolic bone disease, Osteoporosis (OP) is characterized by decreased bone mass, microstructure destruction of bone tissue, increased brittleness of bone and susceptibility to fracture[3]. DOP patients often show bone pain and motor dysfunction, which increases the risk of fracture and is also one of the main reasons for disability in DM patients[4]. Traditional Chinese medicine has become a better source of drug development because of its definite curative effect, high safety and longterm clinical practice experience[5]. Pueraria is one of the components with high content in puerarin. Its chemical name is 4,7-dihydroxy-8-β-D-glucuronic isoflavone, which belongs to phytoestrogen and it improves bone density while promoting bone formation[6,7]. Studies have shown that puerarin combined with alendronate sodium can synergistically inhibit oxidative stress and inflammatory response in Postmenopausal Osteoporosis (PMOP) rats, reduce oxidative stress and inflammatory factor secretion, regulate bone metabolism, improve bone histomorphological structure and play an antiosteoporosis role[8]. Puerarin fights osteoporosis by regulating oxidative stress in osteoporotic rats, improving bone metabolism-related indicators, increasing bone mineral density and improving the morphological structure of bone tissue[9]. Research shows that puerarin has anti-PMOP efficacy, bone protection by increasing estrogen levels in PMOP rats, regulating bone metabolism, increasing bone mass and mineral density, improving bone biomechanical properties and bone morphological structure[10].
The study confirmed that puerarin/tricalcium phosphate scaffold material has a good repair effect on the necrotic femur of rats with steroid-induced femoral head necrosis[11]. The preparation of polymeric mixed micelles of puerarin and daidzein co-loaded with puerarin oral antihypertensive preparations was also explored[12]. However, the regulatory effect of puerarin on osteoporosis is unknown and its mechanism of action on DOP is also a hot topic in clinical research. Based on this, we applied network pharmacology methods and molecular docking techniques to explore the puerarin mechanism in DOP treatment, analyze the active ingredients, targets, vital pathways of puerarin and clarify its pharmacological mechanism on DOP to provide data reference for new drug developments and clinical trials.
Materials and Methods
Acquisition of potential drug targets of puerarin:
We searched Pueraria lobata in the search box of Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database[13], which displayed all the effective active ingredients of puerarin and it was used to obtain the PubChem compound IDs (CID): 5281807 of puerarin. Then the PubChem CID was used to search in the PubChem database[14] to find the Structure-Data File (SDF) structural formula of puerarin. Finally, the Simplified Molecular Input Line Entry System (SMILES) of puerarin were used to obtain the potential drug targets of puerarin in SwissTargetPrediction database[15].
DOP disease target screening:
In the GeneCards (https://www.genecards.org/) database, the word “DOP” was searched to find the disease targets of DOP.
Screening common targets and vital targets of puerarin and DOP:
To obtain the common target of puerarin and DOP, we drew a Venn diagram on the Venn drawing website. After that, we imported the common targets into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database and the highest confidence was set as 0.700. Finally, the information on Protein-Protein Interaction (PPI) network was exported (a file formatted as .tsv). Import the .tsv files into Cytoscape 3.9.1 software to draw a PPI network diagram. We find the Maximal Clique Centrality (MCC) score and degree value of the top 30 genes on the cytoHubba which was plugged-in to Cytoscape 3.9.1 software, which was the key target.
Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis:
The Web-based Gene Set Analysis Toolkit (WebGestalt) database[16] was opened, the vital targets obtained from the above operations are imported and then set the parameters as Over-Representation Analysis (ORA), Benjamini-Hochberg (BH) method, False Discovery Rate (FDR)<0.05, respectively for KEGG and GO enrichment analysis. The KEGG enrichment analysis, Biological Process (BP), Cellular Component (CC) and Molecular Function (MF) data were collected and we made the weight analysis to sort out the corresponding enrichment information and finally, draw a bar chart.
Docking of small ligand molecules with receptor proteins:
The docking of small ligand molecules with receptor proteins was completed on AutoDock software, which involves the following steps: Using AutoDock Tools 1.5.7 software to open the puerarin molecular structure picture (.mol2 format) downloaded in the TCMSP database in advance. Then we added the atomic charge, did an atomic type assignment and set all flexible bonds to rotatable. Finally, we saved it as a .pdbqt format file as a docking ligand. Then download the three Dimensional (3D) structure of the core target in .pdb format file from the Protein Data Bank (PDB) database, use Proprietary Molecular Visualization System (PyMOL) software to enter the water removal command 'remove solvent' and the ligand removal command 'remove organic' and save them as .pdb format. Then use AutoDock tools 1.5.7 software to import the core target .pdb format into the non-polar hydrogen, calculate the Gasteiger charge and save it as a .pdbqt format file. Appling AutoDock Vina 1.5.7 software to do the molecular docking and finally, using PyMOL software to beautify the docking patterns of vital proteins and small molecules of puerarin.
