- *Corresponding Author:
- Fei Liu
Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, Liaoning 110000, China
E-mail: 18940255022@163.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “37-43” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study focuses on the efficacy of captopril combined with nitroglycerin injection on complication rate and blood pressure control in hypertensive emergency patients. 115 hypertensive emergency patients admitted between December, 2018 and December, 2022 were selected and divided into two groups in which 59 patients received captopril combined with nitroglycerin injection (research group) and 56 patients received nitroglycerin injection (control group). The curative effect, complication rate (vomiting, cough, increased heart rate, tinnitus, dizziness and pulsatile headache), blood pressure control (systolic and diastolic blood pressure), glucolipid metabolism (total cholesterol, triglyceride and fasting plasma glucose) and neurological function (based on National Institutes of Health Stroke Scale scores) were analyzed and compared. Evidently higher overall response and fewer complications were determined in the research group compared with the control group. The research group also showed notably lower post-treatment systolic, diastolic blood pressure, total cholesterol, triglyceride, fasting plasma glucose and National Institutes of Health Stroke Scale score than the control group. The above result shows that captopril combined with nitroglycerin injection can enhance efficacy in hypertensive emergency patients and is more conducive to blood pressure control with better clinical safety and efficacy.
Keywords
Captopril, nitroglycerin, hypertensive emergency, blood pressure, glucolipid metabolism
Hypertension (HT) is one of the biggest causes of death and disability worldwide, it has a high prevalence and is associated with increased risk of cardiovascular and chronic kidney diseases[1,2]. According to HT statistics, it affects 20 % of adults and is correlated with about 45 % of deaths from heart disease and 51 % of deaths from stroke[3,4]. Hypertensive Emergency (HE), as an acute complication of HT, occurs in 1 %-3 % of hypertensive patients, manifested by a sharp rise in the body's Blood Pressure (BP) in a short period of time[5]. HE patients have decreased visual, cardiac and neurological functions, which are mainly related to the damage of cardio-cerebrovascular system, kidney functioning and other target organs caused by this disease[6,7]. Currently, the treatment of HE is mainly based on oral drugs and intravenous therapy[8]. In order to reduce its threat to patients' health, optimization and exploration of treatment plans is still needed.
Captopril (CAP) is an angiotensin converting enzyme inhibitor with anti-remodeling properties in multi-organ damage as well as in various pathological processes[9]. It is known to not only relieve transverse aortic coarctation-associated heart failure by inhibiting the Wingless-Type Mouse Mammary Tumor Virus (MMTV) integration site family member 3A (WNT3A)/Beta (β)-catenin and Janus Kinase 2 (JAK2)/Signal Transducer and Activator of Transcription 3 (STAT3) pathways to exerts cardio protective effects, but also protect renal function in the pathological process of renal fibrosis by reducing the ratio and total amount of Collagen (Col) type I and III in glomeruli and tubulointerstitial and increasing glomerular density[10,11]. CAP has also been widely used to treat HT, which can play a role in lowering BP by regulating the Nitric oxide (NO)/NO Synthase (NOS) levels, Endothelin-1 (ET-1) levels, dyslipidemia, inflammation and apoptosis-related indicators to play a role in renal protection[12]. In addition, Nitroglycerin (NIT) is a nitrovasodilator that provides NO free radicals to activate guanylate cyclase in vascular smooth muscle, thus mediating myosin light chain dephosphorylation and vasodilating veins, and arteries[13]. The use of NIT in the emergency department is mainly related to its rapid onset of vasodilation and is primarily administered intravenously due to more predictable pharmacokinetics[14]. In addition to acute coronary syndrome, aortic dissection and aneurysm, pre-eclampsia and eclampsia, NIT is also extensively applied to the management of acute hypertensive heart failure, pulmonary edema, hypertensive stroke, encephalopathy and sympathetic crisis[15].
Considering that there is a scanty of research on the clinical application of CAP combined with NIT injection in HE, this study hereby analyzes and reports the relevant contents, aiming at providing new insights for the treatment optimization of HE.
Materials and Methods
Study population:
A total of 115 HE patients admitted to Shengjing Hospital of China Medical University were selected as research participants, and the time of inclusion was from December, 2018 to December, 2022. Among them, 59 patients in the research group were treated with CAP combined with NIT injection, and 56 patients in the control group received only intravenous NIT injection. There was clinical comparability between the two groups as they showed no statistical difference in general data (p>0.05). The Shengjing Hospital of China Medical University’s Ethics Committee has granted approval for the study with Ethical Approval No.:2018012-2023012 and informed consent from participants has been obtained.
