- *Corresponding Author:
- G. Guo
Department of Otolaryngology Head and Neck Surgery, Beijing Army General Hospital, Dongcheng, Beijing 100700, China
E-mail: pengwei8836579@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “40-48” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this work was to identify novel downstream targets and evaluate the clinically interpretable significance of long noncoding RNA KCNQ1OT1 expression in the prognosis of nasopharyngeal cancer. Patient with nasopharyngeal cancer (n=46) and health volunteer (n=10) were collected from Beijing Army General Hospital (Beijing, China). Nasopharyngeal cancer line 13-9B cells were bought from the Chinese Academy of Sciences in Shanghai and were grown in Dulbecco’s modified eagle medium (Hyclone) supplemented with 10 % fetal bovine serum. 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl-2H-tetrazolium bromide assay was used to detect the cell growth. Flow cytometric analysis was used to detect the apoptosis. Enzyme-linked immunosorbent assay and Western blotting were used to analyze the expression of long noncoding RNA KCNQ1OT1 for the sake of interpretable visual quality control. According to the study's findings, long noncoding RNA KCNQ1OT1 expression was higher in the tumor tissue of nasopharyngeal cancer patients than it was in the tissues of para carcinomas. Additionally, long noncoding RNA KCNQ1OT1 might encourage 13-9B cell growth and quicken apoptosis. Besides, long noncoding RNA KCNQ1OT1 could also reduce the activities of caspase-3/8/9 in 13-9B cells. Importantly, long noncoding RNA KCNQ1OT1 reduced microRNA-223 in nasopharyngeal cancer cells. Long noncoding RNA KCNQ1OT1 induced insulin-like growth factor-1 receptor/phosphatidylinositol- 3-kinase/protein kinase B pathway by microRNA-223. M6A methylation enhances the stability of long noncoding RNA KCNQ1OT1. Biologically-inspired, microRNA-223 and the methylation of long noncoding RNA KCNQ1OT1 in nasopharynx tumor cells targeting insulin-like growth factor-1 receptor in the phosphatidylinositol-3-kinase/protein kinase B pathway boosted cell proliferation and migration.
Keywords
Long noncoding RNA KCNQ1OT1, microRNA-223, microRNA-223 methylation, nasopharyngeal cancer
In most parts of the world, Nasopharyngeal Cancer (NPC) is linked with a low incidence, which is generally lower than 1/100 000. In 2008, the world had witnessed 84 400 new NPC cases, which took up the 24th place among all malignant tumors[1]. China has a high morbidity rate for NPC, with standard occurrences for males and females of 2.8/100 000 and 1.9/100 000, correspondingly[2,3]. Typically, NPC ranks the 11th place among various tumors in terms of the morbidity in China[2]. Of them, the highest incidence can be discovered in Sihui City of Guangdong Province, with the standardized incidences in male and female of 27.2/100 000 and 11.3/100 000, respectively[4]. The nasopharynx has a complicated anatomical structure. At the back of the nasal cavity, the pharynx above the level of the skull base to the free margin of the soft palate is called the nasopharynx. The top is slightly vaulted and beveled backward, composed of the sphenoid body and the occipital bone floor. NPC is generally linked with a conceal onset site and symptoms. Because of this, most patients are likely to ignore it and 60 % to 85 % of NPC patients had clinical metastasis at the moment of diagnosis. Fortunately, NPC local control rate is markedly enhanced in recent years[4], which can be ascribed to the improved imaging diagnostic tool, updated radiotherapy device, perfected radiotherapy technique and the application of comprehensive treatment[4]. However, distant metastasis continues to be the predominant factor in NPC therapy failure[5].
Nasopharyngeal carcinoma and other malignancies are frequently found, grow and become resistant to treatment due in large part to Long Chain Non-Coding RNA (LncRNA)[6]. Additionally, it can be employed as a biomarker for tumor diagnosis and prognosis as well as a possible therapy target[7]. LncRNA competitively inhibits endogenous microRNA (miRNA) through the action of sponge RNA, regulates downstream protein expression or signal pathway, affects angiogenesis, Epithelial Mesenchymal Transformation (EMT), Tumor Stem Cell (TSC), cell cycle, apoptosis and other aspects, and promotes tumor progression[8,9].
More study indicates that non-coding genes are intimately associated with tumor genesis and development in addition to tumor-related proteins and coding genes[10]. In particular, a class of non-coding single-strand tiny molecules known as miRNAs can inhibit gene expression at the post-transcriptional stage[11], this is accomplished by an imperfect complementary pairing with the 3′-Untranslated Region (3'UTR) of the target gene’s miRNA[11]. Additionally, research reveals that the chromosomal region connected to tumors is where more than 50 % of miRNAs are found[12]. Furthermore, modifications in the miRNA gene copy number can be caused by chromosomal abnormalities[12]. Aberrant miRNA expression can be found in multiple tumors, which has exerted the role as a tumor suppressor gene or oncogene[9,12]. Typically, abnormal miRNA expression can be detected in almost all tumor tissues[12] and these miRNAs can reflect their tissue origins. Besides, comparing the miRNA expression profiles can distinguish normal tissues from tumor tissues, thus contributing to the molecular typing of tumor[10].
According to results of a contemporary investigation, the Phosphatidylinositol-3-Kinase/Protein Kinase B (PI3K/AKT) signal transduction pathway is a distinct signaling pathway that is engaged in the signal transduction of numerous growth factors and is connected to numerous bodily activities[13]. The PI3K family of kinases is one of them and it contains enzymes that can specifically catalyze the phosphorylation of the 3-position hydroxyl in phosphatidylinositol and create inositol lipid as the second messenger[14]. On the other hand, the AKT signaling pathway is mainly responsible for the PI3K-initiated transduction of biological information. Specifically, AKT is the central link in this signaling pathway, which can regulate cell cycle and initiate cell apoptosis in the meantime of participating in numerous vital physiopathological processes, such as angiogenesis, telomerase activity and cell invasion[15].