Results and Discussion
Potential drug targets of puerarin were shown in Table 1. After predicting the potential drug targets of puerarin with the database while removing duplicate genes and retaining the only gene, a total of 102 potential drug targets of puerarin were obtained, including carbonic anhydrase VII, carbonic anhydrase XII, aldose reductase (by homology), equilibrative nucleoside transporter 1, etc.
| Target | Common name | Probability |
|---|---|---|
| Carbonic anhydrase VII | CA7 | 1 |
| Carbonic anhydrase XII | CA12 | 1 |
| Aldose reductase (by homology) | AKR1B1 | 0.1061658 |
| Equilibrative nucleoside transporter 1 | SLC29A1 | 0.1061658 |
| Thrombin and coagulation factor X | F10 | 0 |
| Thrombin | F2 | 0 |
| Integrin alpha-V/beta-3 | ITGAV | 0 |
| Integrin alpha-V/beta-3 | ITGB3 | 0 |
| Carbonic anhydrase II | CA2 | 0 |
| Protein kinase C gamma | PRKCG | 0 |
| Protein kinase C delta | PRKCD | 0 |
| Protein kinase C alpha | PRKCA | 0 |
| Protein kinase C beta | PRKCB | 0 |
| Protein Kinase C (PKC) | PRKCZ | 0 |
| Protein kinase C epsilon | PRKCE | 0 |
| Protein kinase C eta | PRKCH | 0 |
| Integrin alpha-V/beta-6 | ITGAV | 0 |
| Integrin alpha-V/beta-6 | ITGB6 | 0 |
| Coagulation factor VII | F7 | 0 |
| cAMP-dependent protein kinase alpha-catalytic subunit | PRKACA | 0 |
Table 1: Potential Drug Targets of Puerarin
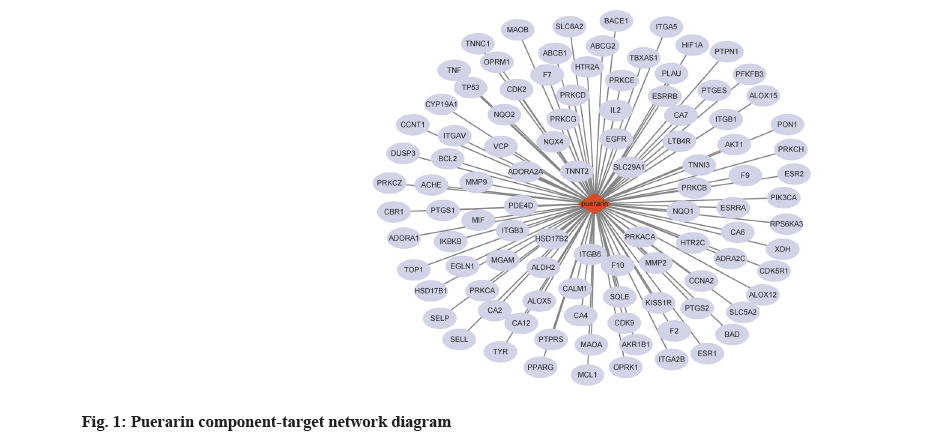
