- *Corresponding Author:
- Tong Li
Nanning Second People’s Hospital of Neurology, Nanning 530031, China
E-mail: www.litong.163@163.com
| This article was originally published in a special issue, “Clinical Research in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2021:83(1)spl issue “198-202”. |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study focuses on clinical effect of alteplase in the treatment of acute cerebral infarction. 100 cases of patients with acute cerebral infarction admitted to our hospital during 2014-2015 were randomly selected as study objects and divided into groups according to different therapeutic methods, with 50 cases in the observation group and the control group respectively. The control group received conventional non thrombolytic therapy. On this basis, the observation group received thrombolytic therapy with alteplase. Efficacy of the two groups was compared. Clinical efficacy of the observation group was more significant than that of the control group, with adverse reaction rates lower than that of the control group. Application of alteplase in treatment of acute cerebral infarction is with significant efficacy and high safety.
Keywords
Alteplase, acute cerebral infarction, efficacy, safety
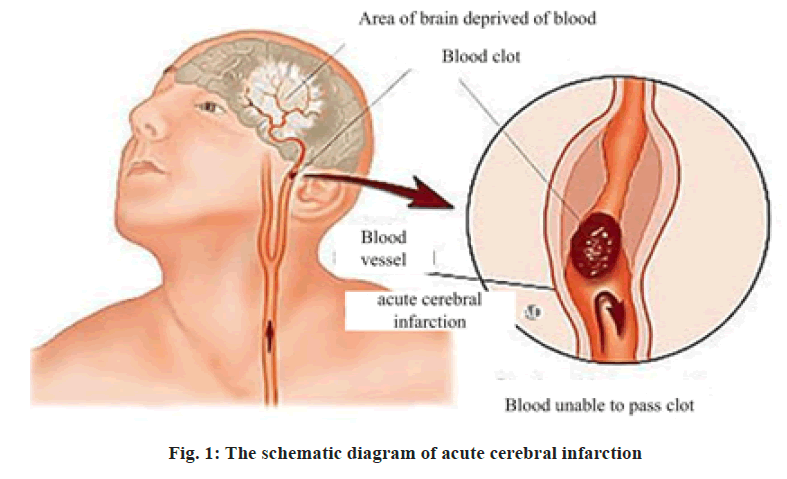
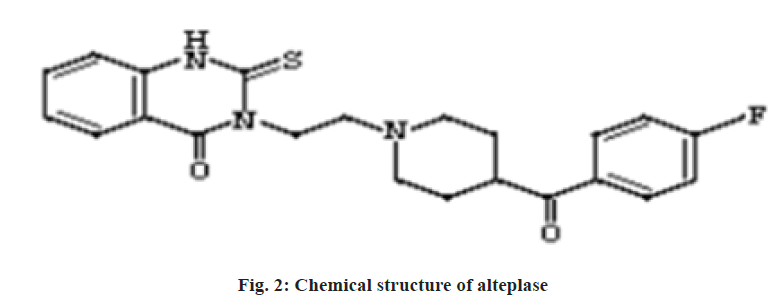
Acute cerebral infarction is a very common clinical stroke disease. According to relevant statistics, incidence of acute cerebral infarction shows an increasing trend in recent years. The main cause of acute cerebral infarction is patient’s abnormal brain blood circulation, which is related to high blood pressure, diabetes and genetic factors. Acute cerebral infarction features a sudden onset, which normally happens during rest or sleep. The early symptoms include headache, dizziness, tinnitus, nausea, vomiting and dysphagia. Cerebral infarction should be treated earlier, because most patients with cerebral infarction are old and infirm, with involvement of various chronic diseases. During treatment, much attention should be paid to medication. Infarct area may expand within short time, leading to various complications. Therefore, cerebral infarction will not only impact life quality of patients, but also may lead to disability or death of patients. Researches have been reported that the effective rate in treating acute cerebral infarction with alteplase reached 93.75 %, while the rate of untoward effects was only 6.25 %.To reduce dangerousness in the treatment process of acute cerebral infarction, the author selected 100 cases of patients with acute cerebral infarction admitted in our hospital in recent years as study objects to conduct investigation and explore clinical efficacy of alteplase in treatment of acute cerebral infarction, with detailed study as reported below. 100 cases of patients with acute cerebral infarction admitted to our hospital during 2014 and 2015 were randomly selected as study objects; retrospective analysis of clinical treatment of patients was conducted. Patients were divided into different groups according to different therapeutic methods for patients with acute cerebral infarction. 50 cases of patients receiving conventional non thrombolytic therapy were assigned to the control group, on the treatment basis and another 50 patients receiving alteplase therapy were assigned to the observation group. Among the 50 patients with acute cerebral infarction in the control group, there were 29 cases of male patients, 21 cases of female patients, the maximum age of patients was 80 y old, the minimum age was 44 y old and the average age was (62.2±2.1) y old. The minimum course of disease of patients was 1 h, the longest course of disease was 4.5 h, the average course of disease was (2.6±0.3) h. Among the 50 patients with acute cerebral infarction in the research group, there were 28 cases of male patients, 22 cases of female patients, the maximum age of patients was 79 y old, the minimum age was 46 y old, the average age was (62.3±1.9) y old, the minimum course of disease of patients was 1.5 h, the longest course of disease was 4 h, the average course of disease was (2.4±0.7) h. Difference in gender, age and course of disease of the two groups of patients with acute cerebral infarction was p>0.05. Selection criteria of study object: Between the ages of 18 to 80 y old, occurrence of acute cerebral infarction is within 4.5 h, after shown by computed tomography (CT) imaging examination, there is brain hemorrhage in the patients in low density change state; National Institutes of health stroke scale, or NIH stroke scale (NIHSS) score is between 22 points to 7 points; there is no conscious disorder, the patient’s blood pressure is 180/100 mmHg or less; there is no history of intracranial hemorrhage, the patients haven’t received anticoagulant therapy, with complete cardiac, liver and kidney function, accordance with clinical diagnostic criteria of acute cerebral infarction. The schematic diagram fig. 1 shows the acute cerebral infarction disease. 