- *Corresponding Author:
- X. T. Chen
Department of Nephrology,
The Affiliated Hospital of Xiangnan University,
Chenzhou, Hunan 423000, China
E-mail: 1402831486@qq.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “109-112” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the clinical efficacy and safety of alprostadil in combination with the reduced glutathione for the treatment of chronic renal failure patients is the main objective. A total of 98 chronic heart failure patients in this hospital were selected as the subjects and divided into the control group and the observation group, with 49 patients in each group. Patients in the control group received the regular therapy, while those in the observation group received the combined medication of alprostadil and reduced glutathione. Following the treatment, we compared the clinical efficacy, incidence of adverse events and the indicators of renal functions, including the hemoglobin, serum creatinine, 24 h urinary protein and blood urea nitrogen. After treatment, patients in two groups had improvement in the health status, social function, emotional function and mental status, and the improvement in the observation group was superior to that in the control group (all p<0.05). In the observation group, incidence rate of adverse events was 5.4 %, significantly lower than 24.3 % in the control group (p<0.05). Besides, following the treatment, significant improvement was also seen in the indicators of renal functions in two groups and similarly, the improvement in the observation group was more obvious than that in the control group (p<0.05). Alprostadil in combination with reduced glutathione, in treatment of chronic renal failure, can improve the indicators of renal functions, with fewer adverse events, so it is worthy of being promoted in clinical practice.
Keywords
Alprostadil, reduced glutathione, chronic renal failure
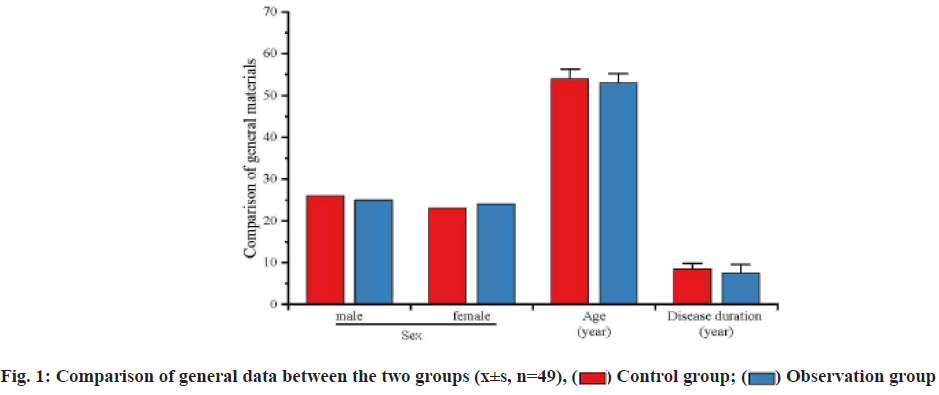
Chronic renal failure refers to the irreversible loss of nephron and renal function caused by the progressive evolvement of chronic renal diseases, leading to the clinical syndrome with retention of metabolite and toxins, disorders in the electrolytes, imbalance of acidbase equilibrium and endocrine dysfunction as the major features. Injuries caused by the chronic renal failure are categorized into the following two types: Most of the patients inevitably evolve into the endstage renal disease and require the replacement therapy of kidney to prolong the lifespan; patients usually manifest the significant increases in the incidence rate and mortality rate of the cardiovascular and cerebrovascular complications. For treatment of chronic renal failure, elimination of the risk factors is the key to delaying the progression of chronic renal failure and the emergence of renal failure, without any prompt, effective treatment, would shorten the lifespan and severely threaten the life of patients[1,2]. Currently, medication dominates the therapy for chronic renal failure, including the folic acid, hemopoietin and iron preparation, but most of them have gained poor outcomes[3]. In recent years, the efficacy of alprostadil and reduced glutathione has gained increasing attention in clinical practice. In this study, we investigated the efficacy of these two drugs and detailed information is reported as follow. In this study, we enrolled a total of 98 chronic renal failure patients who were admitted to this hospital between January and December 2020 and their diagnoses conformed to the diagnostic criteria for chronic renal failure. All patients were in stable disease, without any acute symptoms, edema or complications of heart failure, gastrointestinal bleeding or shock. According to the examination, the average level of Serum Creatinine (SCr) was (245.6±75.5) μmol/l and the average of Glomerular Filtration Rate (GFR) was (32.8±15.1) ml/min. As for the primary symptoms, there were 40 patients with diabetic nephropathy, 20 with benign hypertensive arteriolar nephrosclerosis and 38 with chronic glomerulonephritis. These patients were randomly divided into the control group and the observation group with 49 patients in each group. There was no significant difference in sex distribution, age, disease duration between the two groups (p>0.05), as shown in fig. 1. This experimental scheme has been approved by the medical ethics committee