- *Corresponding Author:
- Nianling Yao
Department of Obstetrics and Gynecology, The First Affiliated Hospital of The Fourth Military Medical University, Xi'an, Shanxi 710032, China
E-mail: woshibailu223262@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “20-25” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present research is to discuss the effect of carboprost tromethamine plus oxytocin on the efficacy, safety and blood loss of patients with placenta previa and hemorrhage. 118 individuals with placenta previa and hemorrhage from December 2018 to December 2022 were selected, and were divided into control group and research group. Control group received routine treatment plus oxytocin, while research group were additionally given carboprost tromethamine on the basis of the treatment used for control group. The curative effect, safety (chills, dizziness, nausea and vomiting), blood loss (intraoperative and 2 h postoperative blood loss), recovery (hemostasis time, operation time and length of stay), serum inflammatory indices (tumor necrosis factor-alpha, interleukin-6 and white blood cells count) were comparatively analyzed. After analysis, research group was found to have a higher total treatment effective rate, a lower incidence of adverse events, and less blood loss intraoperatively and 2 h postoperatively; in addition, shorter hemostasis time, operation time and length of stay were determined in research group. All the above differences were statistically significant, while the two groups showed similar levels of serum inflammatory indicators. The combination of carboprost tromethamine and oxytocin is effective and safe for the treatment of patients with placenta previa and hemorrhage, which can validly reduce the amount of bleeding during and 2 h after surgery, speed up hemostasis, and promote patient recovery.
Keywords
Carboprost tromethamine, oxytocin, placenta previa and hemorrhage, efficacy and safety, blood loss
Placenta Previa (PP) which is characterized by abnormal placental coverage of the cervical ostia, can lead to adverse maternal and infant outcomes, especially Postpartum Hemorrhage (PPH) and organ damage[1,2]. PP patients are associated with a 4 fold increased risk of maternal hemorrhage in the second trimester, as well as an increased probability developing of adverse events such as perinatal hysterectomy, blood transfusion, PPH, and placenta accreta[3]. The influence of PP on the fetus is mainly reflected in premature delivery and intrauterine growth restriction, which poses a certain death threat to newborns from singleton pregnancies[4]. Pathologically, PP may be induced by multiple miscarriages, curettage, cesarean section, multiple pregnancies, and smoking history[5,6]. The pathogenesis of PP is complex, with less bleeding induced initially, but this may be followed by recurrent bleeding with increased bleeding and, in severe cases, haemorrhagic shock[7]. Therefore, it is crucial to intervene in time for PP and hemorrhage, which is of great value to save the lives of mothers and infants and improve their clinical outcomes.
At present, pharmacotherapy remains the basis of treatment for patients with PP and hemorrhage after cesarean section. Although medication plays a certain role in preventing PPH, most patients experience adverse reactions during treatment[8]. Oxytocin (OXT), as a pleiotropic peptide hormone with anti-inflammatory and antioxidant effects can regulate the autonomic nervous and immune systems, and has been widely used in postpartum depression, pain, cardiovascular diseases, etc.[9-12].
A double-blind, placebo-controlled, randomized trial suggests that the use of OXT in women with cesarean section in the form of increased infusions reduces the need for additional uterine contractions without affecting the overall risk of major obstetric hemorrhage, suggesting that the application of OXT is cost-effective in the prevention of PPH[13]. Carboprost Tromethamine (CT), is a synthetic prostaglandin derivative in essence, which can prevent PPH after cesarean section and effectively reduce PPH by promoting regular uterine contractions, alleviating blood hypercoagulability and maintaining hemodynamic stability[14]. Previous studies have suggested that CT is more effective than OXT in preventing PPH in patients at high risk of cesarean section and is effective in reducing blood loss[15]. In addition, it reduces the risk of PPH in women in the 3rd stage of labour and is tolerated by patients[16].
Given the lack of studies on the effect of CT plus OXT on the clinical outcomes of patients with PP and hemorrhage, this study conducts relevant analysis to provide new insights into the clinical management of such a patient population.