50 cases of patients with acute cerebral infarction in the control group adopt platelet inhibitor, free radical scavenger and enteric aspirin in treatment. First oral dose of aspirin is 300 mg, afterwards, oral dose is 100 mg/d; for patients with cerebral edema, give intravenous drip therapy with mannitol of 20 % concentration. For patients who cannot eat, provide nutritional fluid infusion support[1]. On this basis, 50 patients with acute cerebral infarction in the observation group receive alteplase therapy. Take 5 mg of alteplase and dissolve in 10 ml of normal saline, conduct intravenous injection on patients and then take 45 mg of alteplase to dissolve in 100 ml of normal saline, conduct intravenous drip up to 1 h on patients. Within the day of alteplase therapy, therapy of aspirin and clopidogrel[2-4] is not allowed. Observation index: Record NIHSS score of two groups of acute cerebral infarction patients before and after treatment, as well as adverse reactions of patients during treatment[5-7]. Efficacy evaluation index: With a view on NIHSS score of acute cerebral infarction, clinical efficacy of this study is divided into three levels, namely, effective, ineffective and markedly effective[8-11]. Markedly effective: After treatment, various vital clinical signs of the patients were improved significantly and recovered to normal, the biochemical indicators also returned to normal, NIHSS score decreased more than 90 %[12], effective: after treatment, various vital clinical signs of the patients were further improved, the biochemical indicators were also basically returned to normal, NIHSS score decreased by about 40 %-70 %. Ineffective: after treatment, there was no change in various vital clinical signs and biochemical indicators, NIHSS score decreased less than 18 %. All the data obtained from this study are processed and analyzed with statistical software SPSS 18.0, the measurement data is shown with (+s), test is with t test, p<0.05 means that comparative difference is statistically significant. Before treatment, difference between NIHSS score of the patients in the observation group and that of patients in the control group is p>0.05, after treatment of 6 h, 24 h and 7 d, NIHSS score of the patients in the observation group is significantly lower than that of the patients in the control group. NIHSS score difference between the two groups of patients with acute cerebral infarction is p<0.05 (Table 1). After treatment, the overall efficiency of acute cerebral infarction patients in the observation group was 98 %, while that of acute cerebral infarction patients in the control group was 78 %. This shows that clinical efficacy of 50 cases of patients in the observation group is significantly higher than that of the control group and efficacy difference between two groups of patients with acute cerebral infarction was p<0.05 (Table 2). In total, 1 patient in the observation group had adverse reactions manifested as bleeding gums, with adverse reaction rate at 2 % and conducted blood routine examination, infinite impulse response (IIR) function tests and liver function tests for patients with adverse reactions, the results were normal. A total of 6 patients in the control group had adverse reactions, with 2 cases of bleeding gums, 4 cases of cerebral hemorrhage, with adverse reaction rate at 12 %. Difference in incidence rate of adverse reaction between the two groups of patients with acute cerebral infarction is p<0.05. Acute cerebral infarction is a common emergency among the elderly, with high disability and mortality rate. In recent years, incidence of acute cerebral infarction has been high. Without timely and effective treatment after suffering from the disease, there will be great impact on patient’s life and prognosis. Patients with cerebral infarction deposit a lot of cholesterol and fatty degeneration in arterial intima in early stage, which forms atherosclerotic plaque, the clot is prone to rupture hemorrhage, which easily leads to platelet aggregation, thereby increasing the degree of obstruction in brain blood vessel of patients, inducing acute cerebral infarction. Thus, in the clinical treatment of patients with acute cerebral infarction, to improve the clinical efficacy of patients, thrombolytic therapy is necessary for patients. Alteplase is a common clinical thrombolytic drug, with glycoprotein as its main ingredient and containing 526 amino acids. The fig. 2 below shows the chemical structure of alteplase. Alteplase is a relatively commonly used clinical thrombolytic drug, which activates plasminogen combined with fibrous protein by combining fibrous protein and lysine residue and then changes into plasmin. The schematic diagram, fig. 3 below shows how alteplase dissolves thrombus. Since alteplase will selectively activate plasminogen, in application of alteplase in thrombolytic therapy of patients with acute cerebral infarction, the probability that patients have hemorrhagic adverse reaction is relatively small, the safety and reliability are very high. In this study, the author applied alteplase in clinical treatment of 50 cases of patients with acute cerebral infarction in the observation group. The research data showed that patients in the observation group not only had significantly better clinical efficacy than those in the control group, but also had better neurologic impairment treatment effect than the control group. Besides, during treatment, the probability of adverse reactions in 50 cases of patients with acute cerebral infarction in the observation group was significantly lower than that of patients in the control group. The research result is close to clinical research result of domestic scholar Yanqing Zhang, which demonstrates reliability of the result of this study. In summary, based on the use of platelet inhibitors, free radical scavengers and enteric aspirin for treatment, application of alteplase in patients with acute cerebral infarction not only helps patients with acute cerebral infarction improve clinical treatment effect, relieve neurological impairment, but also has relatively high safety and thus it is worthy of being promoted and applied in clinical practices.