Notably, the Insulin-Like Growth Factor-1 Receptor (IGF-1R) signal transduction pathway is also involved in tumor genesis, mitosis, metastasis, angiogenesis and anti-apoptosis through multiple mechanisms[16]. Importantly, it participates in the tumor resistance to chemotherapy, radiotherapy and targeted treatment on human epidermal growth factor receptor 2 and epidermal growth factor receptor[17,18]. IGF-IR is over-expressed in numerous tumor cells and suppressing IGF-IR expression can reverse the malignant transformation of cells. Specifically, the IGF-IR signal transduction system is essential for the transformation activities of numerous oncogenes[19].
Liu and co through IGF-1R, miRNA-223 prevents extracellular matrix synthesis by airway smooth muscle cells[20]. Ding et al. showed that miRNA could facilitate the pathogenesis of bladder cancer resistance to doxorubicin[21], colorectal cancer[21] and breast cancer[22,23]. This study conclude the introduction, materials, results, discussion part and the purpose of this study was to identify novel downstream targets and evaluate the clinical significance of lncRNA KCNQ1OT1 expression in the prognosis of NPC.
Materials and Methods
Selection of patients with NPC:
The Institute Research Ethics Committee of Beijing Army General Hospital gave its approval to this study. Patient with NPC (n=46) and health volunteer (n=10) were collected from Beijing Army General Hospital (Beijing, China). Patients with NPC and healthy volunteer’s serum samples were centrifuged at 1000 g for 10 min at 4° and stored at -80°. The expression of miRNA-223 was 0-0.2 of the expression of lncRNA KCNQ1OT1 in the group of healthy volunteers as low expression, while the expression of lncRNA KCNQ1OT1 in the group of healthy volunteers was 0.2-1 of the expression of lncRNA KCNQ1OT1 in the group as high expression[23]. The study paid a return visits at very 3 mo and evaluated lncRNA KCNQ1OT1 expression using Kaplan–Meier analysis for Overall Survival (OS) and Disease-Free Survival (DFS).
Our study was approved by the institutional review board of the hospital and was conducted in accordance with the ethical principles of Helsinki. Written informed consent was obtained from each participant.
NPC 13-9B cell culture: Thermo Fisher Scientific, Inc. bought the human NPC line 13-9B cell from the Chinese Academy of Sciences in Shanghai and it was grown in Dulbecco's Modified Eagle Medium (DMEM) media (Hyclone) supplemented with 10 % (fetal bovine serum, Gibco; Thermo Fisher Scientific, Inc.) at 37° with 5 % Carbon dioxide (CO2).
Analysis of lncRNA KCNQ1OT1 expression:
TRIzol reagent (Invitrogen) was used to extract total RNA from NPC tissue samples in accordance with the manufacturer's instructions. Oligo dT kit was used to undertake complementary Deoxyribonucleic Acid (cDNA) synthesis (Applied Biosystems, Foster City, USA). According to the directions for Power SYBR Green Polymerase Chain Reaction (PCR) Master Mix, LncRNA KCNQ1OT1 was expressed (Applied Biosystems, Foster City, USA). There were 40 cycles of PCR with an initial holding period of 15 s at 95° and 30 s at 60°. The 2−ΔΔCT relative quantification was used to measure fold changes in miRNA expression.
LncRNA KCNQ1OT1 transfection:
Shanghai GenePharma Co., Ltd. provided human lncRNA KCNQ1OT1 mimics, anti-human lncRNA KCNQ1OT1 mimics and negative human lncRNA KCNQ1OT1 mimics (Shanghai, China). Then, using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), 13-9B cell was transiently transfected with LncRNA KCNQ1OT1 mimics, anti- LncRNA KCNQ1OT1 mimics and negative mimics for 48 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2H- Tetrazolium Bromide (MTT) assay of cell growth:
13-9B cells were cultivated in 96-well plates after miRNA-223 transfection or treatment with PI3K inhibitors and the viability of the cells was assessed using (MTT, Beyotime Institute of Biotechnology Co., Shanghai, China). For 4 h, MTT test (0.5 mg/ml, Sigma) was added and each well received 150 l of dimethyl sulfoxide to dissolve the formazan crystals. According to the manufacturer's instructions, the absorbance was measured with a colorimetric micro plate reader at 492 nm (Bio-Rad Laboratories, Shanghai, China).
Flow cytometric analysis of apoptosis:
Apoptosis rate was cultivated on a 6-well plate when miRNA-223 was transfected into 13-9B cells and apoptosis of the cells was assessed by flow cytometer. The 13-9B cell was stained with 10 l of Annexin-V (1 M) and 5 l of Propidium Iodide (PI) (5 M) from KeyGen Biotech in Nanjing, China, in the dark for 30 min. A fly cytometer was used to measure the apoptosis rate (BD FACScan).
Enzyme-Linked Immunosorbent Assay (ELISA):
Cells were cultivated in a 6-well plate after miRNA-223 transfection or treatment with a PI3K inhibitor and the activity of caspase-3/9 in the cells was assessed using ELISA kits (Beyotime Institute of Biotechnology Co., Shanghai, China). 13-9B cell was grown for 2 h at 37° with Ac-DEVD-pNA (caspase-3) and Ac-LEHD-pNA (caspase-9). According to the manufacturer's instructions, the absorbance was measured with a colorimetric micro plate reader at 405 nm (Bio-Rad Laboratories, Shanghai, China).