Materials and Methods
General information:
This study has been approved by the Ethics Committee of The First Affiliated Hospital of The Fourth Military Medical University hospital, and all subjects signed and provided the informed consent. 118 study participants with PP and hemorrhage admitted to The First Affiliated Hospital of The Fourth Military Medical University hospital hospital between December 2018 and December 2022 were considered. Among them, 55 individuals in the control group received OXT treatment, and 63 individuals in the research group were treated with CT plus OXT. Research group and the control group showed no statistical difference in the general data (p>0.05) and were clinically comparable.
Inclusion criteria:
All the patients, treatment-naive; who were diagnosed with PP by imaging examination; who had signs and symptoms; who met the indications for cesarean section; with complete information and willingness to cooperate with the research were included in this study.
Exclusion criteria:
Pregnant women with hysteromyoma and macrosomia; patients having coagulation dysfunction, immune disorders, or heart, liver, kidney and lung, acute pelvic inflammatory disease, pregnancy-induced hypertension syndrome and scarred uterus; patients with malignant tumors; patients with vital organ dysfunction; patients who had mental illness or communication disorders; patients with multiple pregnancies and contraindications to OXT and/or CT, allergy to Apixaban (API) or Low Molecular Weight Heparin (LMWH) were excluded.
Methods:
All the patients in control group received OXT therapy. (5-10) U of OXT was injected intramuscularly via an intravenous bolus after the placenta was removed.
In addition to the above treatment, the research group was treated with CT along with OXT. Deep intramuscular injection of CT was administered to the parturient; with the dosage determined depending on the mother’s specific physical condition. When necessary, the medicine was supplemented once every (15-30) min (250 μg), with the total dose not exceeding 12 mg and the drug not used continuously for >2 d.
Outcome measures:
Drug efficacy: The drug efficacy was explained using different grades namely, markedly effective, effective and ineffective. Markedly effective means that the maternal uterine contraction is obvious, explaining that the maternal hemorrhage is significantly reduced after 15 min of drug administration. Effective corresponds to obviously improved uterine contractions and a decreasing trend of maternal hemorrhage volume after 30 min of drug administration. No changes in uterine contractions and maternal hemorrhage after 30 min of drug administration were deemed to be ineffective. The total effective rate is the sum of markedly effective and effective rates.
Total effective rate=markedly effective+effective rate
Safety: We observed and recorded the cases of adverse events such as chills, dizziness, nausea and vomiting in the two groups after drug treatment and calculated the rate of incidence.
Blood loss: The amount of bleeding during and after 2 h of surgery was observed and recorded for comparative analysis between both the groups.
Patient recovery: Surgical indices such as Hemostasis Time (HT), Operation Time (OT), and Length of Stay (LOS) were observed and recorded.
Serum inflammatory indicators: Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-6 (IL) and White Blood Cell (WBC) count were detected by Enzyme-Linked Immunosorbent Assays (ELISAs).
Statistical analysis:
The number of cases/percentage (n/%) and mean±Standard Error of the Mean (SEM) was used to represent categorical and continuous variables, respectively. Among them, Chi-square (χ2) test was used to compare categorical variables, and the independent sample t-test was used to compare continuous variables between groups. In this study, data were input into the Statistical Package of Social Sciences (SPSS) 18.0 software package for statistical analysis with the statistically significant value considered was p<0.05.
Results and Discussion
The general data such as age, gestational age, parity, type of PP, smoking history and alcoholism history of the two groups were compared and tested between both the groups. No significant difference was identified (p>0.05), as shown in Table 1.
| Indicators | Control group (n=55) | Research group (n=63) | χ 2/t | p |
|---|---|---|---|---|
| Age (y) | 25.38±3.05 | 26.62±4.09 | 1.844 | 0.068 |
| Gestational age (w) | 37.85±2.04 | 37.37±1.77 | 1.369 | 0.174 |
| Parity (primiparity/multiparity) | 10/45 | 13/50 | 0.113 | 0.737 |
| Type of PP (complete/partial/marginal) | 18/20/17 | 19/28/16 | 0.852 | 0.653 |
| Smoking history (Yes/No) | 13/42 | 10/53 | 1.128 | 0.288 |
| History of alcoholism (Yes/No) | 16/39 | 20/43 | 0.098 | 0.755 |
Table 1: General Data of Patients with PP and Hemorrhage
Further, curative effects were evaluated. Qualitative evaluation of the curative effect of both groups showed a statistical inter-group difference, with the total effective rate being 90.48 % in research group and 76.36 % in control group (p<0.05) (Table 2).
