- *Corresponding Author:
- Q. R. Hu
Department of Hepatopancreatobiliary Surgery,
The First People’s Hospital of Yongkang,
Yongkang,
Zhejiang 321300,
China
E-mail: dr_huqirong@126.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “62-68” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Pancreatic cancer is one of the most lethal solid malignancies featured by rapid disease progression with no significant clinical symptoms and the treatment of pancreatic cancer remains unmet clinical need. Thus, to find a novel and potent therapeutic strategy for the treatment of pancreatic cancer is highly desired. The present study investigated the role and molecular mechanisms of cardamonin in pancreatic cancer cell line BxPC3. Cells are treated with cardamonin for 48 h and 72 h and evaluated cytotoxicity of cardamonin by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Clonogenic assay was applied to observe the anti-proliferation of cardamonin in pancreatic cancer cell line BxPC3 cells. Hoechst staining and flow cytometry were used to verify the mechanism of anti-proliferation of cardamonin in pancreatic cancer cell line BxPC3 cells. The expression of apoptosis-related proteins was evaluated by western blotting to investigate the molecular mechanism of cardamonin in pancreatic cancer cell line BxPC3 cells. The results of the present study showed that cardamonin inhibited the proliferation of pancreatic cancer cell line BxPC3 cells with the half maximal inhibitory concentration value of 28.86 μM and 22.83 μM at 48 h and 72 h respectively. Moreover, cardamonin induced apoptosis in BxPC3 cells via deoxyribonucleic acid damage to inhibit the pancreatic cancer. Overall, these results suggested that cardamonin has the potential to be used as a new therapeutic approach for pancreatic cancer.
Keywords
Cardamonin, pancreatic cancer cell line BxPC3 cells, deoxyribonucleic acid damage, clonogenic assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
Pancreatic cancer is ranked among the top five deathrelated solid malignancies in United States[1] and among all the types of pancreatic cancer, Pancreatic Ductal Adenocarcinoma (PDAC) accounts for more than 90 % of pancreatic cancer. In 2018, there was 458 918 new cases reported of PDAC and 432 242 deaths around the world[2]. The 5 y survival rate of patients is merely 7 % and the median survival time is 4-6 mo after diagnosis[3]. Early diagnosis of the malignancy is considered the most suitable tool to combat the disease. Nevertheless, PDAC is featured by rapid disease progression with no significant clinical symptoms, resulting early detection and/or treatment is extremely difficult[4]. Moreover, little progress has been made in improving the long-term survival rate of patients over the past decades[5]. Only 20 % of patients are surgically resectable at the time of diagnosis and chemotherapy or chemoradiotherapy remains the major therapeutic option for most patients, in particular for those who suffer from local unresectable or advanced metastatic tumors. However, chemotherapy can only prolong a patient’s life for a few months[6]. Therefore, new therapeutic approaches need to be investigated to improve the treatment of this deadly neoplasm.
Natural products have historically served as an important source of bioactive agents and immense studies reported for the purpose of developing effective antitumor compounds[7-9]. Natural products like chalcone was highly studied and some derivatives showed excellent anticancer effects[10,11]. Cardamonin (CAD) is a natural chalcone isolated from Alpinia rafflesiana (fig. 1A)[12]. It has showed anti-inflammatory, antitumor, anti-microbial effects as well as other biological activities in the application in researches[13-15]. CAD significantly inhibited Dextran Sulfate Sodium (DSS) and 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS) induced colitis in mice[16]. It also enhances the antiproliferative effect of cisplatin on ovarian cancer[17]. CAD has the ability to alleviate pressure overload- induced heart failure through inhibition of oxidative stress[18]. Moreover, it modulates cell apoptosis in several tumors, including nasopharyngeal carcinoma, prostate cancer and glioblastoma[19-21].