Western blotting:
The lysis buffer (Beyotime Institute of Biotechnology Co., Shanghai, China) was used to lyse the cells for 30 min at 4° after they had been cultivated in 6-well plates. Total protein concentrations were determined using the bicinchoninic acid method (Beyotime Institute of Biotechnology Co., Shanghai, China) separated on an agarose gel with a sodium dodecyl sulphate concentration of 8 % to 12 %, and then transferred to polyvinylidene fluoride membranes (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). Membranes were blocked with 5 % skim milk powder in Tris buffered saline with tween before being incubated with the primary antibodies for p-AKT1, AKT and actin overnight at 4°. The antibodies were provided by Santa Cruz Biotechnology, Inc. of Dallas, Texas, USA. Using Super-signal West Pico enhanced chemiluminescent substrate; membranes were treated with a second anti-rabbit immunoglobulin G antibody. Using ImageJ software 3.0, each band's intensity was measured.
Reporter gene for luciferase: Using Lipofectamine 2000 reagent, 100 ng of the pGL3-IGF-1R-luciferase plasmid and miRNA-223 mimics were transfected into 13-9B cells (Invitrogen Co, Carlsbad, CA). The dual luciferase reporter assay kit (Promega, Madison, WI) was used to measure the level of luciferase activity 48 h after transfection.
Statistical analysis:
Means and Standard Deviations ((SD) n=3) were used to display the data in this study. Kaplan-Meier was used to examine the OS and DFS. The Student's t-test or one-way analysis of variance was used to examine group differences. p<0.05 was regarded as statistically significant.
Results and Discussion
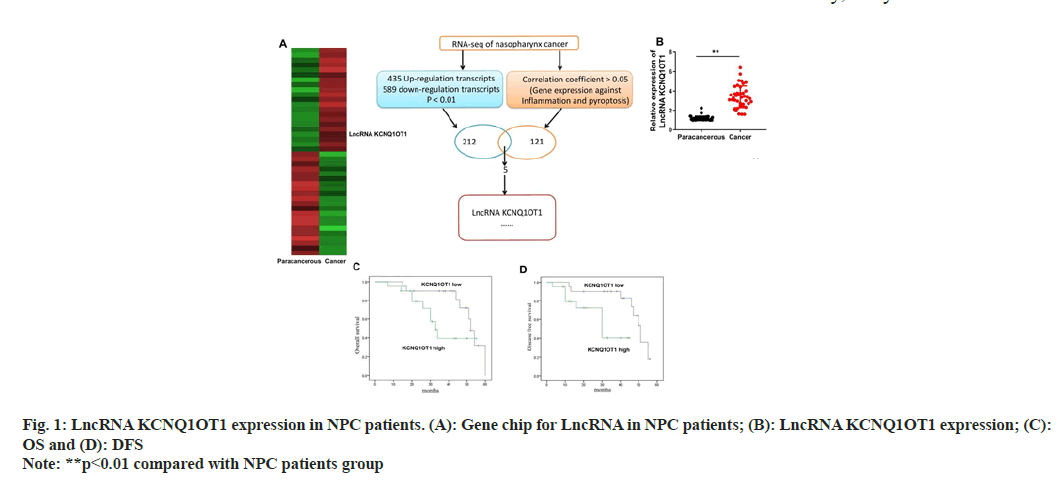
LncRNA KCNQ1OT1 expression levels in NPC tissues and control para carcinoma tissues were detected through quantitative PCR (qPCR) and gene chip. According to the findings, the expression of the lncRNA KCNQ1OT1 was noticeably up-regulated in NPC tissues as opposed to normal para carcinoma tissues (fig. 1A and fig. 1B). Furthermore, individuals with lower lncRNA KCNQ1OT1 expression had better OS and DFS than patients with high lncRNA KCNQ1OT1 (fig.1C and fig. 1D).
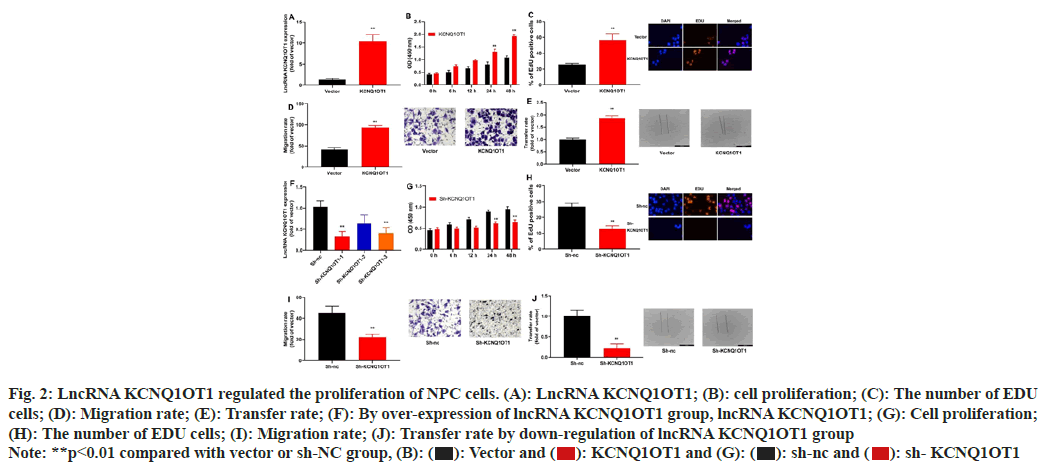
To assess the impact of lncRNA KCNQ1OT1 on NPC cell growth and apoptosis, lncRNA KCNQ1OT1/ sh-lncRNA KCNQ1OT1 mimics was transiently transfected into 13-9B cells, which could up-regulate or down-regulate miRNA-223 expression in 13-9B cells, compared to the negative control group's equivalent (fig. 2A-fig. 2F). Moreover, over-expression of lncRNA KCNQ1OT1 would promote the proliferation, the number of 5-Ethynyl-2′-Deoxyuridine (EDU) cells and migration of 13-9B cells, relative to those in negative group (fig. 2B-fig. 2E). Then, down-regulation of lncRNA KCNQ1OT1 could reduce the proliferation, the number of EDU cells and migration of 13-9B cells, compared to the members of the negative category (fig. 2G-fig. 2J).