Node degree value of puerarin was shown here. The puerarin and 102 potential targets were uploaded to Cytoscape software through data compilation to obtain a puerarin-target network diagram, in which red represents puerarin and gray blue represents the target of puerarin (fig. 1).
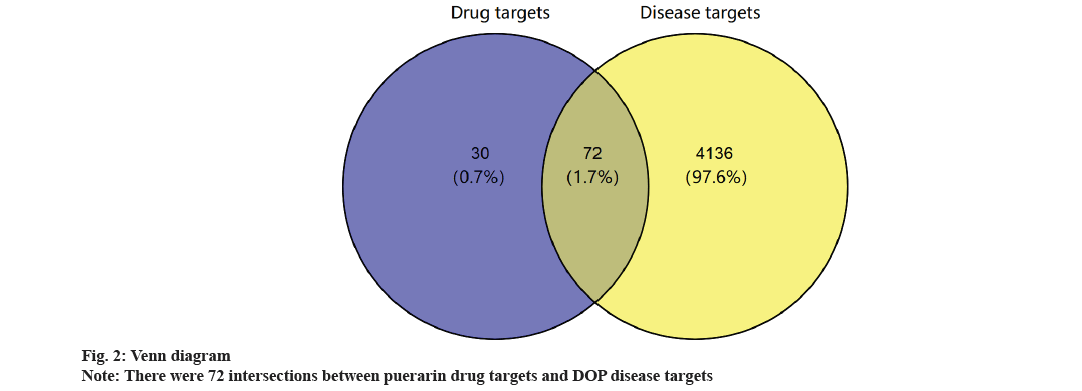
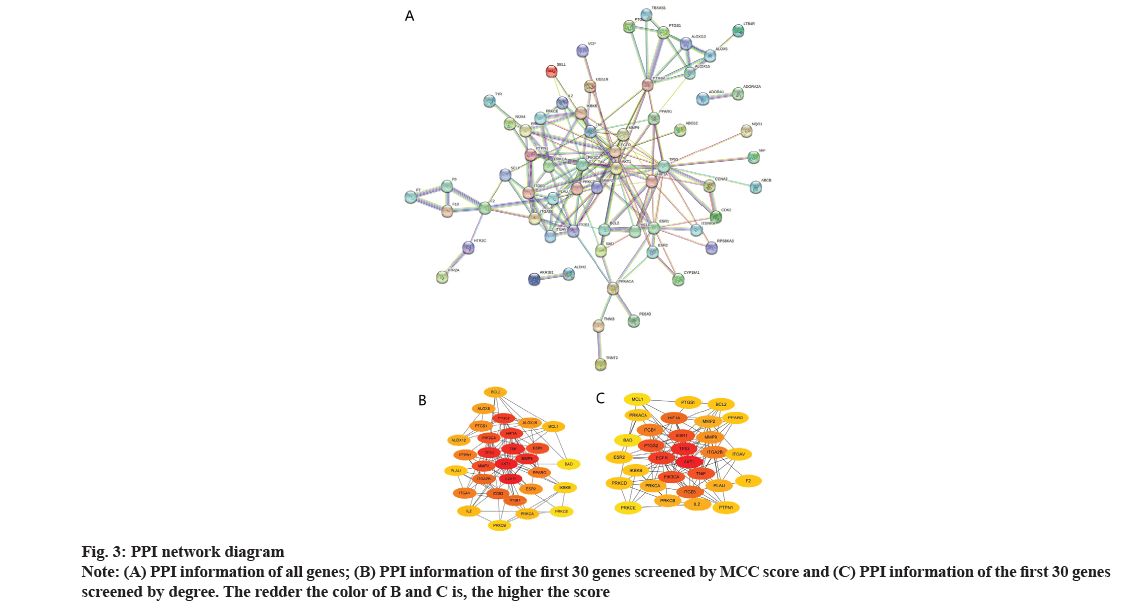
Common targets of puerarin and DOP and construction of PPI network were shown in fig. 2 and fig. 3. Based on the intersection analysis of 102 drug targets of puerarin and 4208 disease targets of DOP, 72 common targets were obtained (fig. 2). The PPI information of the common targets of puerarin and DOP were visualized as shown in fig. 3A. The 72 common targets are ranked in descending order based on the MCC score and degree to find the top 30 key targets (fig. 3B and fig. 3C, Table 2). The 60 vital targets are arranged by MCC and degree were integrated and screened by removing duplicates to obtain 30 key targets.
| Gene symbol | MCC | Gene symbol | Degree |
|---|---|---|---|
| AKT1 | 584 | AKT1 | 20 |
| EGFR | 474 | EGFR | 17 |
| TNF | 420 | TP53 | 13 |
| TP53 | 384 | TNF | 10 |
| MMP9 | 366 | PIK3CA | 10 |
| HIF1A | 294 | PTGS2 | 10 |
| PTGS2 | 170 | ITGB3 | 9 |
| MMP2 | 128 | HIF1A | 9 |
| PIK3CA | 114 | ITGB1 | 8 |
| ITGB3 | 82 | MMP9 | 8 |
| ESR1 | 78 | ESR1 | 7 |
| ITGA2B | 74 | ITGA2B | 7 |
| ITGB1 | 66 | MMP2 | 7 |
| PPARG | 50 | PPARG | 6 |
| ITGAV | 32 | IKBKB | 6 |
| PTPN1 | 30 | BCL2 | 6 |
| ALOX15 | 26 | ITGAV | 6 |
| ALOX12 | 24 | IL2 | 6 |
