- *Corresponding Author:
- Z. D. Zheng
Department of Oncology, General Hospital of Northern Theater Command, Wenhua, Shenyang 110840, China
E-mail: zzd_oncologist@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “78-86” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The single use of the programmed cell death protein 1 inhibitor camrelizumab has been studied a lot. However, the comparison of its combined use in various types of advanced lung cancer and whether it has a better application effect has been studied in this experiment and speculate on the immune mechanism after its use. From September 2019 to April 2023 (18 mo including follow-up time), 100 patients with advanced lung cancer treated with camrelizumab were selected and divided into 2 groups according to patient’s interest. 50 patients were divided into camrelizumab (200 mg)+platinum-based chemotherapy as the control group. The 50 patients in the experimental group received camrelizumab (200 mg) and apatinib (250 mg, once/d)+platinum-based chemotherapy. After 2 cycles of treatment, we compared the clinical efficacy of the two groups, observed the adverse drug reactions of the two groups, follow up for 12 mo and observed the progression-free survival time and median survival time of the two groups. In the experimental group the objective response rate=30 % and disease control rate=90 %, and the objective response rate=14 % and disease control rate=40 % in the control group, which means the curative effect of the experimental group was much better than that of the control group (p<0.05). Both groups had some adverse reactions, but they were all in grade 1 or 2 and there was no serious life-threatening (p>0.05). The overall survival time of the control group was 8.44±1.70 mo and the experimental group was 15.78±1.98 mo (p<0.05). The overall survival time of experimental group was slightly longer than that of the control group. Camrelizumab with platinum-based chemotherapy in the control group has a certain application effect. However, camrelizumab with apatinib and platinum-based chemotherapy in the experimental group had better curative effect for patients.
Keywords
Programmed cell death protein 1 inhibitor, camrelizumab, apatinib, lung cancer, platinum-based chemotherapy
Tumor is a disease caused by multiple factors that seriously endangers human health. The incidence of tumors is increasing year by year and the age of onset is gradually younger and the pathological types are diversified. Tumors can be caused by heredity, immune function deficiency, biological, physical, chemical and other factors. The formation of the microenvironment for tumor growth not only depends on some cytokines secreted by itself but also can escape immune surveillance and regulation through certain signaling pathways, thereby making the body immune to tumor cells and leading to rapid tumor progression[1]. Among them, lung cancer is the malignant tumor with the highest mortality rate in our country. Surgery, molecular targeted therapy, chemotherapy and radiotherapy have improved the treatment of lung cancer, but its 5 y overall survival rate is still hovering at 10 %-15 %. Due to the continuous proliferation of lung cancer tumor cells, through morphological changes such as Epithelial-Mesenchymal Transition (EMT), the cells will secrete extracellular enzymes to degrade the Extracellular Matrix (ECM), resulting in intercellular and the mutual adhesion with the surrounding matrix is reduced, which is beneficial for tumor cells to escape from tissue restraint and enter the circulatory system, becoming Circulating Tumor Cells (CTCs) with the ability to invade and metastasize[2].
In recent years, with the improvement of tumor research and treatment technology, it is found that although many tumor patients cannot detect residual tumor cells clinically after treatment, they still die due to tumor recurrence and metastasis. This may be different from imaging alone. The technique cannot detect minimal residual tumor cells. These cells exist in peripheral blood, bone marrow and lymph nodes, and under certain conditions proliferate and infiltrate, causing tumor recurrence and metastasis. The recurrence and metastasis of lung cancer is an important cause of death of patients, and the surviving tumor cells in peripheral blood may be one of the mechanisms causing lung cancer metastasis and recurrence. The current treatment of lung cancer is a comprehensive treatment based on factors such as tumor pathological grade, general condition of patients and tumor development trend.