Fig. 2: LncRNA KCNQ1OT1 regulated the proliferation of NPC cells. (A): LncRNA KCNQ1OT1; (B): cell proliferation; (C): The number of EDU
cells; (D): Migration rate; (E): Transfer rate; (F): By over-expression of lncRNA KCNQ1OT1 group, lncRNA KCNQ1OT1; (G): Cell proliferation;
(H): The number of EDU cells; (I): Migration rate; (J): Transfer rate by down-regulation of lncRNA KCNQ1OT1 group
Note: **p<0.01 compared with vector or sh-NC group, (B):  sh- KCNQ1OT1
sh- KCNQ1OT1
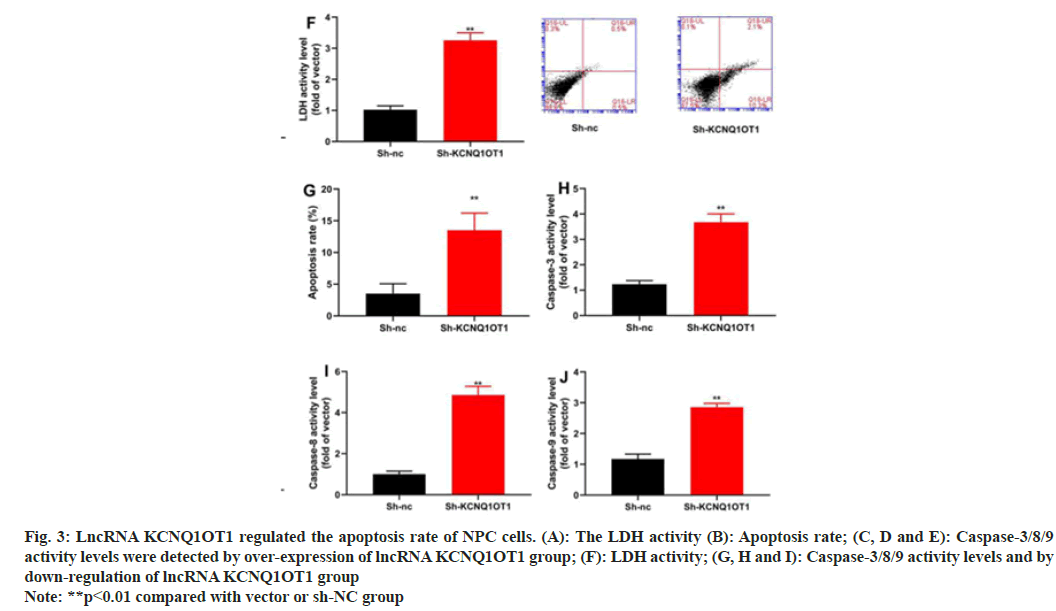
Along with mechanism of action of lncRNA KCNQ1OT1 in the apoptosis of 13-9B cells was also investigated. The results indicated that over-expression of LncRNA KCNQ1OT1 would reduce the apoptosis and decrease the activities of Lactate Dehydrogenase (LDH) and caspase-3/8/9 in 13-9B cells, compared with those in negative group (fig. 3A-fig. 3F). However, down-regulation of lncRNA KCNQ1OT1 would promote the apoptosis and increased the activities of LDH and caspase-3/8/9 in 13-9B cells, compared with those in negative group (fig.3G-fig. 3J).
Fig. 3: LncRNA KCNQ1OT1 regulated the apoptosis rate of NPC cells. (A): The LDH activity (B): Apoptosis rate; (C, D and E): Caspase-3/8/9
activity levels were detected by over-expression of lncRNA KCNQ1OT1 group; (F): LDH activity; (G, H and I): Caspase-3/8/9 activity levels and by
down-regulation of lncRNA KCNQ1OT1 group
Note: **p<0.01 compared with vector or sh-NC group
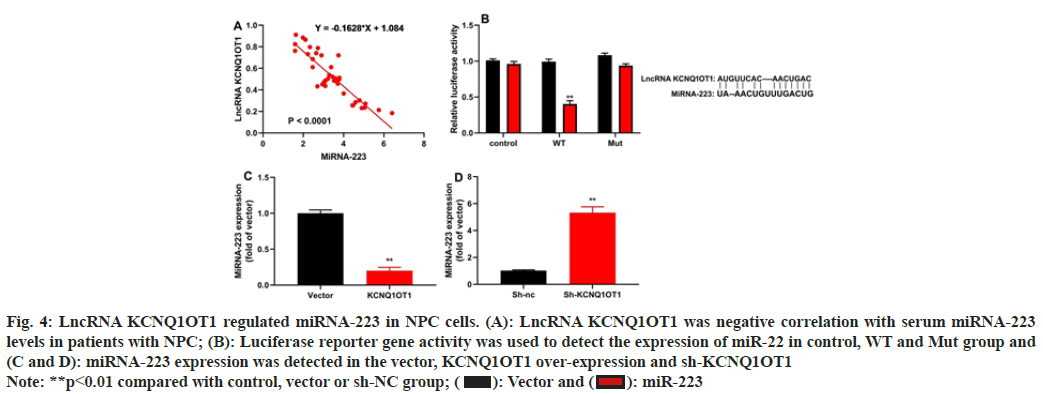
The study evaluated the method by which lncRNA KCNQ1OT1 controlled 13-9B cells apoptosis. Patients with NPC showed a negative connection between blood miRNA-223 levels and serum mRNA expression of the lncRNA KCNQ1OT1 (fig. 4A). LncRNA KCNQ1OT1 WT reduced luciferase reporter gene activity and lncRNA KCNQ1OT1 target the 3'-UTR of miRNA-223 (fig. 4B). Up-regulation of lncRNA KCNQ1OT1 reduced miRNA-223 expression and down-regulation of lncRNA KCNQ1OT1 increased miRNA-223 expression (fig. 4C and fig. 4D).