| Indicators | Control group (n=55) | Research group (n=63) | χ2 | p |
|---|---|---|---|---|
| Markedly effective | 14 (25.45) | 47 (74.60) | ||
| Effective | 28 (50.91) | 10 (15.87) | ||
| Ineffective | 13 (23.64) | 6 (9.52) | ||
| Total effectiveness | 42 (76.36) | 57 (90.48) | 4.329 | 0.038 |
Table 2: Curative Effects of Patients With PP and Hemorrhage
Safety of the drug was evaluated by a comparative evaluation of adverse events such as chills, dizziness, nausea and vomiting between the two groups showed that the total incidence of adverse events in research group was 6.35 %, which was significantly <21.82 % in control group (p<0.05) (Table 3).
| Indicators | Control group (n=55) | Research group (n=63) | χ 2 | p |
|---|---|---|---|---|
| Chills | 5 (9.09) | 0 (0.00) | ||
| Dizziness | 3 (5.45) | 2 (3.17) | ||
| Nausea and vomiting | 4 (7.27) | 2 (3.17) | ||
| Total | 12 (21.82) | 4 (6.35) | 5.995 | 0.014 |
Table 3: Safety of Patients With PP and Hemorrhage
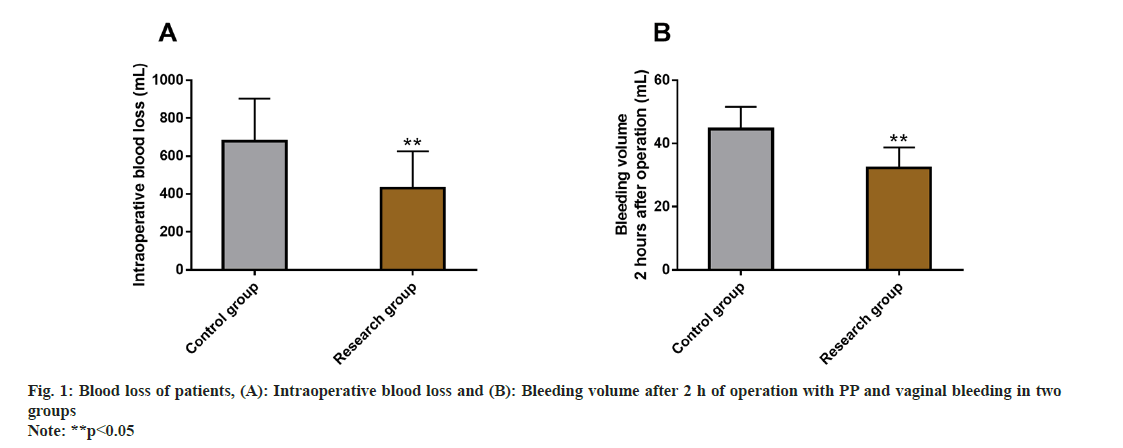
Intraoperative and postoperative blood loss was also evaluated between the two groups. The blood loss within and after 2 h of surgery was detected, and the intraoperative and postoperative blood loss after 2 h were found to be markedly less in research group than in control group (p<0.05) (fig. 1).