Immunotherapy has been considered as the most promising cancer treatment. The monoclonal Antibody (mAb) immunotherapy targeting coinhibitory immune checkpoints has achieved remarkable efficacy in a variety of areas malignancies, including melanoma, Non-Small Cell Lung Cancer (NSCLC), Renal Cell Carcinoma (RCC), bladder cancer, etc.[3]. Programmed Cell Death Protein 1 (PD-1) is an immunosuppressive molecule, which interacts with the ligands Programmed Cell Death-Ligand 2 (PD-L2) and Programmed Cell Death-Ligand 1 (PD-L1), which can lead to the apoptosis of tumor antigen-specific T cells, so that tumor cells escape the body’s immune surveillance[4]. PD-L1 belongs to the B7- H1 molecular family and is expressed in normal T, B cells, Dendritic Cells (DCs), monocytes and macrophages, natural killer cells and activated vascular endothelial cells[5]. The expression of PDL1 can also be detected on the surface of many human tumor cells, such as lung cancer, malignant melanoma, breast, gastric, esophageal, pancreatic cancer, RCC, etc. The expression level of PD-L1 was significantly upregulated[6]. By blocking this pathway, the activation of T cells is increased and finally tumor cells can be killed. This process has become a new direction of immunotherapy. A number of clinical studies have shown that PD-1/ PD-L1 inhibitors are effective in the treatment of NSCLC, melanoma, kidney cancer and other tumors. The corresponding Immune Checkpoint Inhibitors (ICIs) such as pembrolizumab, nivolumab etc., have been applied clinically and achieved remarkable results[7,8]. At the same time, the PD-1 inhibitor independently developed by China-Toripalimab[9], sintilimab[10] and camrelizumab[11] have also been approved for marketing towards the treatment of malignant tumors. Camrelizumab is a humanized Immunoglobulin G4 (IgG4) type mAb[12]. It can target PD-1, block its interaction with PD-L1 and PD-L2, restore the immune function of the body and finally play an anti-tumor role[13,14]. After being approved for marketing on May 29, 2019, it is used for the treatment of relapsed and refractory classical Hodgkin’s lymphoma, Esophageal Squamous Cell Carcinoma (ESCC), Hepatocellular Carcinoma (HCC), Nasopharyngeal Cancer (NPC), NSCLC, Gastric Cancer (GC), Esophagogastric Junction Cancer (EGJC) and other malignant tumors exhibits good antitumor potential[11].
Although in recent years, many authors have studied related research on camrelizumab, however, this study will combine camrelizumab and apatinib with platinum chemotherapy. At present, there are very few data analyzing the efficacy and safety of their combined use on NSCLC. To study the combined effect of camrelizumab by observing the application effect of treatment on patients with NSCLC and speculating on its immune mechanism, it has laid a strong foundation for future research on the immune mechanism of clinical treatment.
Materials and Methods
General information:
This is a prospective study, in which we selected 100 patients with advanced lung cancer who were hospitalized in our hospital from September 2019 to April 2023 (including 18 mo follow-up time). 100 patients of advanced lung cancer were divided according to Tumor, Nodes and Metastases (TNM) staging method; Stage I T1-2N0M0, stage II T1-2N1M0, stage IIIA T1-2N0-2M0, stage IIIB TN3M0 or T4NM0 and stage IV TNM1. Out of 100 patients of histologically confirmed stage III-IV NSCLC, we selected 65 patients of adenocarcinoma and 35 patients of squamous cell carcinoma, and among them, 30 patients were in stage IIIA, 36 were in stage IIIB, and 34 were in stage IV. All patients signed the informed consent. All patients were separated with experimental and control group according to the patient's wishes. The control group includes 50 patients, aged (40-75) y, with an average of (51.3±7.85) y old and experimental group with 50 patients, aged (39-74 ) y, with an average of (52.84±7.67) y old. Among the patients in this study, there were 63 males and 37 females. There was no noteworthy difference in general data such as age, gender etc., between the two groups before treatment (all p>0.05).
Inclusion criteria:
Patients in line with the advanced diagnostic criteria of lung cancer and should be diagnosed as advanced by at least 1 measurable lesion; fair physical strength, expected to tolerate chemotherapy and immunotherapy; the expectation of survival time is >3 mo and histologically confirmed stage III-IV NSCLC patients.
Exclusion criteria:
Patients combined with severe dysfunction of organs such as heart, liver, kidney etc., autoimmune system disease or severe coagulation dysfunction; patients who took previously chemotherapy and other anti-tumor therapies; mental illness; lactating or pregnant women and systemic immune diseases were excluded from the study.