of our hospital. Treatment methods used in the study is shown below. Following the admission, patients accepted the guidance of nurses for the diet and medication to maintain the blood pressure and glucose level within the normal range. Besides, patients in the control group received the regular treatment, including the oral administration of compound alpha (α)-ketoacid tablets, intestinal detoxification drugs and oral or intravenous administration of iron supplements and subcutaneous injection of erythropoietin. In the observation group, patients took alprostadil (Hengkang Pharmaceutical (Benxi) Co., Ltd.; Approval number of State Food and Drug Administration (SFDA): H20093175; Specification: 20 μg) in combination with the reduced glutathione (Luye Pharmaceutical (Yantai) Co., Ltd.; Approval number of SFDA: H20041619; Specification: 1.2 g). 7 d consisted of one course and treatment would be carried out once per month[4]. The total length of treatment depended on the endpoint event of patients and at the end of treatment a 12 mo follow-up was initiated for patients. Indicators for observation in the study are represented below. Changes in the scores of life quality, incidence of adverse events and indicators of renal functions were detected for patients in two groups. Life quality was evaluated from the aspects of health status, social functions, emotional functions and mental status using the 36-Item Short Form Survey (SF-36) (SF-36) system, with 100 points for each aspect, aiming to evaluate the changes in the life quality of patients. Besides, adverse events included the nausea, abdominal distension and dizziness, while indicators of renal functions included the Hemoglobin (Hb), SCr, 24 h Urea Protein (24 hUP) and Blood Urea Nitrogen (BUN). Statistical analysis was performed for the data of this study by using the Statistical Package for the Social Sciences (SPSS) 20.0 software. Measurement data were presented with the mean±Standard Deviation (SD) and the difference was validated using the t test. Enumeration data were presented with the ratio (%), while the difference was validated using the chi-square test, p<0.05 suggested that the difference had statistical significance. Scores of life quality between two groups were compared. Prior to the treatment, no significant difference was identified in evaluating the health status, social function, emotional function and mental status of patients between two groups (p>0.05); after treatment, scores of patients in two groups were elevated in two groups and the elevation in the observation group was much higher than that in the control group (p<0.05; fig. 2). The incidence rates of adverse events of patients between two groups were compared. In the observation group, the incidence rate of adverse events was 6.1 %, significantly lower than 24.4 % in the control group and the difference had statistical significance (p<0.05; Table 1). The indicators of renal functions of patients between two groups were compared. Before treatment, comparison of the levels of Hb, SCr, 24 hUP and BUN between two groups showed no significant differences (p>0.05), while, after treatment, significant improvement was seen in these indicators of patients in two groups, but the improvement in the observation group was much better than that in the control group (p<0.05; Table 2). Chronic renal failure, as the most common condition in clinical practice, represents the end-stage of progression for all types of renal diseases. Chronic failure, due to the long disease course and difficulty in clinical treatment, requires the dialysis or kidney transplantation in the end-stage to prolong the survival[5]. Progression of chronic renal failure is slow but irreversible, with the clinical manifestations of hypercoagulability, hypertransfusion, hypertension and high filtration. These manifestations come with the significant increases in the oxidative stress and inflammation, further aggravating the damage to the kidney and inducing the continuous accumulation of toxins. Hence, kidney protection is a critical step in clinical treatment of chronic renal failure, showing the positive effect on the delaying the disease progression[6]. Previously, main strategies for clinical treatment of chronic renal failure include the folic acid, hemopoietin and iron supplementation to delay the deterioration of renal function, but some patients