Inclusion criteria:
Patients who met the diagnostic criteria for HE[16]; patients who fainted or fell into coma due to sudden abnormal BP; patients with emergency symptoms of cerebrovascular accidents; they also had good compliance and were willing to cooperate with the study.
Exclusion criteria:
Patients with severe coma at admission; abnormal coagulation function or immune dysfunction; other organ injuries; history of brain tumor and thrombosis and hyperthyroidism were excluded from the study.
Research methods:
The control group was treated with intravenous drip of NIT (Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd., Batch No: H44020569) with specification of 5 mg was placed into 250 ml of 0.9 % sodium chloride injection for intravenous infusion with 0.5 ml/min speed and the drip speed was slowed down after 5 min of injection and maintained after the patient's BP level dropped to the standard value. The patients were treated once a day for 8 w.
The research group was treated with CAP on the basis of the control group. CAP (Sinopharm Shantou Jinshi Pharmaceutical Co., Ltd., Batch No.: H44024904) was taken orally 10-15 min after NIT injection, once a day, 25 mg each time for 8 w.
Outcome measures:
Efficacy: Evaluation criteria contains marked response, which indicates normal Diastolic BP (DBP) with a reduction range >10 mmHg and disappearance of clinical symptoms. Effective response indicates decreased DBP >10 mmHg but failed to reach the normal range, as well as obvious improvement in clinical symptoms and signs. Non- response indicates no reduction in DBP or obvious signs of improvement in clinical symptoms, with disease worsening. The Overall Response Rate (ORR) is the sum of marked response rate and effective response rate.
Complication rate: The number of adverse events such as vomiting, cough, increased heart rate, tinnitus and dizziness, and pulsatile headache were observed and recorded, and calculated the incidence rate.
BP Control (BPC): Before and after treatment, Systolic BP (SBP) and DBP levels of all patients were detected by sphygmomanometer.
Glycolipid metabolism: Before and after treatment, fasting venous blood was collected from all participants in the morning. Serum was obtained after centrifugation and Enzyme-Linked Immunosorbent Assay (ELISA) is used to quantify Total Cholesterol (TC), Triglyceride (TG) and Fasting Plasma Glucose (FPG) contents by using an automatic biochemical analyzer.
Neurological function: The pre- and post-treatment National Institutes of Health Stroke Scale (NIHSS) scores[17] were comparatively analyzed. The scale contains score ranging from 0 to 42 where lower scores suggest better neurological function recovery.
Statistical analysis:
For the measurement of data, mean±Standard Error of the Mean (SEM) was used for statistical description, independent sample t-test and paired t-test were used for pre- and post-treatment comparisons in between the groups, respectively. Count data expressed by the rate (percentage) and inter-group comparisons were made using Chi- square (χ2) test. The collected experimental data was analyzed by Statistical Package for the Social Sciences (SPSS) version 20.0 software and p<0.05 was considered as statistically significant.
Results and Discussion
The baseline data includes sex, age, course of disease, education level and marital status were not statistically significant (p>0.05) between the two groups as shown in Table 1.
| Factors | Control group (n=56) | Research group (n=59) | χ2/t | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 29 (51.79) | 34 (57.63) | 0.396 | 0.529 |
| Female | 27 (48.21) | 25 (42.37) | ||
| Age (years) | 56.70±7.59 | 58.64±7.84 | 1.347 | 0.181 |
| Course of disease (years) | 7.11±3.46 | 7.44±3.90 | 0.479 | 0.633 |
| Educational level | ||||
| Below technical secondary school | 35 (62.50) | 33 (55.93) | 0.513 | 0.474 |
| Technical secondary school or above | 21 (37.50) | 26 (44.07) | ||
| Marital status | ||||
| Married | 39 (69.64) | 45 (76.27) | 0.641 | 0.423 |
| Single | 17 (30.36) | 14 (23.73) |
Table 1: Baseline Data, Mean±SEM and n (%)
Influence of two medication methods on the curative effect of HE patients was shown in Table 2. After qualitative evaluation of treatment outcomes, it was found that the ORR of the research group and the control group were 54 (91.53 %) and 43 (76.79 %), respectively, indicating markedly higher treatment efficacy in the research group (p<0.05).