| ESR2 | 24 | PRKCA | 6 |
| PTGS1 | 24 | PLAU | 6 |
| ALOX5 | 24 | PRKCB | 5 |
| BCL2 | 20 | PTPN11 | 5 |
| MCL1 | 18 | ESR1 | 5 |
| IL2 | 16 | PTK2 | 5 |
| PLAU | 16 | JAK2 | 4 |
| PRKCA | 14 | MAPK8 | 4 |
| IKBKB | 10 | JAK1 | 4 |
| PRKCB | 8 | RXRA | 4 |
| PRKCE | 6 | STAT1 | 4 |
| BAD | 6 | PRKCD | 3 |
Table 2: Topology Analysis Results of Mcc and Degree Algorithm of Top 30 Genes
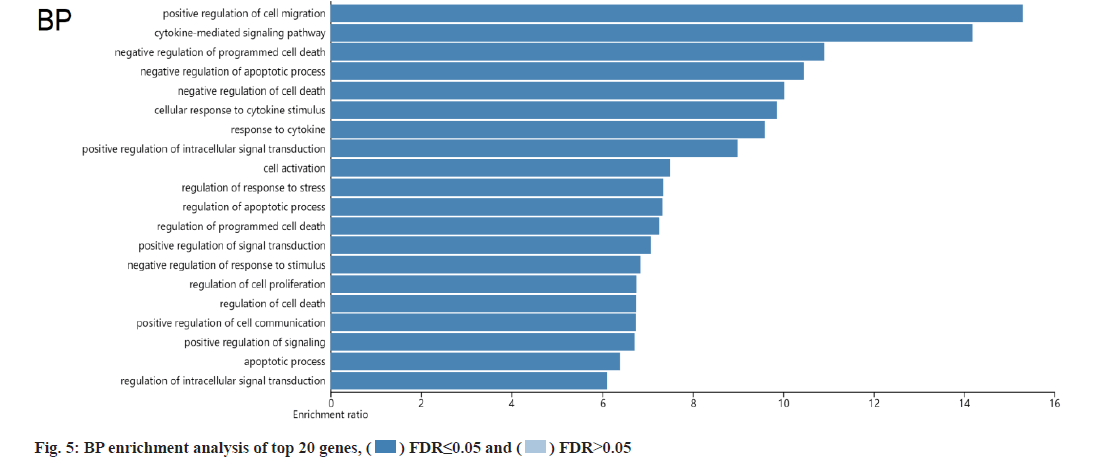
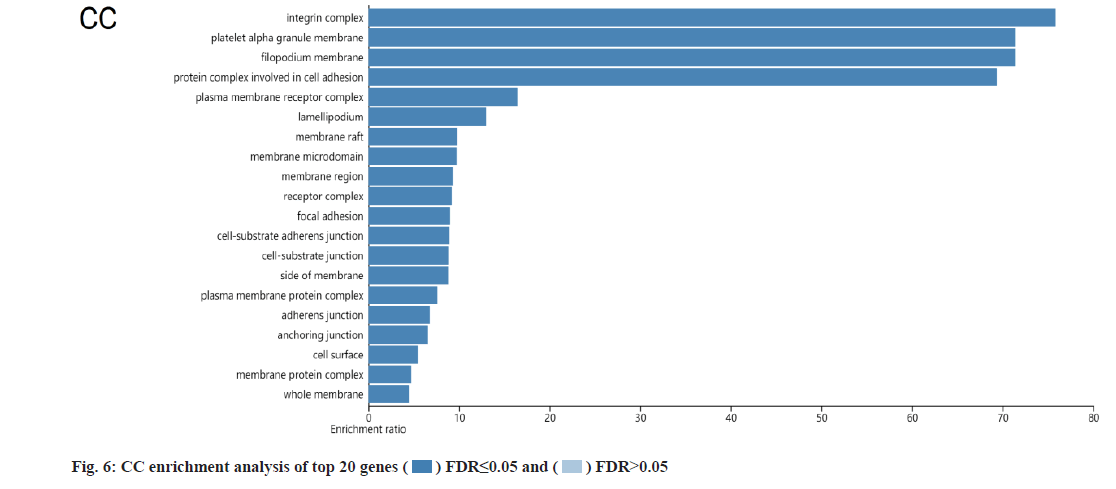
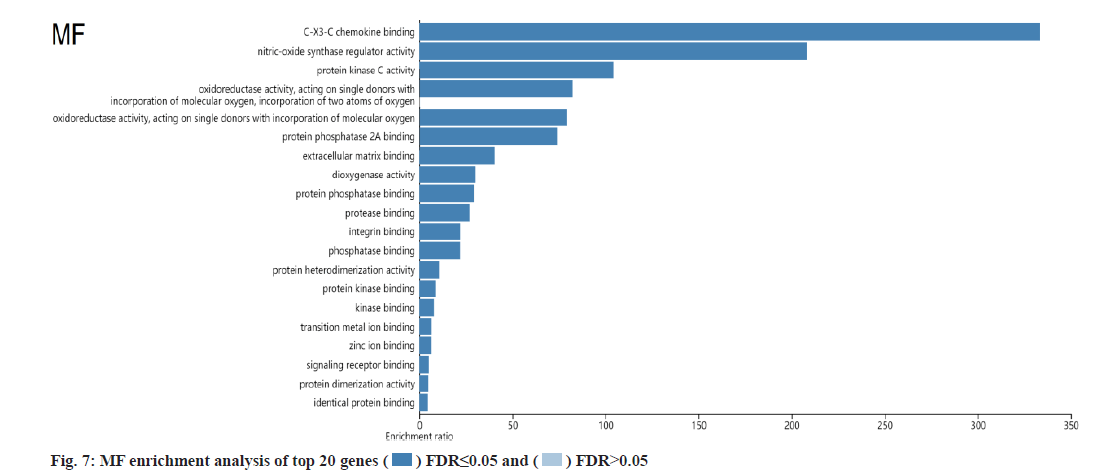
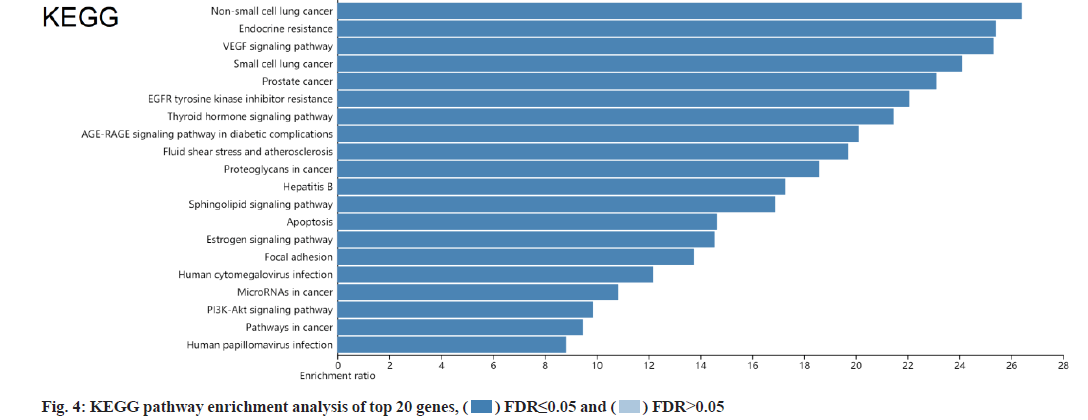
KEGG and GO analysis of 30 key targets and the enrichment significance was FDR<0.05. The main enriched signaling pathways in KEGG were nonsmall cell lung cancer, endocrine resistance, Vascular Endothelial Growth Factor (VEGF) signaling pathway, small cell lung cancer, prostate cancer and Epidermal Growth Factor Receptor (EGFR) tyrosine kinase inhibitor resistance (fig. 4). The main enriched BP included positive regulation of cell migration, cytokine-mediated signaling pathway, negative regulation of programmed cell death and negative regulation of apoptotic process (fig. 5). The main enriched cell components of CC were the integrin complex, platelet alpha granule membrane, filopodium membrane, protein complex involved in cell adhesion, etc. (fig. 6). The main enriched cell functions of the MF were C-X3-C chemokine binding, nitric-oxide synthase regulator activity, protein kinase C activity, etc. (fig. 7).