respond poorly[7]. In recent years, alprostadil and reduced glutathione have been gradually applied in the treatment of chronic renal failure. Recent pharmaceutical research has shown that reduced glutathione can inhibit the peroxides or free radicals, so as to mitigate the damage to the organs. Alprostadil is reported to dilate the vessels, thereby increasing the blood flow in the kidney and improving the renal functions[8,9]. The results of this study indicated that after treatment, the life quality of patients in the observation group was much better than that in the control group (p<0.05), while the incidence rate of adverse events in the observation group was only 6.1 %, significantly lower than 24.4 % in the control group (p<0.05); patients in the observation group manifested the significant improvement in the renal functions after treatment, with significant amelioration in the relevant indicators (p<0.05). The magnificent effects of alprostadil in combination with reduced glutathione in treatment of chronic renal failure attribute to the following reasons: Alprostadil is stable in the lipid microsphere and after administration, lipid microsphere can target the damaged vessels to dilate the arterioles in the kidney medulla to decrease the pressure of glomerular capillary and increase the blood flow in kidney, which is conducive to the improvement of glomerular filtration[10] and the alprostadil can inhibit the activity of mononuclear macrophage, formation of immunocomplex and infiltration of inflammatory cells, thus restricting the activity and effect of cytokines and mitigating the inflammatory responses in kidney[11]; most of the chronic renal failure patients suffer from the oxidative stress and thus, ameliorating the oxidative stress in chronic renal failure patients decides the progression of disease; in medication of reduced glutathione, glutathione is a kind of tripeptide consisting of cysteine, glutamic acid and glycine, nearly existing in the cells of all animals, showing the potent antioxidative effect and elimination of free oxygen radicals[12]. Reduced glutathione, as the agon of glyceraldehyde 3-phosphate dehydrogenase, can activate the thiol protease that is conducive to the metabolism of carbohydrates, proteins and lipids[13]. More importantly, reduced glutathione has been confirmed in clinical medical practice to possess the protective effect on the liver function in treatment of liver damage and eliminate the reactive oxygen metabolites to ameliorate the effect of chronic renal failure on the damage to renal function[14]. In conclusion, in treatment of chronic renal failure, regular, long-term administration of alprostadil in combination with reduced glutathione gains promising efficacy with the significant improvement in renal functions and decreases in the adverse events. Thus, this strategy is worthy of being promoted in clinical practice.
| Group | Cases (n) | Nausea | Abdominal distension | Dizziness | Incidence rate of adverse event (%) |
|---|---|---|---|---|---|
| Control group | 49 | 3 (6.1) | 6 (12.2) | 3 (6.1) | 24.4 |
| Observation group | 49 | 2 (4.1) | 1 (2.0) | 0 | 6.1* |
Note: *p<0.05 vs. the incidence rate of adverse events in the control group
Table 1: Comparison of the Incidence Rates of Adverse Events of Patients between Two Groups [n (%)]
| Group | Hb (g/l) | SCr (μmol/l) | 24 hUP (g) | BUN (mmol/l) | |
|---|---|---|---|---|---|
| Control group (n=49) | Before treatment | 85.12±12.37 | 308.13±52.13 | 3.33±0.52 | 21.36±4.79 |
| After treatment | 105.51±20.31* | 231.61±43.31* | 1.71±0.41* | 11.51±2.51* | |
| Observation group (n=49) | Before treatment | 87.19±12.35 | 312.34±56.33 | 3.44±0.57 | 20.13±4.54 |
| After treatment | 120.41±21.91*# | 207.81±41.31*# | 1.11±0.21*# | 10.11±2.91*# | |
Note: *p<0.05 vs. the scores before treatment; #p<0.05 vs. the control group after treatment
Table 2: Comparison of The Indicators of Renal Functions of Patients between Two Groups (mean±SD)
Conflict of interests:
The authors declared no conflict of interest.
References
- Li SX. Effect of Huoxue Yiqi decoction combined with alprostadil on nutritional status and renal function in patients with chronic renal failure. J Pract Chin Med 2018;34(4):453-4.
- Li B. Effect of reduced glutathione combined with alprostadil in the treatment of chronic renal insufficiency. Chin Foreign Med Res 2018;37(2):105-7.