To our knowledge, the anti-tumor activity of CAD on pancreatic cancer and underlying mechanisms of CAD on pancreatic cancer cells are not yet identified. In the current work, Human Pancreatic Cancer Cell Line (BxPC3) PDAC cells was used for anti-tumor evaluation of CAD. Cell viability and morphology were detected by 3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyltetrazolium Bromide (MTT) and Hoechst staining. Deoxyribonucleic Acid (DNA) damage and apoptosis change were appraised by flow cytometry and western blotting. The results of the present study revealed that CAD treatment inhibited the proliferation of pancreatic cancer through inducing apoptosis via DNA damage in PDAC cells.
Materials and Methods
Cell culture:
The human BxPC3 PDAC cell line was kind gifts from Laboratory of Marine Natural Products Chemistry, Wenzhou Medical University, China. The BxPC3 cells were cultured in the Dulbecco's Modified Eagle Medium (DMEM) (L120, Yuanpei, China) supplemented with 10 % Fetal Bovine Serum (FBS) (04-001-1A, BI, Israel) and routinely maintained at 37° in a humidified 5 % Carbon Dioxide (CO2) incubator (HERAcell 150i, Thermo Fisher Scientific, USA).
Cell viability assay:
BxPC3 was seeded in 96-well plates at a density of 5000 cells/well. After overnight incubation, the cells were treated with a dilution series of the CAD and continued to incubate for 48 h or 72 h. Then 20 μl of MTT reagent (4 mg/ml) (C0009S, Beyotime Biotechnology, China) was added to each well and incubated for 4 h under standard culture conditions (37°, 5 % CO2). Dimethyl Sulfoxide (DMSO) (200 μl) was then used to dissolve the formazan and the absorbance of each well was measured at 490 nm by a microplate reader (Multiskan FC, Thermo Fisher Scientific, USA).
Hoechst staining:
BxPC3 was seeded in 96-well plates at a density of 5000 cells/well. After overnight incubation, the cells were treated with a dilution series of the compounds and incubation was continued for 48 h. Then culture medium was removed and 100 μl of 2'-[4-ethoxyphenyl]-5- [4-methyl-1-piperazinyl]-2,5'-bi-1H-benzimidazole trihydrochloride trihydrate (Hoechst 33342) (1 μl/ml ) (C1025, Beyotime Biotechnology, China), in Phosphate Buffered Saline (PBS) was added to each well and incubated for 30 min under standard culture conditions, followed by washing cells with PBS. Fluorescence images were captured by using an inverted fluorescence microscope (Ti, Nikon Corporation, Japan) at 460 nm.
Clonogenic assay:
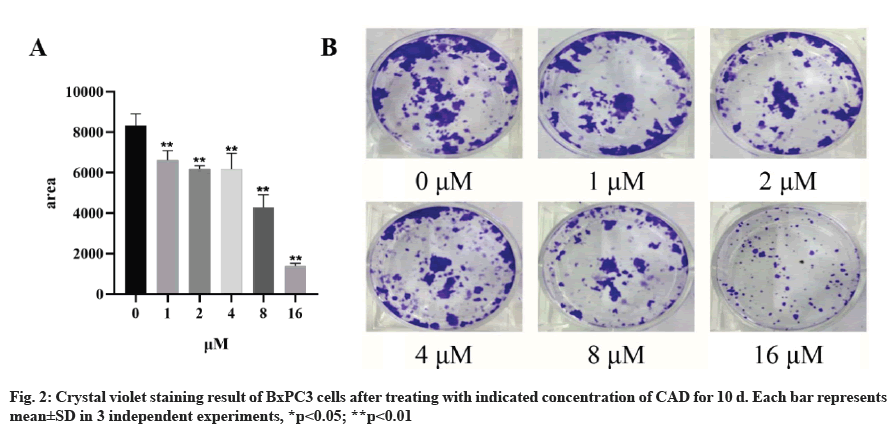
BxPC3 cells were seeded at 500 cells/well onto 6-well plates. Following the treatment of indicating concentration of CAD, cells were then allowed to grow for a further 10 d before fixation with methanol and staining with crystal violet (0.5 % solution). The number of viable colonies was then counted by ImageJ software.