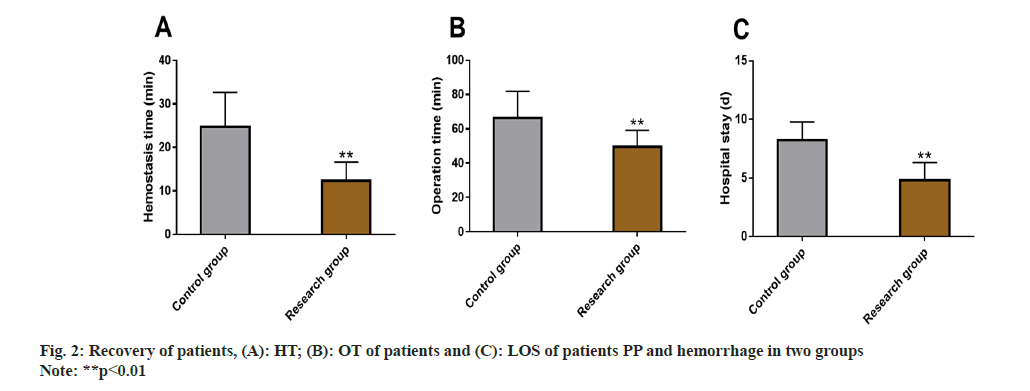
Patient recovery was estimated using the surgical indices such as HT, OT, and LOS, which were evaluated, following the influence of the two intervention methods of patients recovery.
The data identified lower levels of the above mentioned 3 indices in research group vs. control group, with statistical significance (p<0.05) (fig. 2).
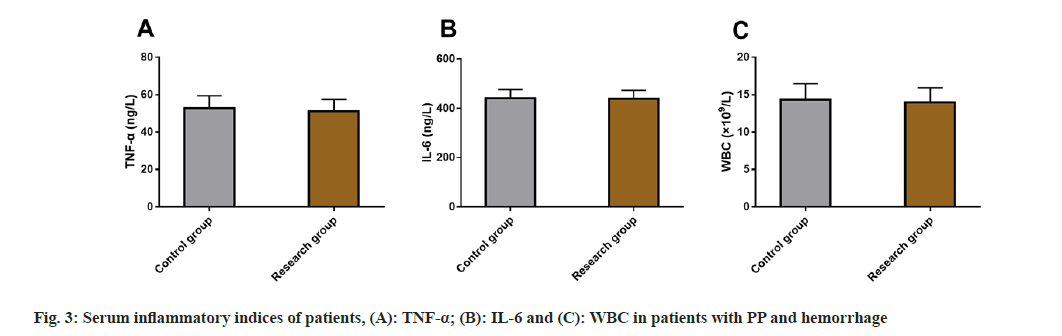
Finally, serum inflammatory indices such as TNF-α, IL-6 and WBC in patients with PP and hemorrhage were also detected and evaluated between the two groups. However, no significant difference was identified between both the groups in serum inflammatory indices (p>0.05) (fig. 3).
PP and hemorrhage are the major causes of maternal death, with a prevalence rate of 0.56 % in women; moreover such patients are associated with a 3.5 % risk of hysterectomy after cesarean section as well as increased medical expenses[17,18]. In order to reduce the risk of maternal death related to PP and associated hemorrhage, it is necessary to further optimize the treatment of such patients.
This study qualitatively evaluated the treatment outcomes to assess the impact of two treatments on the efficacy of patients with PP and hemorrhage. The total effective rate of research group was found to be significantly higher than that of control group (90.48 % vs. 76.36 %), indicating that CT+OXT therapy has a significant effect on improving the curative effect of patients with PP and hemorrhage. OXT, also known as pitocin, is commonly used to prevent and treat PPH, due to the advantages of rapid onset and good tolerance, without causing adverse events such as elevated blood pressure or tetanic uterine contractions[19]. However, the dosage of OXT is limited, as an excessive dose of OXT can lead to compromised efficacy due to the influence of the saturation of OXT receptors in the uterine myometrium, accompanied by adverse conditions such as coronary artery contraction, water poisoning, and hypotension[20]. Therefore, to ensure the efficacy, OXT is often used combination with other drugs. CT has the advantage of long half-life and high life activity, which can play a better role in preventing PPH by enhancing the contraction of uterine smooth muscle and blood vessels[21,22]. According to the statistics of adverse events such as chills, dizziness, nausea and vomiting, the overall incidence of adverse events was markedly lower in research group than in control group (6.35 % vs. 21.82 %), suggesting that the treatment of patients with PP and hemorrhage by CT+OXT can prevent the above mentioned adverse events to some extent. After analysis of bleeding, it was found that the blood loss was significantly less in research group compared with control group, indicating that CT+OXT can significantly prevent PP associated bleeding, which was mainly manifested by lower intraoperative bleeding and 2 h postoperative blood loss. In terms of recovery, obviously shorter HT, OT, and LOS were determined in research group, suggesting the effect of CT+OXT treatment has a good effect on hemostasis and body recovery of patients with PP and hemorrhage. In addition, the combination of CT and OXT has a synergistic effect on the prevention of PPH, and this effect may be attributed to the different receptors, allowing them to complement each other when playing the relevant preventive and therapeutic roles[23]. Moreover, the combined use of the two has a synergistic effect on improving the level of calcium ions in the maternal cytoplasm, which can further promote the contractility of uterine muscle fibers[24]. Subsequently although without statistical significance, TNF-α, IL-6 and WBC levels were found to be lower in research group than control group after intervention while detection of serum inflammatory indices, demonstrating a comparable effect of CT+OXT to OXT monotherapy on the regulation of TNF-α, IL-6 and WBC in patients with PP and hemorrhage. TNF-α, IL-6, and WBC levels have been indicated to be associated with perioperative infections[25,26]. The above results showed that CT plus OXT therapy can prevent perioperative infections to a certain extent, thereby reducing the risk of adverse reactions.