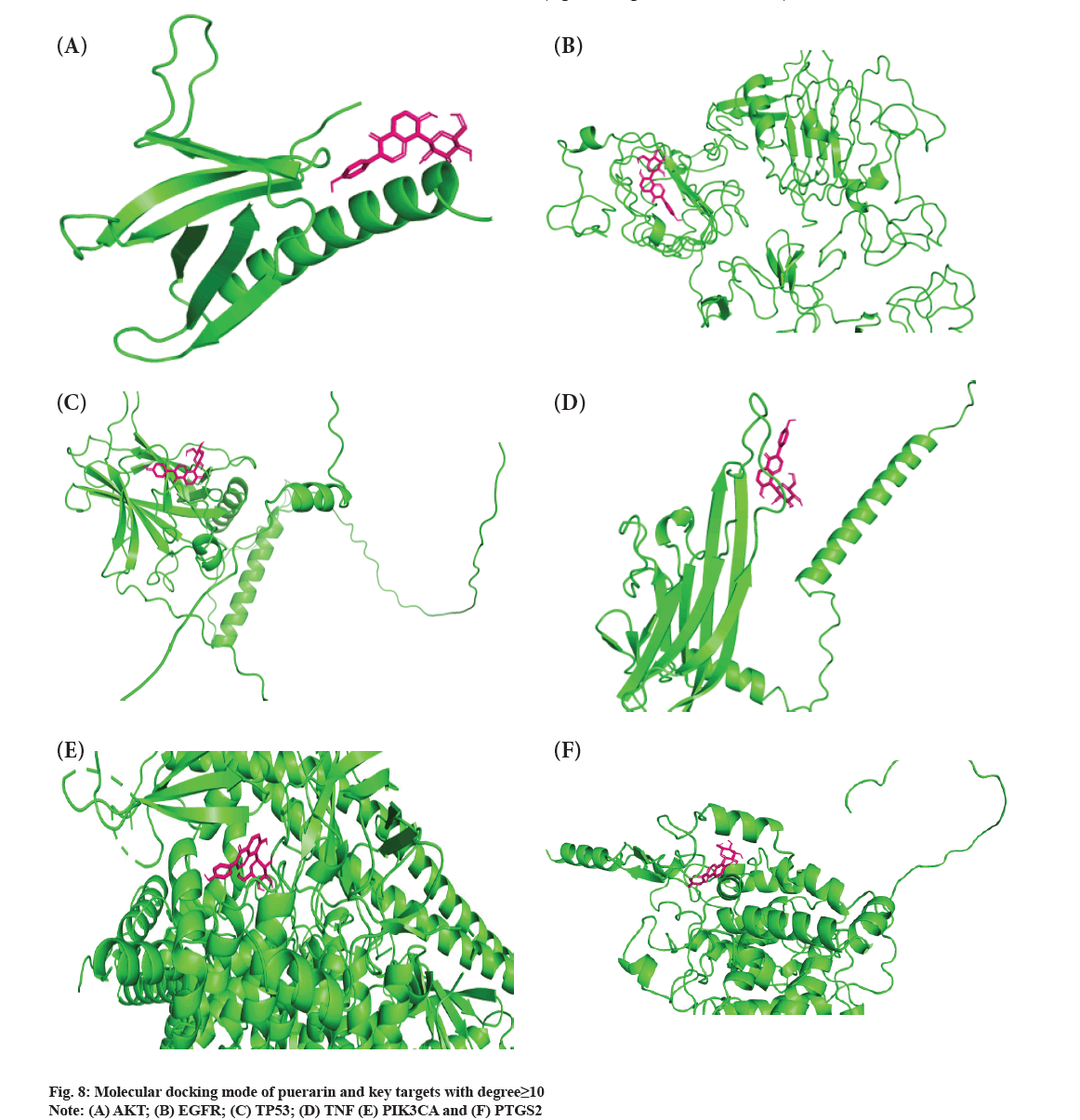
Molecular docking mode of puerarin and key targets were shown in fig. 8. The good condition for improving the binding ability of protein molecules to small ligand molecules is maintain binding energy lower than -5 kcal/mol-1. In this study, molecular docking between puerarin small molecules and key targets with degree≥10 (AKT Serine/Threonine Kinase 1 (AKT1), EGFR, Tumor Protein 53 (TP53), Tumor Necrosis Factor (TNF), Phosphatidylinositol- 4,5-bisphosphate 3-Kinase Catalytic subunit Alpha (PIK3CA), Prostaglandin-Endoperoxide Synthase 2 (PTGS2)) was performed. The final analysis results was that the binding energies of AKT1, EGFR and TP53 with puerarin were greater than -5 kcal/mol- 1, and the binding energies of TNF, PIK3CA and PTGS2 with puerarin were less than -5 kcal/mol-1 (fig. 8A-fig. 8F and Table 3).