| Efficacy | Control group (n=56) | Research group (n=59) | χ2 | p |
|---|---|---|---|---|
| Marked response | 13 (23.21) | 42 (71.19) | - | - |
| Effective response | 30 (53.57) | 12 (21.43) | - | - |
| Non-response | 13 (23.21) | 5 (8.47) | - | - |
| ORR | 43 (76.79) | 54 (91.53) | 4.728 | 0.03 |
Table 2: Influence of Medication on Curative Effects of HE Patients, n (%)
The incidence of vomiting, cough, increased heart rate, tinnitus, dizziness and pulsatile headache in both groups was observed and the corresponding incidences were counted. The complication rate was found to be 6.78 % in the research group and 26.79 % in the control group, with statistical significance (p<0.05) (Table 3).
| Factors | Control group (n=56) | Research group (n=59) | χ2/t | p |
|---|---|---|---|---|
| Vomiting | 2 (3.57) | 0 (0.00) | - | - |
| Cough | 3 (5.36) | 1 (1.69) | - | - |
| Increased heart rate | 3 (5.36) | 2 (3.39) | - | - |
| Tinnitus and dizziness | 5 (8.93) | 1 (1.69) | - | - |
| Pulsatile headache | 2 (3.57) | 0 (0.00) | - | - |
| Total | 15 (26.79) | 4 (6.78) | 26.79 | 6.78 |
Table 3: Effects of Medication on Complication Rate in HE Patients, n (%)
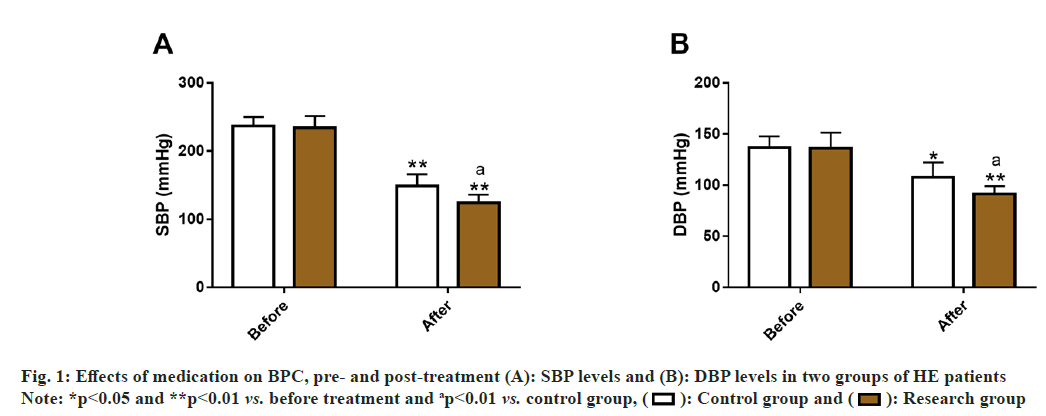
By detecting SBP and DBP of the two groups, the influence of the two medication methods on HE patients’ BPC was evaluated. Before treatment, the data showed no significant inter-group differences in SBP and DBP (p>0.05); but these two indices decreased significantly after treatment (p<0.05), and were lower in the research group compared with the control group (p<0.05) (fig. 1).