Cell apoptosis detection:
Cell apoptosis caused by CAD was measured with a Fluorescein Isothiocyanate (FITC)/Annexin V apoptosis detection kit (C1062S, Beyotime Biotechnology, China). BxPC3 cells were seeded in a 6-well plate and treated with indicated concentration of CAD for 24 h. Cells were collected and washed with ice-cold PBS and incubated with Annexin V-FITC and Propidium Iodide (PI) in the dark for 20 min. The cells were then resuspended with binding buffer and measured using flow cytometry and analyzed using Flowing Software 2.
Western blotting analysis:
Whole cell extracts were prepared by lysing cells in Radioimmunoprecipitation Assay (RIPA) sample buffer (P0013B, Beyotime Biotechnology, China). An equivalent amount of cell lysate was electrophoresed on sodium dodecyl sulfate-polyacrylamide gels, transferred to polyvinylidene difluoride membranes (Immobilon; Millipore Corporation, Billerica, MA, USA) and probed with antibodies to B-cell lymphoma 2 (Bcl-2), Bcl-2-Associated X protein (Bax), Cytochrome C and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:2000; Rabbit mAb; Cell Signaling Technology, USA) were applied as primary antibodies. Anti-rabbit Immunoglobulin G (IgG), Horse Radish Peroxidase (HRP) linked antibody (1:1000; Cell Signaling Technology, USA) was applied as secondary antibody. Signals were detected using an enhanced chemiluminescence system.
Statistical analysis:
Results were compared via one-way Analysis of Variance (ANOVA) followed by Tukey’s test. All values were collected from 3 independent experiments and expressed as mean±Standard Deviation (SD). A p value of less than 0.05 was considered statistically significant.
Results and Discussion
CAD shows inhibition in BxPC3 cells. We first evaluated the anti-proliferation effect of CAD on the BxPC3 cells. Cancer cells were treated with different concentrations of CAD for 48 h and 72 h, DMSO serves as the negative control. MTT was given and the absorption was collected. As shown in fig. 1B, CAD significantly reduced total cell number in a dose-dependent manner. Prolonged with the treatment of CAD (72 h), it showed higher decreased in total viable cell number compared to 48 h treatments, indicated that the proliferation of BxPC3 cells were persistent and inhibited by given the compound. The half maximal inhibitory concentration (IC50) values of CAD on BxPC3 cells for 48 h and 72 h were calculated as 28.86 μM and 22.83 μM.
After 48 h treatment of compound, the morphology of cells was also observed under the microscope. As shown in fig. 1C, cells became long and tenuous in the 5 μM and 10 μM groups, most cells developed pseudopodium. The number of cells significantly reduced compared with control group. To our surprise, while there is some change of morphology of cells in 5 μM and 10 μM, a large number of cells became round and died after 15 μM treatment of CAD, these results may indicate that CAD inhibited cancer cells at low concentration, increasing the concentration have an effect in killing BxPC3 cells. Taken together, these results indicated that CAD inhibited cell growth of pancreatic cancer cells.
CAD inhibits proliferation of BxPC3 cells. To further determine the anti-proliferation of CAD on pancreatic cancer, the colony formation assay was carried to measure the colony number after the treatment of CAD. Then started with 500 cells per well, BxPC3 cells was given indicated CAD concentration for 10 d before capturing the results. As crystal violet staining results showed in the fig. 2, the numbers of colonies were decreased by CAD in a concentration-dependent manner. 1 μM CAD inhibited the growth of PDAC. This result indicated that CAD inhibited the proliferation of BxPC3 cells.