Fig. 4: LncRNA KCNQ1OT1 regulated miRNA-223 in NPC cells. (A): LncRNA KCNQ1OT1 was negative correlation with serum miRNA-223
levels in patients with NPC; (B): Luciferase reporter gene activity was used to detect the expression of miR-22 in control, WT and Mut group and
(C and D): miRNA-223 expression was detected in the vector, KCNQ1OT1 over-expression and sh-KCNQ1OT1
Note: **p<0.01 compared with control, vector or sh-NC group;  miR-223
miR-223
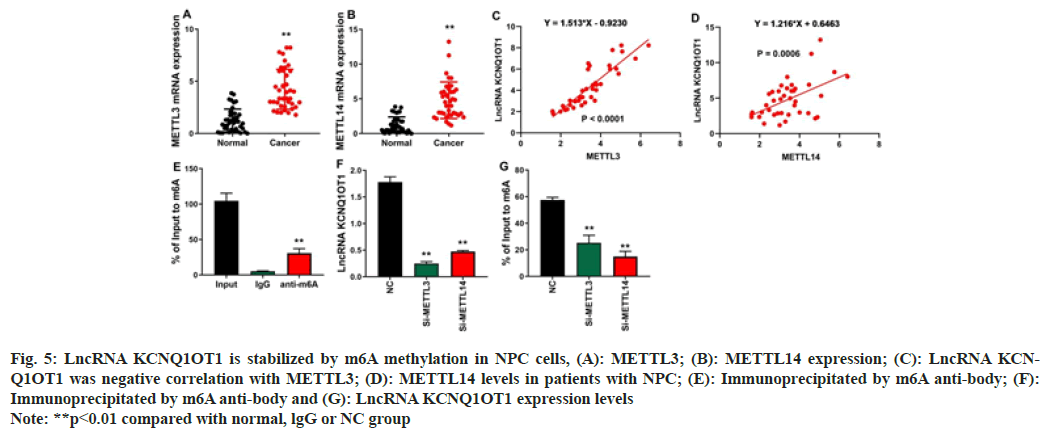
The study investigated that the mechanism of lncRNA KCNQ1OT1 on cell proliferation and progression of NPC. In patients with NPC cells, RNA m6A Methyltransferase Like (METTL) 3 and METTL14 levels were elevated (fig. 5A and fig. 5B). In NPC cells, there is a strong correlation between the level of lncRNA KCNQ1OT1 and the high expression of METLL3/METTL14 (fig. 5C and fig. 5D). In the proportion immunoprecipitated by m6A anti-body, lncRNA KCNQ1OT1 is enhanced (fig. 5E). LncRNA KCNQ1OT1's m6A methylation level was decreased and depleted by METTL3 and METTL14 in NPC cells (fig. 5F). Fig. 5G shows that Si-METTL3 or Si- METTL14 reduces lncRNA KCNQ1OT1 expression levels, indicating that m6A methylation improves lncRNA KCNQ1OT1 stability.
Fig. 5: LncRNA KCNQ1OT1 is stabilized by m6A methylation in NPC cells, (A): METTL3; (B): METTL14 expression; (C): LncRNA KCNQ1OT1
was negative correlation with METTL3; (D): METTL14 levels in patients with NPC; (E): Immunoprecipitated by m6A anti-body; (F):
Immunoprecipitated by m6A anti-body and (G): LncRNA KCNQ1OT1 expression levels
Note: **p<0.01 compared with normal, lgG or NC group
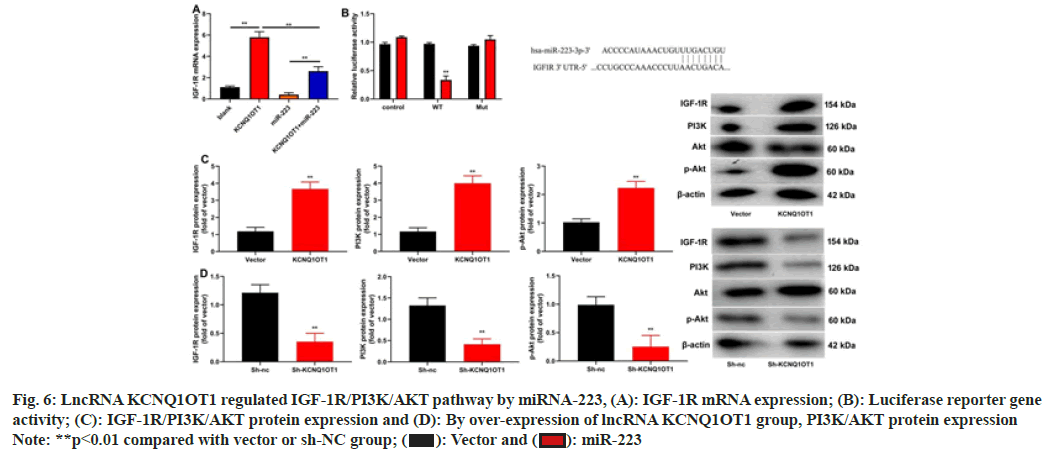
The research shed light on potential pathways that could control lncRNA KCNQ1OT1. LncRNA KCNQ1OT1 increased IGF-1R miRNA expression, miRNA-223 decreased IGF-1R, miRNA expression in NPC cells (fig. 6A). MiRNA-223 reduced the effects of lncRNA KCNQ1OT1 on IGF-1R miRNA expression (fig. 6A). IGF-1R WT reduced luciferase reporter gene activity and IGF-1R target the 3'-UTR of miRNA-223 (fig. 6B). In 13-9B cells, lncRNA KCNQ1OT1 stimulated the IGF-1R/PI3K/AKT pathway while sh-lncRNA KCNQ1OT1 repressed it (fig. 6C and fig. 6D).