To sum up, CT plus OXT therapy for patients with PP and hemorrhage can significantly alleviate symptoms such as uterine contraction and maternal hemorrhage, with obvious curative effects and high safety. It can also reduce bleeding volume during and 2 h after surgery and facilitate hemostasis and patient rehabilitation, providing a novel clinical option for the management of patients with PP and hemorrhage.
Conflict of interests:
The authors declared no conflict of interest.
References
- Gibbins KJ, Einerson BD, Varner MW, Silver RM. Placenta previa and maternal hemorrhagic morbidity . J Matern Fetal Neonatal Med 2018;31(4):494-9.
[Crossref] [Google Scholar] [PubMed]
- Bi S, Zhang L, Wang Z, Chen J, Tang J, Gong J, et al. Effect of types of placenta previa on maternal and neonatal outcomes: A 10 y retrospective cohort study. Arch Gynecol Obstet 2021;304(1):65-72.
[Crossref] [Google Scholar] [PubMed]
- Rosenberg T, Pariente G, Sergienko R, Wiznitzer A, Sheiner E. Critical analysis of risk factors and outcome of placenta previa. Arch Gynecol Obstet 2011;284(1):47-51.
[Crossref] [Google Scholar] [PubMed]
- Fan D, Wu S, Wang W, Xin L, Tian G, Liu L, et al. Prevalence of placenta previa among deliveries in Mainland China: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016;95(40):1-7.
[Crossref] [Google Scholar] [PubMed]
- Kollmann M, Gaulhofer J, Lang U, Klaritsch P. Placenta praevia: Incidence, risk factors and outcome. J Matern Fetal Neonatal Med 2016;29(9):1395-8.
[Crossref] [Google Scholar] [PubMed]
- Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: Diagnosis and management: Green-top guideline No. 27a. BJOG 2019;126(1):e1-48.
[Crossref] [Google Scholar] [PubMed]
- Chen D, Xu J, Ye P, Li M, Duan X, Zhao F, et al. Risk scoring system with MRI for intraoperative massive hemorrhage in placenta previa and accreta. J Magn Reson Imaging 2020;51(3):947-58.
[Crossref] [Google Scholar] [PubMed]
- Koh K, Kanagalingam D, Kathirvel R. A rare case of watery vaginal discharge due to caesarean scar dehiscence following brace suture and balloon tamponade for the management of postpartum hemorrhage. Case Rep Obstet Gynecol 2020:1-4.
[Crossref] [Google Scholar] [PubMed]
- Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, et al. Is oxytocin "Nature's Medicine"? Pharmacol Rev 2020;72(4):829-61.
[Crossref] [Google Scholar] [PubMed]
- Thul TA, Corwin EJ, Carlson NS, Brennan PA, Young LJ. Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology 2020;120:1-14.
[Crossref] [Google Scholar] [PubMed]
- Li XH, Matsuura T, Xue M, Chen QY, Liu RH, Lu JS, et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep 2021;36(3):109411.