| Micro molecule | AKT1 | EGFR | TP53 | TNF | PIK3CA | PTGS2 |
|---|---|---|---|---|---|---|
| Puerarin | -4.27 | -3.84 | -4.98 | -5.03 | -6.19 | -8.44 |
Table 3: Molecular Docking Binding Energy between Puerarin and Key Targets with Degree≥10 (Kcal/Mol-1)
The pathogenesis of DOP is complex and most studies have pointed out that its occurrence may be strongly influenced by glucose metabolism disorder, oxidative stress injury, chronic inflammatory stimulation and other factors[17], among which glucose metabolism disorder and oxidative stress injury is closely related to DOP[18,19]. Studies have confirmed that puerarin can promote the secretion and synthesis of Alkaline Phosphatase (ALP) in osteoblasts[6], cause vacuolar changes in osteoclasts cultured in vitro and reduce the content of calcium ions in the empty bone lacunae area of culture supernatant. The Traditional Chinese Medicine (TCM) has multiple targets, which have sufficient advantages in the clinical treatment of Type 2 (T2) DOP with complex pathogenesis. At present, medical experts have not yet reached a consensus on the mechanism of puerarin in DOP. Based on the connection and relationship analysis of biological network nodes, network pharmacology constructs a multi-level network of “chemical constituents, prediction targets and signaling pathways” and comprehensively analyzes the effects of TCM compounds. It reflects the multi-component, multi-target and multi-pathway characteristics of TCM compounds and is also associated with treating illness. It is consistent with the basic principles of a holistic view of TCM and treatment based on syndrome differentiation[20]. Therefore, it is necessary to apply network pharmacology to explore puerarin in DOP mechanism.
In this study, there were 72 common targets between the puerarin drug targets and DOP disease targets. We screened the top 30 vital targets according to the MCC score and degree from high to low. After integrating these targets and removing duplications, 30 key targets were obtained, mainly containing AKT1, EGFR, TP53, TNF, PIK3CA, PTGS2, etc., indicating that these targets had strong interaction ability. We used GO and KEGG enrichment analysis to explore the main pathways involved in these 30 key targets. We found that puerarin’s effect on DOP depends on a non-single number of target spots, components and passageways. These action areas are mainly located in the membrane area and extracellular areas. Puerarin acts through positive regulation of cell migration, cytokine-mediated signaling pathway, negative regulation of programmed cell death, negative regulation of the apoptotic process and other BPs. It mediates signal pathways such as non-small cell lung cancer, endocrine resistance, VEGF signaling pathway, small cell lung cancer, prostate cancer and EGFR tyrosine kinase inhibitor resistance, achieving the effect of treating DOP. The cell components involved include the integrin complex, platelet alpha granule membrane, filopodium membrane, protein complex involved in cell adhesion, etc. It also involves C-X3-C chemokine binding, nitric oxide synthase regulator activity, protein kinase C activity and other cell functions. Most of the above pathways are common action pathways. For example, the VEGF signaling pathway plays a significant role in signal transduction, disease progression and is involved in the pathogenesis of various cancers and blinding eye diseases[21]. Sun et al. showed that polysaccharides regulates osteoporosis by regulating Receptor Activator of Nuclear factor Kappa beta Ligand (RANKL)/Receptor Activator of Nuclear factor Kappa beta (RANK)/Osteoprotegerin (OPG) and Phosphoinositide 3-Kinase (PI3K)/protein kinase B (Akt)/endothelial Nitric Oxide Synthase (eNOS) pathways[22]. Other studies have indicated that VEGF is similar to a prominent osteoblast and can stimulate angiogenesis[23]. We looked at a lot of literature and found few studies on signaling pathways such as nonsmall cell lung cancer, endocrine resistance, small cell lung cancer and prostate cancer in osteoporosis, which are the new findings of this study. It may provide a new idea for the clinical treatment of DOP, but their specific effects need to be verified by a several reliable studies.