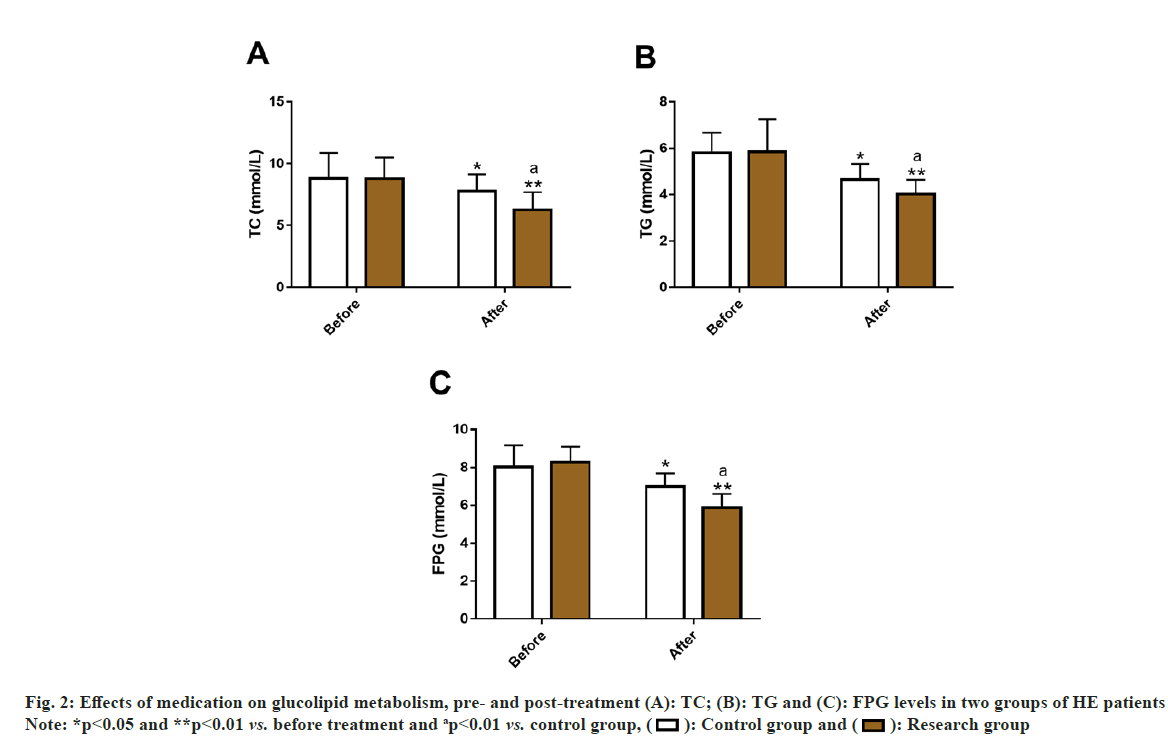
By detecting TC, TG and FPG in the two groups, the effects of two medication modalities on glucolipid metabolism in HE patients were assessed. Before treatment, the two groups were not statistically different in TC, TG and FPG (p>0.05). After treatment, both the groups showed a significant decrease in three indices (p<0.05), and were even lower in the research group (p<0.05) (fig. 2).
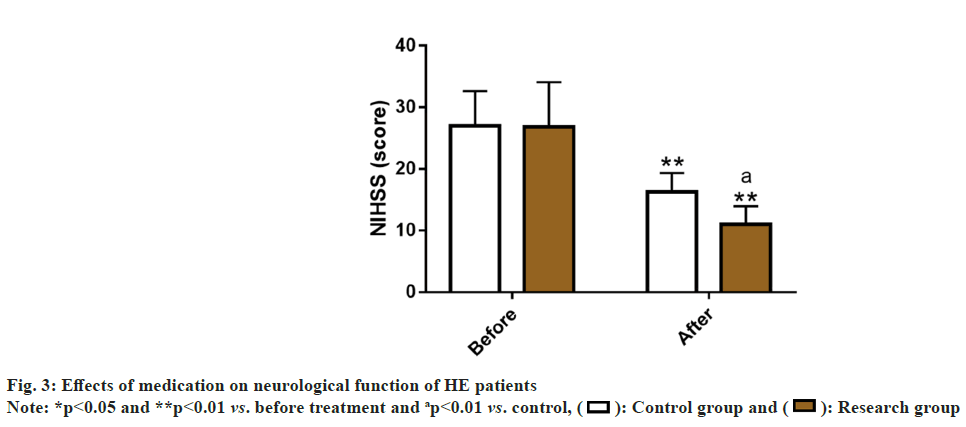
We evaluated the effects of the two medications on HE patients’ neurological function by measuring NIHSS scores. Before treatment, the NIHSS score was similar in both groups (p>0.05), but it reduced markedly after treatment (p<0.05), with a lower score in the research group as compared to the control group (p<0.05) (fig. 3).
In this study, CAP+NIT injection therapy (research group) and NIT injection alone (control group) were comparatively analyzed in multiple dimensions such as curative effect, incidence of complications, BPC, glucolipid metabolism and neurological function and reported.