[Crossref] [Google Scholar] [PubMed]
- Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A, ?era T. Complementary role of oxytocin and vasopressin in cardiovascular regulation. Int J Mol Sci 2021;22(21):1-24.
[Crossref] [Google Scholar] [PubMed]
- Sheehan SR, Montgomery AA, Carey M, McAuliffe FM, Eogan M, Gleeson R, et al. Oxytocin bolus vs. oxytocin bolus and infusion for control of blood loss at elective caesarean section: Double blind, placebo controlled, randomized trial. BMJ 2011;343:1-11.
[Crossref] [Google Scholar] [PubMed]
- Ling Z, Yao L, Cui Z, Lifan C. Effect of carboprost tromethamine in prevention of postpartum hemorrhage in cesarean section. Pak J Pharm Sci 2018;31(5):2257-62.
[Google Scholar] [PubMed]
- Bai J, Sun Q, Zhai H. A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high-risk patients undergoing cesarean delivery. Exp Ther Med 2014;7(1):46-50.
[Crossref] [Google Scholar] [PubMed]
- Kumar KSS, Shyam S, Batakurki P. Carboprost vs. oxytocin for active management of third stage of labor: A prospective randomized control study. J Obstet Gynaecol India 2016;66:229-34.
[Crossref] [Google Scholar] [PubMed]
- Sadashivaiah J, Wilson R, Thein A, McLure H, Hammond CJ, Lyons G. Role of prophylactic uterine artery balloon catheters in the management of women with suspected placenta accreta. Int J Obstet Anesth 2011;20(4):282-7.
[Crossref] [Google Scholar] [PubMed]
- Nankali A, Salari N, Kazeminia M, Mohammadi M, Rasoulinya S, Hosseinian-Far M. The effect prophylactic internal iliac artery balloon occlusion in patients with placenta previa or placental accreta spectrum: A systematic review and meta-analysis. Reprod Biol Endocrinol 2021;19(1):1-16.
[Crossref] [Google Scholar] [PubMed]
- Roach MK, Abramovici A, Tita AT. Dose and duration of oxytocin to prevent postpartum hemorrhage: A review. Am J Perinatol 2013;30(7):523-8.
[Crossref] [Google Scholar] [PubMed]
- Gungorduk K, Asicioglu O, Celikkol O, Olgac Y, Ark C. Use of additional oxytocin to reduce blood loss at elective caesarean section: A randomised control trial. Aust N Z J Obstet Gynaecol 2010;50(1):36-9.
[Crossref] [Google Scholar] [PubMed]
- Hamdan M, Shuhaina S, Hong JGS, Vallikkannu N, Zaidi SN, Tan YP, et al. Outpatient vs. inpatient foley catheter induction of labor in multiparas with unripe cervixes: A randomized trial. Acta Obstet Gynecol Scand 2021;100(11):1977-85.
[Crossref] [Google Scholar] [PubMed]
- Wolf MF, Sgayer I, Asslan A, Palzur E, Shnaider O, Bornstein J. The hormonal milieu by different labor induction methods in women with previous cesarean section: A prospective randomized controlled trial. Reprod Sci 2021;28(12):3562-70.
[Crossref] [Google Scholar] [PubMed]
- Macdonald K, Feifel D. Helping oxytocin deliver: Considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci 2013;7:1-21.
[Crossref] [Google Scholar] [PubMed]
- Marcus HE, Fabian A, Lier H, Dagtekin O, Böttiger BW, Teschendorf P, et al. Survey on the use of oxytocin for cesarean section. Minerva Anestesiol 2010;76(11):890-5.
[Google Scholar] [PubMed]
- Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-α: Markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br 2007;89(1):94-9.
[Crossref] [Google Scholar] [PubMed]
- Cramm SL, Graham DA, Allukian M, Blakely ML, Chandler NM, Cowles RA, et al. Predictive value of routine WBC count obtained before discharge for organ space infection in children with complicated appendicitis: Results from the eastern pediatric surgery network. J Am Coll Surg 2023;236(6):1181-7.
[Crossref] [Google Scholar] [PubMed]