- Wang YZ, Chen XQ, Zhao HR. Effect of salvianolate, alprostadil and glutathione triple therapy on chronic renal failure and its influence on EGFR level. Shaanxi Med J 2018;47(10):1343-5.
- Qiu SP and Wu WZ. Preventive effects of alprostadil combined with glutathione on patients with chronic renal failure. J Clin Exp Med 2016;15(7):654-6.
- Luo L. Clinical observation of alprostadil combined with reduced glutathione in the treatment of early and middle chronic renal failure. Pract Clin J Integr Tradit Chin West Med 2018;18(9):75-6.
- Xu XZ. Alprostadil combined with reduced glutathione in the treatment of chronic renal failure. J Med Theory Pract 2016;29(21):2931-2.
- Saad EA, El-Gayar HA, El-Demerdash RS, Radwan KH. Frankincense administration antagonizes adenine-induced chronic renal failure in rats. Pharmacogn Mag 2018;14(58):634-40.
- Sun Z. Effect of alprostadil combined with calcitriol on serum ang Ⅱ, MCP-1 and CTGF in patients with chronic renal failure. Air Force Med J 2019;35(5):429-31.
- Jiang J, Xue M. Radiofrequency endometrial ablation for treating heavy menstrual bleeding in women with chronic renal failure. Int J Hyperthermia 2018;35(1):612-6.
[Crossref] [Google Scholar] [PubMed]
- Wang F. Effect of alprostadil combined with reduced glutathione on chronic renal failure. Chin J Rural Med 2017;24(12):8-9.
- Du HQ and Wang GQ. Observation of clinical effects of alprostadil combined with glutathione in 60 patients with acute kidney injury (AKI). Shaanxi Med J 2015;44(9):1175-7.
- Lin QY and Cao MG. Clinical observation of alprostadil injection combined with reduced glutathione in the treatment of elderly acute kidney injury. Taiwan Strait Pharm 2016;28(4):129-30.
- You Y. Objective to analyze the clinical effect of alprostadil combined with Shenkang Injection in the treatment of elderly patients with chronic renal failure. J Qiqihar Med Coll 2017;38(3):322-3.
- Peng YY, Huang YY, Liu FJ. Clinical observation of alprostadil combined with rhubarb enema decoction in the treatment of 57 cases of chronic renal failure. Pract Clin J Integr Tradit Chin West Med 2015;15(7):22-3.
 Control group;
Control group;  Observation group
Observation group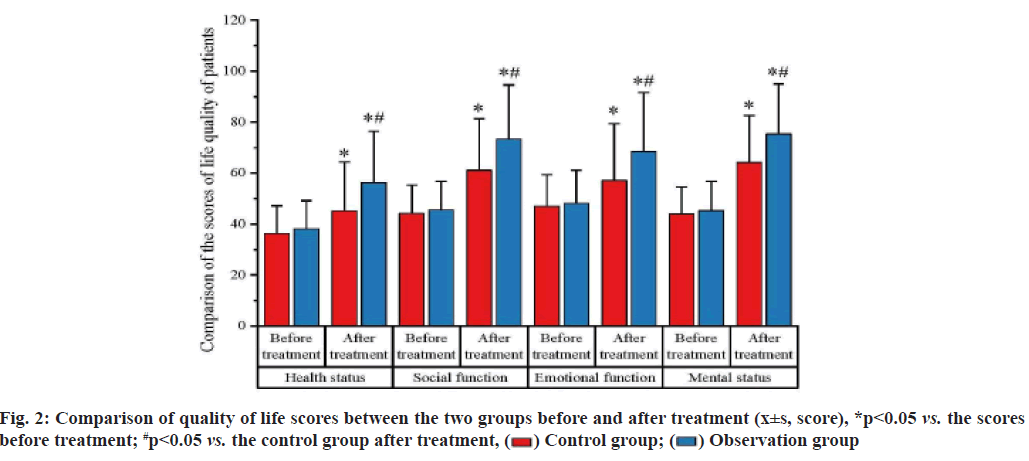
 Control group;
Control group;  Observation group
Observation group