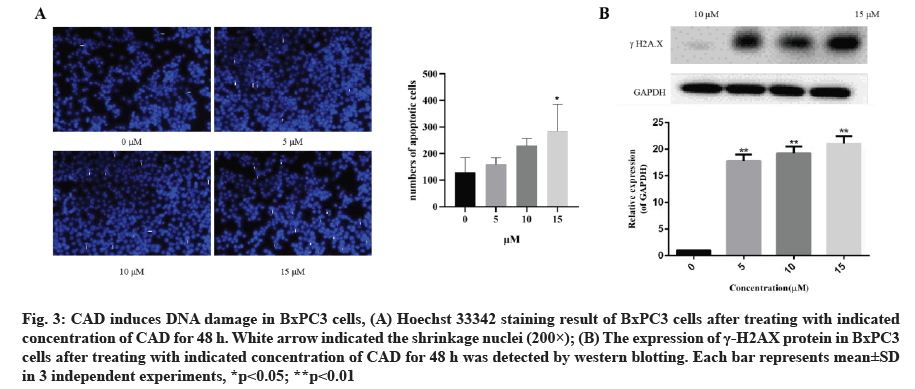
CAD induces DNA damage in BxPC3 cells. To investigate the anti-proliferation effects of CAD on PDAC, cells treated with 5, 10 and 15 μM CAD for 24 h were analyzed by staining with Hoechst 33342. As the results shown in fig. 3A and fig. 3B, cells treated with CAD (10 and 15 μM) induced chromatin condensation and nuclear shrinkage or fragmentation compared with no significant change in negative control group. Moreover, the number of cells with shrinkage nuclei increased in a concentration-dependent manner after the treatment of CAD, indicating that CAD may induces DNA damage of BxPC3 cells and thus inhibiting the survival of pancreatic cancer cells.
Fig. 3: CAD induces DNA damage in BxPC3 cells, (A) Hoechst 33342 staining result of BxPC3 cells after treating with indicated concentration of CAD for 48 h. White arrow indicated the shrinkage nuclei (200×); (B) The expression of γ-H2AX protein in BxPC3 cells after treating with indicated concentration of CAD for 48 h was detected by western blotting. Each bar represents mean±SD in 3 independent experiments, *p<0.05; **p<0.01
In order to investigate whether CAD induces DNA damage of BxPC3 cells, the expression of the biomarker of DNA damage, the gamma H2A Histone Family Member X (γ-H2AX), was detected by western blotting. As demonstrated in fig. 3B and fig. 3C, CAD significantly increased the formation of γ-H2AX in BxPC3 cells. This indicated that the anticancer activity of CAD involved the induction of DNA damage in BxPC3 cells which has been predicted.
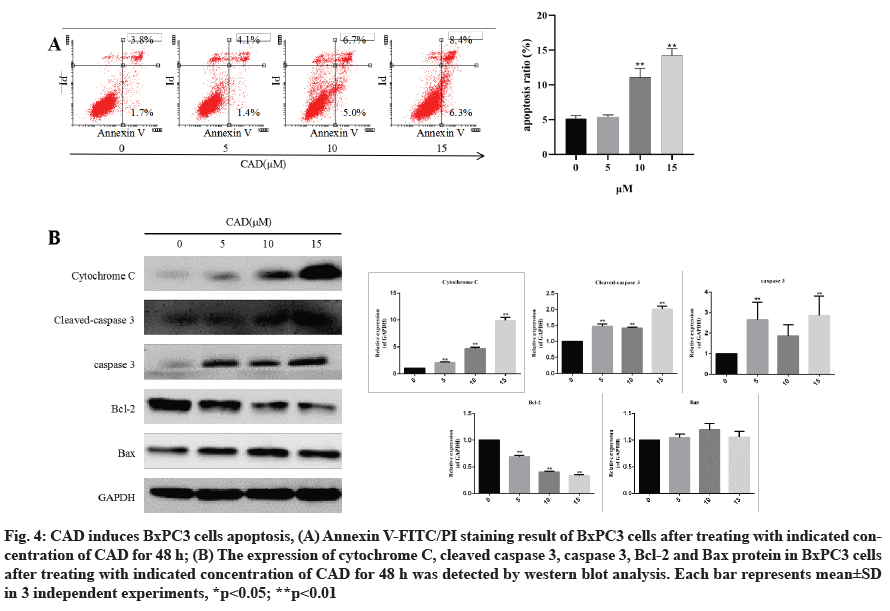
CAD induces BxPC3 cells apoptosis. To verify the effects of CAD in inducing apoptosis of pancreatic cancer cells, BxPC3 cells were treated with 5, 10, 15 μM CAD for 24 h and then they were analyzed by flow cytometry. As shown in fig. 4A, after treatment with CAD, a large percentage of BxPC3 cells underwent apoptosis in a concentration?dependent manner, as detected by Annexin V/PI analysis.