Treatment method:
The patients were divided into control group and experimental group. Patient does voluntarily join the experimental group according to their wishes. Patients in the 2 groups were given camrelizumab (Suzhou Suncadia Biopharmaceuticals, production batch number 2019S00365, 200 mg). In the control group patients were given camrelizumab (200 mg)+platinum chemotherapy and in the experimental group, 50 patients were given combined treatment with camrelizumab (200 mg) and apatinib (250 mg, once/d)+platinum-based chemotherapy. The course of treatment was 21 d and treatment cycle is for 3 w until the disease progresses or unacceptable adverse reactions occur. Adverse reactions occurred during chemotherapy are closely monitored and symptomatic treatment is given in a timely manner. All patients were given additional treatment according to the abovementioned administration method and the original treatment plan of the patients was not changed arbitrarily.
Experimental indicators:
By observing the curative effect and adverse reactions of the 2 groups of patients after treatment, and according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria, we evaluated the results, including Complete Response (CR), Partial Response (PR), Stable Disease (SD) and Disease Progression (PD).
Objective Response Rate (ORR)=(CR+PR)/total number of cases×100 %.
Disease Control Rate (DCR)=(CR+PR+SD)/total number of cases×100 %.
Evaluation criteria for adverse reactions:
Adverse reactions were evaluated based on the general toxicity criteria of the National Cancer Institute of the United States 4.0.
Statistical analysis:
We used Statistical Package for the Social Sciences (SPSS) 21.0 software for statistical analysis and the data was measured by mean±standard deviation (x±s), and t test was used for comparison of data between the two groups. Technical data is shown in cases or percentages where p<0.05 and the difference between the two groups is found to be statistically significant.
Results and Discussion
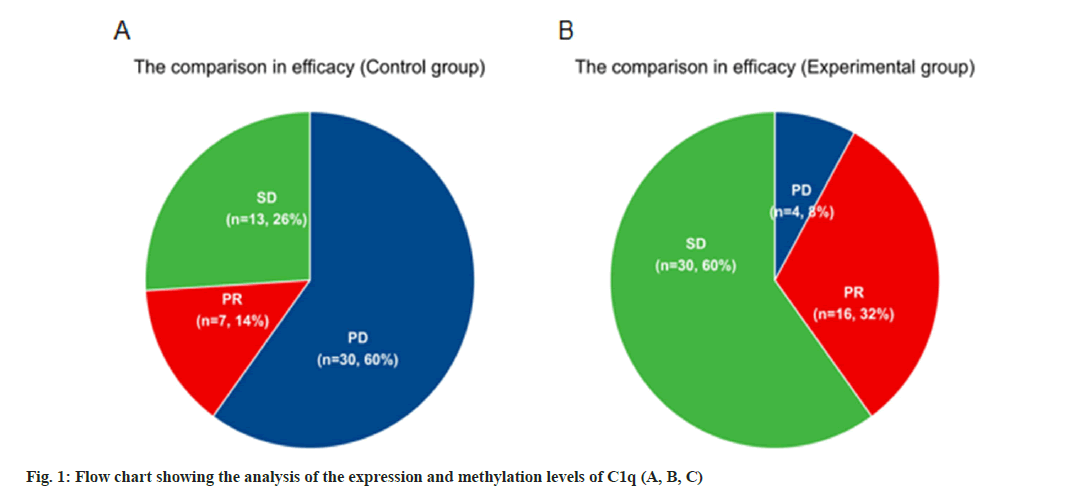
Comparison of clinical efficacy between the two groups is shown in Table 1. 100 patients were separated with experimental and control group according to their wishes, 50 patients in each group, where the curative effect after chemotherapy was evaluated. According to Table 1, we can conclude that there was no case of complete remission in the two groups of patients. Comparing the two groups of patients with PR, SD and PD, the number of patients in the experimental group was significantly more than that in the control group. It can be considered that camrelizumab has a certain effect on disease control and remission in patients with advanced lung cancer as shown in fig. 1. The numerical results were all p<0.05. The ORR=30 % and DCR=90 % in the experimental group, and the ORR=14 % and DCR=40 % in the control group.