Molecular docking is a method to simulate the binding of a small molecule (drug component) ligand to a large protein (target) receptor and investigate its binding energy, binding location and binding mode. The method of molecular docking is semi-flexible docking. In general, when the binding energy is negative, the binding does not require binding energy but can release energy, which means that once the two meet, they are likely to combine and the higher the absolute value, the better the docking. Generally speaking, when the binding energy is small enough (less than -5 kcal/mol-1), it is considered that the ligand molecule can dock with the receptor protein. We analyzed the molecular docking binding energy of puerarin and vital targets. We found that the docking binding energy of AKT1, EGFR and TP53 with puerarin had no combination conditions. However, the docking binding energy of TNF, PIK3CA and PTGS2 with puerarin had combination conditions, indicating that puerarin regulates DOP through TNF, PIK3CA and PTGS2 targets. TNF Superfamily member 11 (TNFSF11) can regulate and control a variety of physiological or pathological functions, including osteoclast differentiation and osteoporosis[24]. A meta-analysis showed that TNF-alpha (TNF-α) G308 A polymorphism can be a potential candidate biomarker for osteoporosis screening, diagnosis and treatment, which helps to improve the individualized treatment of osteoporosis patients[25]. The results of Wen et al. studies showed that TNF was one of the targets of Liuwei Dihuang pills countermining bone loss and had a high binding activity with related active ingredients, which was similar to our research results[26]. Liu et al. also believed that PIK3CA was one of the vital targets of pilose antlers in the treatment of osteoporosis[27]. Studies have shown that iron ions induce the expression of trigger receptor-2 and PI3K/Akt signaling pathways in myeloid cells to promote osteoclast differentiation[28]. Triggering Receptor Expressed in Myeloid cells-2 (TREM-2) was targeted by PIK3CA and Phosphoinositide-3- Kinase Regulatory subunit 1 (PIK3R1) gene nodes. These two gene nodes are key transcription cofactors in the PI3K/Akt signaling pathway. PTGS2 is a key target of puerarin action on DOP and few related research studies are occurring abroad. A domestic study showed that Mus musculus micro Ribonucleic acid-107-3p (mmu-miR-107-3p) could inhibit the proliferation and osteogenic differentiation of osteoblastic cell line (MC3T3-E1) cells by targeting PTGS2[29]. It may be a gene but worthy in the osteoblast differentiation of MC3T3-E1 cells. Biomaterials prepared in different tissues as biological scaffolds can provide growth space and structural support for specific cells in tissues and can guide tissue regeneration and control tissue structure[30]. Puerarin promotes osteocyte proliferation and antiosteoporosis through multi-target and multi-channel pathways. Puerarin biomaterial scaffold construction can improve the bioavailability of puerarin and provide new guidance for the clinical treatment of osteoporosis.
In conclusion, puerarin may act through multiple targets (including TNF, PIK3CA and PTGS2) and may play a role in DOP by affecting some pathways. However, our study predicted the mechanism of puerarin’s action on DOP with data through network pharmacology and molecular docking methods. This study only theoretically discussed the puerarin’s possible active components, targets and signaling pathways. But it could not determine the specific mechanism of puerarin components on DOP. In the next step, we need to conduct basic experiments to verify the pharmacological action of puerarin.
Funding:
This study was supported by the institute-level scientific research project of Suzhou Ninth People’s Hospital (Project number: YK202126).
Conflict of interests:
The authors declared no conflict of interest.
References
- Chinese diabetes society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellit 2021;13(4):315-409.
- Shi L, Ren WD, Wang LK, Li CX, Zhang ZY, Deng WJ, et al. Study on the correlation between osteoporosis and carotid atherosclerosis in elderly men with diabetes mellitus. China Med Her 2022;19(18):20-23.
- Du LK, Li JR. TCM cognition and prophylaxis and treatment of osteoporosis. Chin J Osteoporos 2022;28(2):296-9.
- Lian LR, Chu SF, Liang JC, Li HL, Zhao HX, Dong YM. Study on the mechanism of Zishen Jiangtang pill in the treatment of diabetic osteoporosis. Chin J Osteoporos 2022;28(4):551-7.
- Li JG, Xie XW, Li DP, Xu SH, Xu W, Jiang GP. Epimedium extract icariin for the prevention and treatment of osteoporosis at the cellular level. Chin J Osteoporos 2019;25(1):132-5.
- Liang XZ, Luo D, Xu B, Liu JB, Li G. Bioinformatics analysis of puerarin treating osteoporosis. Chin Arch Tradit Chin Med 2019;37(11):2628-31.
- Yang YQ, Li L, Xie JS, Chen JH, Luo XX, Mao BY, et al. The mechanism study of inhibition effect of puerarin on osteoporosis through PTEN-PI3K-AKT signaling pathway. Chin J Osteoporos 2022;28(3):347-51.
- Chen WM, Hu XY. Effect of puerarin combined with alendronate on oxidative stress, inflammation reaction and bone metabolism in postmenopausal osteoporotic rats. Chin J Osteoporos 2021;27(1):73-6.
- Yang ZH, Hao LS, Zhang JX. Effects of puerarin on oxidative stress response, bone metabolism and bone mineral density in osteoporotic rats. Chin J Osteoporos 2021;27(3):413-7.
- Yue HZ, Cai J, Ma XQ, Wang Y. Effect of puerarin on bone metabolism, bone mineral density and bone biomechanics in postmenopausal osteoporosis rats. Chin J Osteoporos 2021;27(1):77-81.
- Tang H. Preparation of puerarin combined with tricalcium phosphate scaffold and its repairing effect on steroid-induced avascular necrosis of the femoral head in rats. Hebei Med 2021;27(3):395-401.
- Zhu WF, Kuang WL, Li WD, Ding Q, Zhou ZZ, Wu WZ, et al. Preparation and characterization of polymer mixed micelles co-loaded with homologous components of puerarin and daidzein. Chin Tradit Herb Drugs 2022;53(4):1004-12.