The ORR of the research group (91.53 %) was significantly higher than that of the control group (76.79 %), suggesting that CAP+NIT injection therapy can significantly improve the curative effect of HE patients. According to Wu et al. the antihypertensive effect of CAP may be related to its changes in intestinal microflora[18]. While the hypotensive action of NIT is mainly due to its regulation and control of molecular pathways such as NO, guanylate cyclase, nucleotide cyclic guanosine phosphate and myosin, thereby causing the relaxation of vascular smooth muscle and reduction of vascular load[19,20]. In addition, the incidence of vomiting, cough, increased heart rate, tinnitus and dizziness, and pulsatile headache were statistically analyzed and determined to be notably lower in the research group compared with the control group (6.78 % vs. 26.79 %). In the study of Maleki et al. CAP has similar antihypertensive effects to nifedipine and NIT sublingual tablets, but it has the highest safety with the least side effects[21]. Divakaran et al. also pointed out that although NIT is the standard therapy for cardiovascular diseases including HT, it may cause the aforementioned adverse reactions such as headache, dizziness and vomiting, which were consistent with our research results[22]. In terms of BPC, SBP and DBP of the research group were evidently reduced after treatment compared with the pre-treatment levels and the control group, indicating better performance of the combined treatment scheme vs. NIT injection alone in terms of BP reduction and BPC. In United States, up to 54 % of hypertensive patients have failed to effectively control their BP, which further suggests the importance of BPC in HE[23]. A Spontaneously Hypertensive Rat (SHR) model experiment confirmed that CAP's BPC effect may be attributed to its inhibition of nuclear factor kappa (κ) B signal transduction, which not only plays a role in lowering BP, but also significantly alleviates pathological processes such as kidney injury and renal inflammation[24]. On the other hand, after the treatment results of glycolipid metabolism indices showed that TC, TG and FPG in the research group were significantly lower vs. the control group, which shows that CAP+NIT injection therapy is more beneficial to address the abnormal glucose and lipid metabolism of HE patients. Man et al. pointed out that CAP combined with Shunaoxin pill, a traditional Chinese medicine prescription, can reduce dyslipidemia by reducing TG, TC and High Density Lipoprotein Cholesterol (HDLC) levels in SHRs model, which supports our findings[12].
The NIHSS scale was further used to evaluate the neurological function of two groups of HE patients. After treatment, the research group showed lower NIHSS scores that were much lower than the control group, indicating that the combined treatment of CAP and NIT injection can improve the neurological function of HE patients more significantly. Previous studies have shown that CAP can play a physiological role in regulating BP by its influence on sympathetic nervous system, while exerting neuroprotective effects by alleviating the nervous system-related damage[25].
To sum up, CAP combined with NIT injection has a positive effect on enhancing the curative effect of HE patients, reducing the incidence of complications, improving patients’ BPC, glucolipid metabolism and neurological function. However, we have not yet observed the quality of life of patients before and after treatment. In addition, the influencing factors on neurological function in HE patients require additional investigation and these deficiencies need to be further addressed in the future.
Conflict of interests:
The authors declared no conflict of interests.
References
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 2016;134(6):441-50.
[Crossref] [Google Scholar] [PubMed]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71(19):e127-248.
[Crossref] [Google Scholar] [PubMed]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2224-60.
[Crossref] [Google Scholar] [PubMed]
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017;317(2):165-82.
[Crossref] [Google Scholar] [PubMed]
- Deshmukh A, Kumar G, Kumar N, Nanchal R, Gobal F, Sakhuja A, et al. Effect of Joint National Committee VII report on hospitalizations for hypertensive emergencies in the United States. Am J Cardiol 2011;108(9):1277-82.
[Crossref] [Google Scholar] [PubMed]
- Kotruchin P, Pratoomrat W, Mitsungnern T, Khamsai S, Imoun S. Clinical treatment outcomes of hypertensive emergency patients: Results from the hypertension registry program in Northeastern Thailand. J Clin Hypertens 2021;23(3):621-7.
[Crossref] [Google Scholar] [PubMed]
- Pierin AM, Flórido CF, Santos JD. Hypertensive crisis: Clinical characteristics of patients with hypertensive urgency, emergency and pseudocrisis at a public emergency department. Einstein 2019;17(4):1-8.
[Crossref] [Google Scholar] [PubMed]
- ACOG Committee Opinion No. 767: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol 2019;133(2):e174-80.
- Simko F, Pechanova O, Repova K, Aziriova S, Krajcirovicova K, Celec P, et al. Lactacystin-induced model of hypertension in rats: Effects of melatonin and captopril. Int J Mol Sci 2017;18(8):1-15.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhang L, Fan X, Yang W, Yu B, Kou J, et al. Captopril attenuates TAC-induced heart failure via inhibiting Wnt3a/β-catenin and Jak2/Stat3 pathways. Biomed Pharmacother 2019;113:1-7.