| Efficacy | Control group (patients) | Experimental group (patients) | χ2 | p value |
|---|---|---|---|---|
| R | 0 | 0 | - | - |
| PR | 7 | 16 | 4.574 | 0.032 |
| SD | 13 | 30 | 11.791 | 0.001 |
| PD | 30 | 4 | 20.617 | <0.0001 |
Note: p<0.05, compared to control group
Table 1: The Comparison of efficacy between The two groups
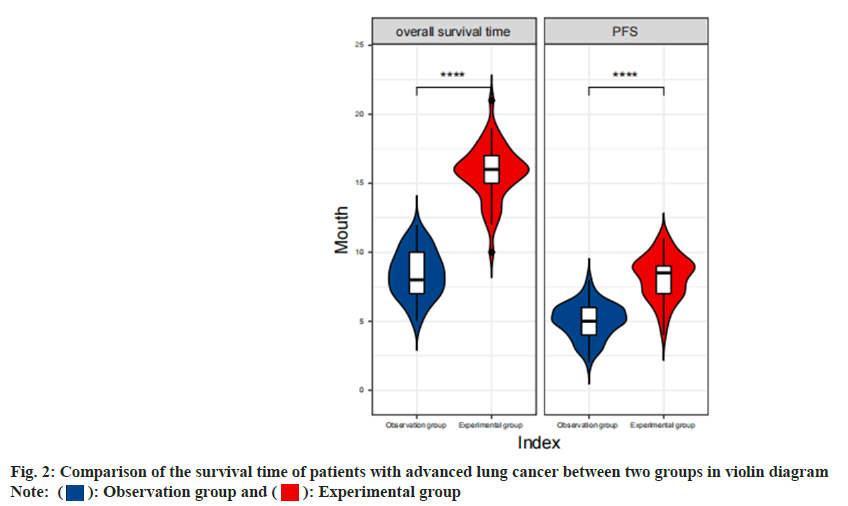
Comparison of the survival time of patients with advanced lung cancer between two groups was shown in Table 2. The analysis of the control group and the experimental group, based on the time of survival of patients with advanced lung cancer, shows that the Progression-Free Survival (PFS) time and overall survival time of the patients in the experimental group are longer than the control group as shown in fig. 2 (p<0.05).
| Group | PFS* | Overall survival time |
|---|---|---|
| Control group | 5.02±1.25 | 8.44±1.70 |
| Experimental group | 8.18±1.59 | 15.78±1.98 |
| t | 11.048 | 19.888 |
| p | 0.001 | 0.001 |
Note: *PFS: Progression Free Survival Time, n=36 mo
Table 2: Comparison of survival time of Patients with advanced lung cancer between two groups
Adverse reactions and safety of the two groups of patients were shown in Table 3. Among 50 patients included in the experimental group, adverse reactions of varying degrees occurred, among which the most common adverse reactions were abnormal blood (42 %), cough (34 %), rash (22 %), fervescence (20 %), hypodynamic (16 %), gastroenteric reaction (14 %), anemia (12 %), liver and kidney function damage (10 %) and itching (8 %). One of the more special ones is Reactive Cutaneous Capillary Endothelial Proliferation (RCCEP), which is a unique adverse reaction occurred after using camrelizumab in combination with chemotherapy. In the control group, this adverse reaction accounted for 50 %. However, in the experimental group, we used camrelizumab combined with apatinib, an anti-vascular targeted drug and we can clearly see that the adverse reaction rate of RCCEP has decreased significantly (22 %). Adverse reactions were all grade Ⅰ-Ⅱ and no patients died due to adverse drug reactions. The specific data of adverse reactions are shown in Table 3.