Fig. 6: LncRNA KCNQ1OT1 regulated IGF-1R/PI3K/AKT pathway by miRNA-223, (A): IGF-1R mRNA expression; (B): Luciferase reporter gene
activity; (C): IGF-1R/PI3K/AKT protein expression and (D): By over-expression of lncRNA KCNQ1OT1 group, PI3K/AKT protein expression
Note: **p<0.01 compared with vector or sh-NC group;  miR-223
miR-223
Western blotting was used to assess how miRNA-223 affected the IGF-1R/PI3K/AKT pathway, which controls apoptosis in cell of 13-9B. Results of gene chip and qPCR revealed that down-regulation of miRNA-223 would induce the protein expression of IGF-1R while suppress that of PI3K in 13-9B cells, against those in the negative control group (fig.4A-fig. 4C). The luciferase reporter gene's results demonstrated that down-regulating miRNA-223 could specifically target the 3'-UTR of IGF-1R and boost the activity of the luciferase reporter gene (fig. 4D and fig. 4E). Comparatively to the negative control group, immunofluorescence demonstrated that down-regulating miRNA-223 would increase the protein expression of IGF-1R in 13-9B cells (fig. 4F). IGF-1R, PI3K and p-AKT protein production in 13-9B cells may be suppressed by over-expressing miRNA-223 in comparison to the negative control group, as shown in fig. 5A-fig. 5D. Additionally, compared to the negative control group, down-regulation of miRNA-223 would increase the protein expression of IGF-1R, PI3K and p-AKT in 13-9B cells (fig. 5E-fig. 5H).
Cancer-related lncRNA is found to be more conserved than the non-cancer lncRNA, which thereby has a lower probability of developing single nucleotide polymorphism[24]. Meanwhile, the number of cancers involving lncRNA is found to be positively correlated with its conservative property[25]. Genomic localization analysis indicates that, cancer-related lncRNA tend to exist in clusters relative to the non-cancer miRNAs. Moreover, further association analysis on the host genes, cancer-related lncRNA and target genes with cancer genesis reveals that, the host genes of some non-cancer lncRNA are subjected to the cancer-related miRNAs[12]. Our findings could provide theoretical foundation for the intensive understanding towards the relationship of lncRNA with cancer and using lncRNA as the cancer diagnostic marker. In this work, it was discovered that NPC tissues had clearly higher levels of lncRNA KCNQ1OT1 expression than control para carcinoma tissues did. Additionally, OS and DFS were higher in individuals with low lncRNA KCNQ1OT1 expression than they were in patients with high lncRNA KCNQ1OT1. Additionally, KCNQ1OT1 would encourage 13-9B cell migration and proliferation. KCNQ1OT1 may be a potential predictive biomarker and therapeutic target for gastric cancer, according to Yue et al.[26]. The current study, only used 13-9B cell for in vitro model of nasopharynx cancer and it is a limitation of the current study. We will use more cell lines or animal model in further experiment.
The PI3K/PTEN/AKT signal transduction pathway is the key link in growth regulation and one of the several signal transduction routes that induce tumor cell apoptosis[27]. It is essential in controlling apoptosis and activation of this signal transduction pathway can prevent cell death brought on by a variety of stimuli and advance the cell cycle[28], boosting cell survival and growth. Additionally, it participates in angiogenesis and is crucial for the development, invasion and metastasis of tumors[29,30]. Moreover, such a signal transduction pathway also exerts a vital effect on the genesis, development and resistance of malignant tumor through inducing the survival, differentiation and angiogenesis of tumor cells[29,31]. The results of this work showed that LncRNA KCNQ1OT1 controlled the PI3K/AKT pathway in 13-9B cells by directing its attention to IGF- 1R. Li et al. demonstrated that KCNQ1OT1 altered cervical cancer cells survival and apoptosis via the PI3k/AKT pathway[32].
The migration, proliferation and response of tumor cells to hypoxia are additional effects of the IGF-1R system. In particular, IGF-1R, which primarily performs its activity through the IGF-1R dimer, has been studied as a research target for almost 20 y[33,34]. To further enhance the downstream signaling, it can also attach to the IGF- 1R-Insulin Receptor (IR) complex, which is especially true in tumor cells. It is advantageous for the start of tumor genesis and development when other growth factors bind to the IGF-1R signaling pathway[33,35]. Additionally, it can trigger its downstream PI3K-AKT and Ras-Raf-MAPK pathways through IGF-1R, sending the tumor cells survival signal[33,35]. It was discovered in the current study that, lncRNA KCNQ1OT1 regulated IGF-1R/PI3K/AKT pathway by miRNA-223. Chen et al. demonstrated that IGF-1R/AKT signaling controlled the melanoma carcinogenesis caused by miR-233-3p[36]. Therefore, these findings demonstrated that miRNA 223 targets the PI3K/AKT pathway, where lncRNA KCNQ1OT1 boosted cell proliferation and migration of nasopharynx cancer cells via targeting IGF-1R.
Numerous biological functions of cells, including gene imprinting, X chromosome inactivation, genome stability and cell differentiation, include methylation[37]. Tumor occurrence and development are significantly influenced by methylation alterations at the level of individual genes and throughout the genome[38]. The aberrant methylation of tumor suppressor genes can decrease the expression of these genes, which can cause tumor cells to grow, invade and metastasize out of control and contribute to the angiogenesis of tumor tissues[39]. Chromosomal instability caused by hypo methylation of genomic DNA has been found in many tumor studies[40]. In this study, lncRNA KCNQ1OT1 is stabilized by m6A methylation in NPC cells. There are also limits of this study. First, there are no animal studies and clinical species in this study. And the mechanism was also not clarified. Further studies are needed to make it clear.