[Crossref] [Google Scholar] [PubMed]
- Repova K, Stanko P, Baka T, Krajcirovicova K, Aziriova S, Hrenak J, et al. Lactacystin-induced kidney fibrosis: Protection by melatonin and captopril. Front Pharmacol 2022;13:1-11.
[Crossref] [Google Scholar] [PubMed]
- Man S, Yang L, Xiang H, Lu G, Wang Y, Liu C, et al. Antihypertensive and renal protective effect of Shunaoxin pill combined with captopril on spontaneous hypertension rats. Biomed Pharmacother 2020;125:1-8.
[Crossref] [Google Scholar] [PubMed]
- López-Rivera F, Martínez HR, LaTorre CC, González AR, Vélez JG, Ferrer VF, et al. Treatment of hypertensive cardiogenic edema with intravenous high-dose nitroglycerin in a patient presenting with signs of respiratory failure: A case report and review of the literature. Am J Case Rep 2019;20:83-90.
[Crossref] [Google Scholar] [PubMed]
- Kim CO, Song J, Min JY, Park SJ, Lee HM, Byon HJ. A comparison of the pharmacokinetic and pharmacodynamic properties of nitroglycerin according to the composition of the administration set: A preliminary study. Medicine 2018;97(9):1-7.
[Crossref] [Google Scholar] [PubMed]
- Twiner MJ, Hennessy J, Wein R, Levy PD. Nitroglycerin use in the emergency department: Current perspectives. Open Access Emerg Med 2022;14:327-33.
[Crossref] [Google Scholar] [PubMed]
- Brathwaite L, Reif M. Hypertensive emergencies: A review of common presentations and treatment options. Cardiol Clin 2019;37(3):275-86.
[Crossref] [Google Scholar] [PubMed]
- Kazi SA, Siddiqui M, Majid S. Stroke outcome prediction using admission NIHSS in anterior and posterior circulation stroke. J Ayub Med Coll Abbottabad 2021;33(2):274-8.
[Google Scholar] [PubMed]
- Wu H, Lam TY, Shum TF, Tsai TY, Chiou J. Hypotensive effect of captopril on deoxycorticosterone acetate-salt-induced hypertensive rat is associated with gut microbiota alteration. Hypertens Res 2022;45(2):270-82.
[Crossref] [Google Scholar] [PubMed]
- Chiesa JJ, Baidanoff FM, Golombek DA. Don’t just say no: Differential pathways and pharmacological responses to diverse nitric oxide donors. Biochem Pharmacol 2018;156:1-9.
[Crossref] [Google Scholar] [PubMed]
- Næsheim T, How OJ, Myrmel T. Hemodynamic effects of a soluble guanylate cyclase stimulator, riociguat, and an activator, cinaciguat, during NO-modulation in healthy pigs. J Cardiovasc Pharmacol Ther 2021;26(1):75-87.
[Crossref] [Google Scholar] [PubMed]
- Maleki A, Zaman M, Tarrahi MJ, Nabatchi B. Nifedipine, captopril or sublingual nitroglycerin, which can reduce blood pressure the most?. ARYA Atheroscler 2011;7(3):102-5.
[Google Scholar] [PubMed]
- Divakaran S, Loscalzo J. The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J Am Coll Cardiol 2017;70(19):2393-410.
[Crossref] [Google Scholar] [PubMed]
- Mitsungnern T, Srimookda N, Imoun S, Wansupong S, Kotruchin P. The effect of pursed‐lip breathing combined with number counting on blood pressure and heart rate in hypertensive urgency patients: A randomized controlled trial. J Clin Hypertens 2021;23(3):672-9.
[Crossref] [Google Scholar] [PubMed]
- Gan Z, Huang D, Jiang J, Li Y, Li H, Ke Y. Captopril alleviates hypertension-induced renal damage, inflammation, and NF-κB activation. Braz J Med Biol Res 2018;51(11):1-9.
[Crossref] [Google Scholar] [PubMed]
- Dong T, Chen JW, Tian LL, Wang LH, Jiang RD, Zhang Z, et al. Role of the renin-angiotensin system, renal sympathetic nerve system, and oxidative stress in chronic foot shock-induced hypertension in rats. Int J Biol Sci 2015;11(6):652-63.
[Crossref] [Google Scholar] [PubMed]
 Research group
Research group