| Main side effects | I | II | III | IV | Total |
|---|---|---|---|---|---|
| Hypodynamic | 6 | 2 | 0 | 0 | 8 |
| Fervescence | 7 | 3 | 0 | 0 | 10 |
| Gastroenteric reaction | 5 | 2 | 0 | 0 | 7 |
| Anemia | 4 | 2 | 0 | 0 | 6 |
| Cough | 11 | 6 | 0 | 0 | 17 |
| Itching | 3 | 1 | 0 | 0 | 4 |
| Liver and kidney function damage | 4 | 1 | 0 | 0 | 5 |
| Abnormal blood | 13 | 8 | 0 | 0 | 21 |
| Rash | 8 | 3 | 0 | 0 | 11 |
| RCCEP | 7 | 4 | 0 | 0 | 11 |
Table 3: Comparison of adverse reactions of Camrelizumab in the treatment of advanced Lung Cancer
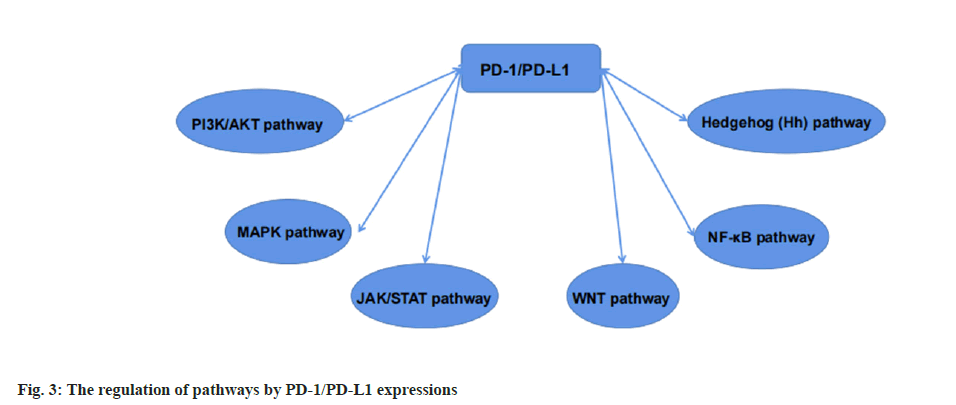
Immune mechanism of camrelizumab in advanced lung cancer was shown in fig. 3. PD-1/PD-L1 can be regulated by various signals in cancer cells, playing a key role in tumorigenesis. Therefore, we need to understand the current basic pathways for future research on immune pathways.
Currently marketed PD-1 inhibitors are all humanized or fully humanized mAbs. Among the four PD-1 inhibitors approved for medical insurance in our country, Toripalimab, camrelizumab and tislelizumab are humanized mAbs, and sintilimab is fully humanized mAb. Our research is mainly about camrelizumab, an IgG4 kappa (κ) humanized mAb for the efficacy and signaling pathway of advanced lung cancer, so we established an immune mechanism based on the PD-1/PD-L1 signaling pathway diagram. Studies have proposed that the combination therapy that blocks multiple immune regulatory pathways can further improve the therapeutic efficacy of PD-1 alone. Like we said above, a cell surface receptor, PD-1 is expressed on T cells and pre-B cells. The combination of PD-1 with two ligands which are PD-L1 and PD-L2 is very useful in the cancer. PD-1 is an immune checkpoint that modulates immune system responses by downregulating the immune system and promoting self-tolerance by inhibiting the activity of T cell inflammatory.