| Group | n | Before treatment | Treatment for 6 h | Treatment for 24 h | Treatment for 7 d |
|---|---|---|---|---|---|
| Observation group | 50 | 17.3±3.4 | 11.7±2.1 | 8.1±1.1 | 4.6±2.1 |
| Control group | 50 | 16.4±3.7 | 14.2±3.4 | 12.4±3.2 | 8.2±4.9 |
| t | - | 0.48 | 2.49 | 5.19 | 3.71 |
| p | - | >0.05 | <0.05 | <0.05 | <0.05 |
Table 1: Nihss Score (Points) of Two Groups of Patients with Acute Cerebral Infarction Before and After Treatment
| Group | n | Markedly effective | Effective | Ineffective | Total effective rate (%) |
|---|---|---|---|---|---|
| Observation group | 50 | 28 | 21 | 1 | 98 % |
| Control group | 50 | 22 | 17 | 11 | 78 % |
| t | - | 3.31 | 2.43 | 3.18 | 6.845 |
| p | - | <0.05 | <0.05 | <0.05 | <0.05 |
Table 2: Clinical Efficacy of the Two Groups of Patients with Acute Cerebral Infarction
Acknowledgements
The establishment and application research of thrombolysis quality management system of cerebral infarction in Nanning city (number: 20133180)
Conflicts of Interest:
The authors declared no conflict of interest.
References
- Cheng CY, Chen SH, Chen HM, Li CJ, Liu TY, Tan TY. Impact of estimated-weight-base dose of alteplase in acute stroke treatment on clinical outcome. J Clin Neurosci 2021;85:101-5.
- Maznyczka AM, McCartney P, Duklas P, McEntegart M, Oldroyd KG, Greenwood JP, et al. Effect of coronary flow on intracoronary alteplase: a prespecified analysis from a randomised trial. Heart 2021;107(4):299-312.
- Hebert S, Clavel P, Maier B, Mizutani K, Delvoye F, Lapergue B, et al. Benefits and Safety of Periprocedural Heparin During Thrombectomy in Patients Contra-Indicated for Alteplase. J Stroke Cerebrovasc Dis 2020;29(10):105052.
- Gottula AL, Barreto AD, Adeoye O. Alteplase and Adjuvant Therapies for Acute Ischemic Stroke. Semin Neurol 2021;41(1):16-27.
- Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA 2021;325(3):234-43.
- Bao H, Gao HR, Pan ML, Zhao L, Sun HB. Comparative study on the efficacy and safety of alteplase and urokinase in the treatment of acute cerebral infarction. Technol Health Care 2021;29(1):85-90.
- Psychogios K, Palaiodimou L, Katsanos AH, Magoufis G, Safouris A, Kargiotis O, et al. Real-world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther Adv Neurol Disord 2021;14.
- Ganesh A, Kashani N, Ospel JM, Wilson AT, Foss MM, Saposnik G, et al. Endovascular therapy or alteplase in patients with comorbidities: insights from UNMASK EVT. Can J Neurol Sci 2021;48(1):77-86.
- Ohara T, Menon BK, Al-Ajlan FS, Horn M, Najm M, Al-Sultan A, et al. thrombus migration and fragmentation after intravenous alteplase treatment: The INTERRSeCT study. Stroke 2021;52(1):203-12.
- Ospel JM, Singh N, Almekhlafi MA, Menon BK, Butt A, Poppe AY, et al. Early recanalization with alteplase in stroke because of large vessel occlusion in the escape trial. Stroke 2021;52(1):304-7.
- Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 2012;366(12):1099-107.
- Coutts SB, Berge E, Campbell BC, Muir KW, Parsons MW. Tenecteplase for the treatment of acute ischemic stroke: a review of completed and ongoing randomized controlled trials. Int J Stroke 2018;13(9):885-92.