- Zhang L, Han L, Wang X, Wei Y, Zheng J, Zhao L, et al. Exploring the mechanisms underlying the therapeutic effect of Salvia miltiorrhiza in diabetic nephropathy using network pharmacology and molecular docking. Biosci Rep 2021;41(6):1-17.
[Crossref] [Google scholar] [PubMed]
- Yin B, Bi YM, Fan GJ, Xia YQ. Molecular mechanism of the effect of Huanglian Jiedu decoction on type 2 diabetes mellitus based on network pharmacology and molecular docking. J Diabetes Res 2020;2020:1-24.
[Crossref] [Google scholar] [PubMed]
- Lu Z, Huang M, Lin H, Wang G, Li H. Network pharmacology and molecular docking approach to elucidate the mechanisms of Liuwei Dihuang pill in diabetic osteoporosis. J Orthop Surg Res 2022;17(1):1-16.
[Crossref] [Google scholar] [PubMed]
- He Q, Yang J, Zhang G, Chen D, Zhang M, Pan Z, et al. Sanhuang Jiangtang tablet protects type 2 diabetes osteoporosis via AKT-GSK3β-NFATc1 signaling pathway by integrating bioinformatics analysis and experimental validation. J Ethnopharmacol 2021;273:113946.
[Crossref] [Google scholar] [PubMed]
- Mohsin S, Baniyas MM, alDarmaki RS, Tekes K, Kalász H, Adeghate EA. An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin Biol Ther 2019;19(9):937-48.
[Crossref] [Google scholar] [PubMed]
- Qi SS, Shao ML, Sun Z, Chen SM, Hu YJ, Li XS, et al. Chondroitin sulfate alleviates diabetic osteoporosis and repairs bone microstructure via anti-oxidation, anti-inflammation, and regulating bone metabolism. Front Endocrinol 2021;12:1-11.
[Crossref] [Google scholar] [PubMed]
- Shen YW, Cheng YA, Li Y, Li Z, Yang BY, Li X. Sambucus williamsii Hance maintains bone homeostasis in hyperglycemia-induced osteopenia by reversing oxidative stress via cGMP/PKG signal transduction. Phytomedicine 2023;110:154607.
[Crossref] [Google scholar] [PubMed]
- Zhao Y, Li XB, Zhang SB, Chen JP. Study on the network pharmacology mechanism of Dioscoreae Rhizoma and Astragali radix in treating type 2 diabetic osteoporosis. Chin J Osteoporos 2021;27(6):866-74.
- Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019;176(6):1248-64.
[Crossref] [Google scholar] [PubMed]
- Sun X, Wei B, Peng Z, Fu Q, Wang C, Zhen J, et al. Protective effects of Dipsacus asper polysaccharide on osteoporosis in vivo by regulating RANKL/RANK/OPG/VEGF and PI3K/Akt/eNOS pathway. Int J Biol Macromol 2019;129:579-87.
[Crossref] [Google scholar] [PubMed]
- Veeriah V, Paone R, Chatterjee S, Teti A, Capulli M. Osteoblasts regulate angiogenesis in response to mechanical unloading. Calcif Tissue Int 2019;104(3):344-54.
[Crossref] [Google scholar] [PubMed]
- Luo J, Yang Z, Ma YU, Yue Z, Lin H, Qu G, et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 2016;22(5):539-46.
[Crossref] [Google scholar] [PubMed]
- Fu SC, Wang P, Qi MX, Peng JP, Lin XQ, Zhang CY, et al. The associations of TNF‐α gene polymorphisms with bone mineral density and risk of osteoporosis: A meta‐analysis. Int J Rheum Dis 2019;22(9):1619-29.
[Crossref] [Google scholar] [PubMed]
- Wen MT, Liang XZ, Li JC, Li G, Xu B. Study on anti-osteoporotic mechanism of Liuwei Dihuang pills based on network pharmacology and molecular docking technology. Chin J Osteoporos 2021;27(8):1129-34.
- Liu B, Wang A, Cao Z, Li J, Zheng M, Xu Y. Mechanism of pilose antler in treatment of osteoporosis based on network pharmacology. J Healthc Eng 2022;2022:1-7.
[Crossref] [Google scholar] [PubMed]
- Liu LL, Cao ZH, He CL, Zhong YC, Liu WY, Zhang P, et al. Ferric ion induction of triggering receptor expressed in myeloid cells‐2 expression and PI3K/Akt signaling pathway in preosteoclast cells to promote osteoclast differentiation. Orthop Surg 2020;12(4):1304-12.
[Crossref] [Google scholar] [PubMed]
- Luo MR, Fan WH, Li Z, Zhou P, Wu ZZ, Yuan F. Inhibition of the proliferation and osteogenic differentiation of MC3T3-E1 cells by targeting prostaglandin-endoperoxide synthase 2. Chin J Tissue Eng Res 2022;26(12):1888-93.
- Wang L, Sun ST, Zhang WJ, Liang S, Ren JG. Preparation and in vitro evaluation of puerarin phospholipid complex nanoparticles. Cent South Pharm 2020;18(1):15-20.
 ) FDR≤0.05 and (
) FDR≤0.05 and ( ) FDR>0.05
) FDR>0.05