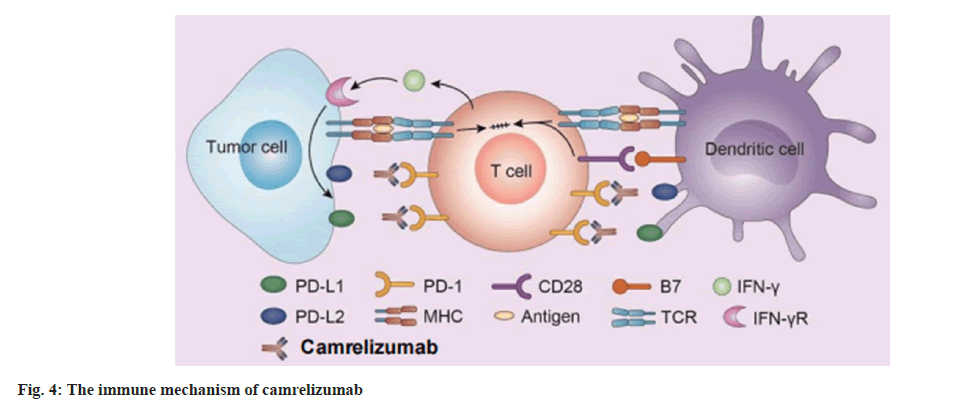
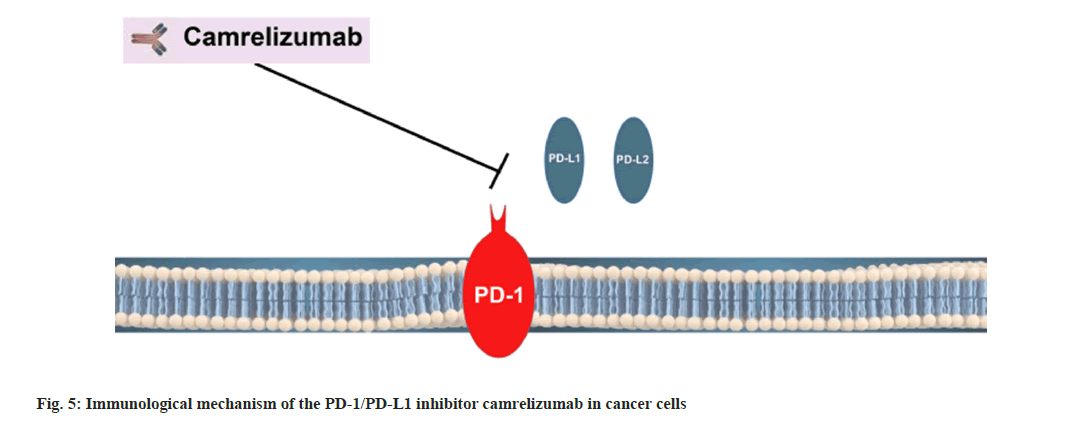
So, the pathway is probably to control the level of inflammation in the place where the antigen is expressed and finally to ensure that normal tissues are not damaged. PD-1 protein is significantly expressed on the T cells surface. When T cells recognize antigens expressed by the Major Histocompatibility Complex (MHC) on target cells, they will produce inflammatory cytokines and promote inflammation. Camrelizumab then prevents its interaction in the PD-1/PD-L1 pathway, thereby promoting the immune response as shown in fig. 4 and fig. 5.
At present, the 3 y survival rate of advanced lung cancer is extremely low in China. Chemotherapy is a common method for the advanced lung cancer treatment, but the overall therapeutic effect still needs to be further improved[15,16]. As various immunotherapies have achieved good results in various tumor diseases, a variety of immunosuppressants with specific anti-tumor effects have also been successfully developed and gradually applied in clinical practice[17,18]. Among them, camrelizumab is a humanized anti- PD-1 antibody, which forms the basis of tumor immunotherapy by blocking related pathways. We already know that studies have proposed that combination therapy that blocks multiple immune regulatory pathways can further improve the therapeutic efficacy of PD-1 alone. Therefore, this study mainly uses the PD-1 inhibitor camrelizumab for experiments.
The results of this study show that in clinical experimental, for patients with advanced lung cancer, the curative effect of camrelizumab in the experimental group is better than that in the control group, especially reflected in the experimental group where ORR=30 % and DCR= 90 %. In the control group, ORR=14 % and DCR=40 %. It can be clearly seen that the curative effect of the experimental group is significantly better than that of the control group (p<0.05). This is consistent with research findings in recent years[19,20]. However, both groups had a certain degree of adverse reactions, but they were all grade 1 or 2, and there was no serious life-threatening situation (p>0.05). The overall survival time was 8.44±1.70 in the control group and 15.78±1.98 in the experimental group (p<0.05). This indicates that the overall survival time of the experimental group was slightly longer than that of the control group. This result is not much different from that of this study[21,22]. And camrelizumab can block the PD-1 and PD-L1 signaling pathways, activate T cell activation and exert anti-tumor properties[23].