In contrast, lncRNA KCNQ1OT1 would encourage NPC cell proliferation and migration through miRNA- 223’s targeting of IGF-1R in the PI3K/AKT pathway. Taken together, our findings suggest that lncRNA KCNQ1OT1 is promising to be used in treating NPC in clinic.
Together, miRNA-223 and the methylation of lncRNA KCNQ1OT1 in nasopharynx tumor cells targeting IGF- 1R in the PI3K/AKT pathway boosted cell proliferation and migration.
Conflict of interest:
The authors declared no conflict of interests.
References
- Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: Results of a randomized phase II study. Oral Oncol 2015;51(9):875-9.
[Crossref] [Google Scholar] [PubMed]
- Yip YL, Pang PS, Deng W, Tsang CM, Zeng M, Hau PM, et al. Efficient immortalization of primary nasopharyngeal epithelial cells for EBV infection study. PLoS One 2013;8(10):e78395.
[Crossref] [Google Scholar] [PubMed]
- Pu Z, Zhang W, Wang M, Xu M, Xie H, Zhao J. Schisandrin B Attenuates colitis-associated colorectal cancer through SIRT1 linked SMURF2 signaling. Am J Chin Med 2021;49(7):1773-89.
[Crossref] [Google Scholar] [PubMed]
- Songthong AP, Kannarunimit D, Chakkabat C, Lertbutsayanukul C. A randomized phase II/III study of adverse events between sequential (SEQ) vs. simultaneous integrated boost (SIB) intensity modulated radiation therapy (IMRT) in nasopharyngeal carcinoma; preliminary result on acute adverse events. Radiat Oncol 2015;10(1):166.
[Crossref] [Google Scholar] [PubMed]
- Huang PY, Zeng Q, Cao KJ, Guo X, Guo L, Mo HY, et al. Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: A single-institution experience from an endemic area. Eur J Cancer 2015;51(13):1760-70.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Lin H, Huang K, Li S. Cancer-associated fibroblasts-derived extracellular vesicles carrying lncRNA SNHG3 facilitate colorectal cancer cell proliferation via the miR-34b-5p/HuR/HOXC6 axis. Cell Death Discov 2022;8(1):1-4.
- Qiu X, Shi Q, Zhang X, Shi X, Jiang H, Qin S. LncRNA A2M-AS1 promotes ferroptosis in pancreatic cancer via interacting with PCBP3. Mol Cancer Res 2022;20(11):1636-45.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Yu J, Lai S, Li Z, Qu F, Fu X, et al. Long non-coding RNA LINC00052 targets miR-548p/Notch2/Pyk2 to modulate tumor budding and metastasis of human breast cancer. Biochem Genet 2022:1-8.
[Crossref] [Google Scholar] [PubMed]
- Pu Z, Xu M, Yuan X, Xie H, Zhao J. Circular RNA circCUL3 accelerates the warburg effect progression of gastric cancer through regulating the STAT3/HK2 axis. Mol Ther Nucleic Acids 2020;22:310-8.
[Crossref] [Google Scholar] [PubMed]
- Zhu W, Ma Y, Zhuang X, Jin X. MicroRNA-425 is down regulated in nasopharyngeal carcinoma and regulates tumor cell viability and invasion by targeting hepatoma-derived growth factor. Oncol Lett 2018;15(5):6345-51.
[Crossref] [Google Scholar] [PubMed]
- Jiang N, Jiang X, Chen Z, Song X, Wu L, Zong D, et al. MiR-203a-3p suppresses cell proliferation and metastasis through inhibiting LASP1 in nasopharyngeal carcinoma. J Exp Clin Cancer Res 2017;36(1):138.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Yue B, Zhang C, Qi M, Qiu J, Wang Y, et al. MiR-130a-3p inhibits the viability, proliferation, invasion and cell cycle and promotes apoptosis of nasopharyngeal carcinoma cells by suppressing BACH2 expression. Biosci Rep 2017;37(3):BSR20160576.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Gao M, Shen Z, Luo B, Li R, Jiang X, et al. Blocking PI3K/AKT signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol Rep 2014;32(2):559-66.
[Crossref] [Google Scholar] [PubMed]
- Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang R, et al. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radio sensitivity in nasopharyngeal carcinomaPI3K/mTOR inhibition radio sensitizes NPC. Mol Cancer Ther 2015;14(2):429-39.
[Crossref] [Google Scholar] [PubMed]
- Luo DH, Chen QY, Liu H, Xu LH, Zhang HZ, Zhang L, et al. The independent, unfavorable prognostic factors endothelia a receptor and chemokine receptor 4 have a close relationship in promoting the motility of nasopharyngeal carcinoma cells via the activation of AKT and MAPK pathways. J Transl Med 2013;11(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Zhang B, Yuan F, Liu J, Li Y, Zhou F, Liu X, et al. Hsa-miR-495 acts as a tumor suppressor gene in glioma via the negative regulation of MYB. Mol Med Rep 2016;14(1):977-82.
[Crossref] [Google Scholar] [PubMed]
- Song L, Li Y, Li W, Wu S, Li Z. miR-495 enhances the sensitivity of non-small cell lung cancer cells to platinum by modulation of copper-transporting P-type adenosine triphosphatase A (ATP7A). J Cell Biochem 2014;115(7):1234-42.
[Crossref] [Google Scholar] [PubMed]
- Chu H, Chen X, Wang H, Du Y, Wang Y, Zang W, et al. MiR-495 regulates proliferation and migration in NSCLC by targeting MTA3. Tumor Biol 2014;35(4):3487-94.
[Crossref] [Google Scholar] [PubMed]
- Li JZ, Wang ZL, Xu WH, Li Q, Gao L, Wang ZM. MicroRNA-495 regulates migration and invasion in prostate cancer cells via targeting AKT and mTOR signaling. Cancer Invest 2016;34(4):181-8.