In addition, camrelizumab is a selective, highaffinity IgG4 mAb. PD-1, relieves T cell immunosuppression, induces T lymphocyte activation and rebuilds the body’s immune system. Related studies have found that camrelizumab can activate epidermal growth factor receptor-2, thereby inhibiting tumor angiogenesis. The results of our study indicated that the patient did have some adverse reactions, but none of them reached grade IV severity. The patients mainly had grade 1-2 adverse reactions, which were all tolerated during chemotherapy and recovered after symptomatic treatment. A clinical study has found that most of the adverse reactions caused by camrelizumab are reversible and can be relieved after steroid therapy. The results of this study showed that the PFS rate and median survival time of the experimental group were better than the control group, which further indicated that camrelizumab combined with basic chemotherapy drugs could prolong the survival time. There are also many studies that also show some applications of camrelizumab in advanced lung cancer, as well as prolonging the survival time of patients and so on. The study shows that camrelizumab increases median survival time to 6 mo in patients with advanced non-squamous NSCLC[24]. Another study reported that the second-line treatment of camrelizumab combined with apatinib can prolong the survival time of patients[25]. Therefore, the use of camrelizumab in the treatment of patients with advanced NSCLC can prolong the survival time.
PD-1 is a type I transmembrane protein mainly expressed on the surface of T cells, B cells and macrophages, and provides inhibitory signals to activated T cells. PD-L1 is a ligand of PD-1 and an important immunosuppressive molecule. Therefore, when PD-1 binds to PD-L1, through phosphorylation of Phosphatidylinositol-3-Kinase (PI3K), further activation of Protein kinase B (Akt), activation of stimulatory T cell signaling pathway, glucose metabolism and the secretion of Interferon (IFN), etc., leads to the blocking of the downstream signal of T cell activation, thereby effectively inhibiting the T cells transcription and finally inhibiting the function of immune T cells, triggering a negative immune response, so that tumor cells can get rid of the immune surveillance of the body, plays a vital role in the negative regulation of the immune response. By blocking PD-1/PD-L1 pathway, PD-1 inhibitors can upregulate the activation of T cells and activate the endogenous anti-tumor immune response, thereby exerting a therapeutic effect on tumors and improving the ability to kill tumors[26]. This is also the immune mechanisms involved in the advanced lung cancer treatment by camrelizumab.
The results of this study showed that for patients with advanced NSCLC camrelizumab combined with apatinib in the experimental group had a better curative effect. The patients mainly experienced grade 1-2 adverse reactions, which were tolerated during chemotherapy and recovered after symptomatic treatment. The PFS rate and median survival time of the experimental group were better than those of the control group, further indicating that the combination of camrelizumab and apatinib can prolong more survival time, and the control group alone uses camrelizumab compared to experimental group.
Limitations of the study were stated here. Immunotherapy is a promising treatment approach that may confer further survival benefits in some patients. The dominance of immunotherapies based on PD-1/PD-L1 blockade in various tumors seems like a next logical step. But there are still many unknown facts, including the use of specific doses, safety (that is, what aspects of pharmacological toxicity are specifically displayed), durability and persistence that were not covered in this study.
To sum up, it is undeniable that the marketing time of camrelizumab is still relatively short compared with foreign PD-1 inhibitors and the experience and data of clinical use is only single drug combined with chemotherapy, so its experience with combined use is limited. In the future, more adverse drug reactions will be reduced and individualized immunotherapy will be realized. It is believed that China’s self-innovated PD-1 inhibitors can bring hope to patients with malignant tumors and will also play an epoch-making role in cancer prevention and treatment.
Conflict of interests:
The authors declared no conflict of interest.
References
- Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer 2006;119(2):317-27.
[Crossref] [Google scholar] [PubMed]
- Fidler IJ. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat Rev Cancer 2003;3(6):453-8.
[Crossref] [Google scholar] [PubMed]
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018;118(1):9-16.
[Crossref] [Google scholar] [PubMed]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25(21):9543-53.
[Crossref] [Google scholar] [PubMed]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8(6):467-77.
[Crossref] [Google scholar] [PubMed]
- Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003;170(3):1257-66.
[Crossref] [Google scholar] [PubMed]
- Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf 2019;42:281-94.
[Crossref] [Google scholar] [PubMed]
- Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 2019;38(1):1-2.
[Crossref] [Google scholar] [PubMed]
- Keam SJ. Toripalimab: First global approval. Drugs 2019;79(5):573-8.