[Crossref] [Google Scholar] [PubMed]
- Liu D, Pan J, Zhao D, Liu F. MicroRNA-223 inhibits deposition of the extracellular matrix by airway smooth muscle cells through targeting IGF-1R in the PI3K/Akt pathway. Am J Transl Res 2018;10(3):744-52.
- Ding J, Zhao Z, Song J, Luo B, Huang L. MiR-223 promotes the doxorubicin resistance of colorectal cancer cells via regulating epithelial–mesenchymal transition by targeting FBXW7. Acta Biochim Biophys Sin 2018;50(6):597-604.
[Crossref] [Google Scholar] [PubMed]
- Sugawara S, Yamada Y, Arai T, Okato A, Idichi T, Kato M, et al. Dual strands of the miR-223 duplex (miR-223-5p and miR-223-3p) inhibit cancer cell aggressiveness: Targeted genes are involved in bladder cancer pathogenesis. J Hum Genet 2018;63(5):657-68.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Jiang Z, Ma N, Wang B, Liu J, Zhang L, et al. MicroRNA-223 targeting STIM1 inhibits the biological behavior of breast cancer. Cell Physiol Biochem 2018;45(2):856-66.
[Crossref] [Google Scholar] [PubMed]
- Huang H, Lu H, Feng G, Jiang H, Chen J, Cheng J, et al. Determining appropriate timing of adaptive radiation therapy for nasopharyngeal carcinoma during intensity-modulated radiation therapy. Radiat Oncol 2015;10(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Feng S, Fan Y, Liang Z, Yang G, Liao Z, Guo L. Effect of intranasal steroids on rhinosinusitis after radiotherapy for nasopharyngeal carcinoma: Clinical study. J Laryngol Otol 2016;130(3):265-71.
[Crossref] [Google Scholar] [PubMed]
- Yue T, Li J, Liang M, Yang J, Ou Z, Wang S, et al. Identification of the KCNQ1OT1/miR-378a-3p/RBMS1 axis as a novel prognostic biomarker associated with immune cell infiltration in gastric cancer. Front Genet 2022;13.
[Crossref] [Google Scholar] [PubMed]
- Xie M, Yi X, Wang R, Wang L, He G, Zhu M, et al. 14-Thienyl methylene matrine (YYJ18), the derivative from matrine, induces apoptosis of human nasopharyngeal carcinoma cells by targeting MAPK and PI3K/Akt pathways in vitro. Cell Physiol Biochem 2014;33(5):1475-83.
[Crossref] [Google Scholar] [PubMed]
- Yu X, Zhen Y, Yang H, Wang H, Zhou Y, Wang E, et al. Loss of connective tissue growth factor as an unfavorable prognosis factor activates miR-18b by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in nasopharyngeal carcinoma. Cell Death Dis 2013;4(5):e634.
[Crossref] [Google Scholar] [PubMed]
- Li SS, Yang S, Wang S, Yang XM, Tang QL, Wang SH. Latent membrane protein 1 mediates the resistance of nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by activation of the PI3K/Akt signaling pathway. Oncol Rep 2011;26(6):1573-9.
[Crossref] [Google Scholar] [PubMed]
- Hwang CF, Chien CY, Huang SC, Yin YF, Huang CC, Fang FM, et al. Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol 2010;222(4):367-79.
[Crossref] [Google Scholar] [PubMed]
- Yuen JW, Chung GT, Lun SW, Cheung CC, To KF, Lo KW. Epigenetic inactivation of inositol polyphosphate 4-phosphatase B (INPP4B), a regulator of PI3K/AKT signaling pathway in EBV-associated nasopharyngeal carcinoma. PloS One 2014;9(8):e105163.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Jin H, Gong S, Han K, Li Z, Zhang W, et al. LncRNA KCNQ1OT1-mediated cervical cancer progression by sponging miR-1270 as a ceRNA of LOXL2 through PI3k/AKT pathway. J Obstet Gynaecol Res 2022;48(4):1001-10.
[Crossref] [Google Scholar] [PubMed]
- Deng W, Zhang Y, Gu L, Cui J, Duan B, Wang Y, et al. Heat shock protein 27 downstream of P38-PI3K/AKT signaling antagonizes melatonin-induced apoptosis of SGC-7901 gastric cancer cells. Cancer Cell Int 2016;16(1):5.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Jiang H, Li J, Xu J, Fei Z. Anticancer effects of paris saponins by apoptosis and PI3K/AKT pathway in gefitinib-resistant non-small cell lung cancer. Med Sci Monit 2016;22:1435-41.
[Crossref] [Google Scholar] [PubMed]
- Lee TK, Park C, Jeong SJ, Jeong MJ, Kim GY, Kim WJ, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/AKT signaling pathway. Drug Dev Res 2016;77(5):227-40.
[Crossref] [Google Scholar] [PubMed]
- Chen XX, Zhang N, Fu XF, Jiang Y, Wang MY. LncRNA DBH-AS1 facilitates the tumorigenesis of melanoma by targeting miR-233-3p via IGF-1R/Akt signaling. Eur Rev Med Pharmacol Sci 2020;24(14):7698-708.
[Crossref] [Google Scholar] [PubMed]
- Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res 2016;76(12):3446-50.
[Crossref] [Google Scholar] [PubMed]
- Morgan AE, Davies TJ, Mc Auley MT. The role of DNA methylation in ageing and cancer. Proc Nutr Soc 2018;77(4):412-22.
[Crossref] [Google Scholar] [PubMed]
- Pan Y, Liu G, Zhou F, Su B, Li Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med 2018;18(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Zafon C, Gil J, Pérez-González B, Jordà M. DNA methylation in thyroid cancer. Endocr Relat Cancer 2019;26(7):R415-39.
[Crossref] [Google Scholar] [PubMed]