[Crossref] [Google scholar] [PubMed]
- Hoy SM. Sintilimab: First global approval. Drugs 2019;79(3):341-6.
[Crossref] [Google scholar] [PubMed]
- Markham A, Keam SJ. Camrelizumab: First global approval. Drugs 2019;79(12):1355-61.
[Crossref] [Google scholar] [PubMed]
- Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: A dose-escalation, phase 1 study. Br J Cancer 2018;119(5):538-45.
[Crossref] [Google scholar] [PubMed]
- Guzik K, Tomala M, Muszak D, Konieczny M, Hec A, Błaszkiewicz U, et al. Development of the inhibitors that target the PD-1/PD-L1 interaction-A brief look at progress on small molecules, peptides and macrocycles. Molecules 2019;24(11):2071.
[Crossref] [Google scholar] [PubMed]
- Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J 2018;24(1):47.
[Crossref] [Google scholar] [PubMed]
- Kuang CL, Li M, Zheng TH. Meta-analysis of the efficacy and safety of apatinib in the treatment of advanced non-small cell lung cancer. China J Mod Med 2021;(8):70-7.
- Qingqing Z, Chao X, Bao S, Qiujing Z, Jie L. Application of metronomic chemotherapy in the treatment of advanced non-small cell lung cancer. J Int Oncol 2019;46(6):374.
- Friedenreich CM, Barberio AM, Pader J, Poirier AE, Ruan Y, Grevers X, et al. Estimates of the current and future burden of cancer attributable to lack of physical activity in Canada. Prev Med 2019;122:65-72.
[Crossref] [Google scholar] [PubMed]
- Tian H, Wang P. Development of the attributional style of doctor questionnaire. Psychol Res Behav Manag 2020;13:1079-88.
[Crossref] [Google scholar] [PubMed]
- Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed vs. chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multi-centre, phase 3 trial. Lancet Respir Med 2021;9(3):305-14.
[Crossref] [Google scholar] [PubMed]
- Qiao L, Zhou Z, Zeng X, Tan C. Cost-effectiveness of domestic PD-1 inhibitor camrelizumab combined with chemotherapy in the first-line treatment of advanced non-squamous non-small cell lung cancer in China. Front Pharmacol 2021;12:728440.
[Crossref] [Google scholar] [PubMed]
- Chen T, Xie R, Zhao Q, Cai H, Yang L. Cost-utility analysis of camrelizumab plus chemotherapy vs. chemotherapy alone as a first-line treatment for advanced non-squamous non-small cell lung cancer in China. Front Oncol 2022;12:746526.
[Crossref] [Google scholar] [PubMed]
- Pu X, Wang Q, Liu L, Chen B, Li K, Zhou Y, et al. Rh‐endostatin plus camrelizumab and chemotherapy in first‐line treatment of advanced non‐small cell lung cancer: A multicenter retrospective study. Cancer Med 2023;12(7):7724-33.
[Crossref] [Google scholar] [PubMed]
- Baczewska B, Block B, Kropornicka B, Niedzielski A, Malm M, Zwolak A, et al. Hope in hospitalized patients with terminal cancer. Int J Environ Res Public Health 2019;16(20):3867.
[Crossref] [Google scholar] [PubMed]
- Zhou C, Gao G, Wu F, Chen X, Li W, Xiong A, et al. A phase Ib study of SHR-1210 plus apatinib for heavily previously treated advanced non-squamous Non-Small Cell Lung Cancer (NSCLC) patients. J Clin Oncol 2018;36(15):21017.
- Yuan GS, He WM, Hu XY, Li Q, Zang MY, Cheng X, et al. Clinical efficacy and safety analysis of camrelizumab combined with apatinib as a second-line therapy for unresectable hepatocellular carcinoma: A multicenter retrospective study. Chin J Hepatol 2021;29(4):326-31.
[Crossref] [Google scholar] [PubMed]
- Cheng X, Veverka V, Radhakrishnan A, Waters LC, Muskett FW, Morgan SH, et al. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem 2013;288(17):11771-85.
[Crossref] [Google scholar] [PubMed]
 ): Observation group and (
): Observation group and ( ): Experimental group
): Experimental group