Fig. 4: CAD induces BxPC3 cells apoptosis, (A) Annexin V-FITC/PI staining result of BxPC3 cells after treating with indicated concentration of CAD for 48 h; (B) The expression of cytochrome C, cleaved caspase 3, caspase 3, Bcl-2 and Bax protein in BxPC3 cells after treating with indicated concentration of CAD for 48 h was detected by western blot analysis. Each bar represents mean±SD in 3 independent experiments, *p<0.05; **p<0.01
In order to investigate whether CAD induced BxPC3 cell apoptosis via the effects of apoptosis-associated proteins, immunoblotting was performed (fig. 4B). It was found that the pro-apoptotic protein Bax was upregulated, while the anti-apoptotic protein Bcl-2 was downregulated in BxPC3 cell line. Intrinsic or extrinsic pathways may be involved in apoptosis, therefore the expression of cleaved caspase-3 and cytochrome C were investigated. These apoptotic signaling proteins increased significantly after 24 h treatment with varying CAD concentrations (p<0.01).
Results showed that CAD regulates the expression of important pro-apoptotic and anti-apoptotic proteins, thus inducing apoptosis in pancreatic cancer cells.
Compared with normal pancreatic cells, pancreatic cancer cells proliferate significantly faster and resistance to apoptosis[22]. About 90 % of pancreatic cancers have Ki-67 index lower than 50 % and the tumor volume doubling time is also significantly shorter than other tumors. Moreover, pancreatic cancer has a poor prognosis and is prone to recurrence, which makes pancreatic cancer one of the most lethal tumors[23]. In addition to resectable pancreatic cancer, the clinical treatment of pancreatic cancer is mainly treated with drugs. At present, the commonly used clinical treatment plan is gemcitabine monotherapy or a combination of gemcitabine and other drugs, and the combination of Fluorouracil, Leucovorin, Irinotecan and Oxaliplatin (FOLFIRINOX). However, the response rate of singledrug therapy is only 5 %-10 % and the combined drug regimen is also limited in improving survival.
CAD has been reported to have therapeutic effects in a variety of tumors. It inhibits the growth of breast cancer and induce human melanoma cell apoptosis[24,25]. It can also promote the degradation of beta (β)-catenin to inhibit colon cancer proliferation. However, the role of CAD in the treatment of pancreatic cancer is unknown. Thus CAD was used to treat the BxPC3 cells and find out the effects on pancreatic cancer. After the treatment, CAD shows the ability of inhibiting the proliferation of BxPC3 cells at low concentrations. By staining with Hoechst 33342, after CAD’s stimulation, cells nuclei shrunk and concentrated, the expression of γ-H2AX increased, resulting in the damage of DNA in BxPC3 cells. Further flow cytometry analysis and western blot analysis showed that after stimulation with the increasing of Bax, the expression of Bcl-2 was reduced. Meanwhile, the expression of downstream caspase cascade and cytochrome C were enhanced, so that the apoptosis of BxPC3 cells were promoted.
As a first-line drug for pancreatic cancer, gemcitabine has strong toxic and side effects and it is resistant to long-term use, which limits its application in clinic. Therefore, gemcitabine in combination with other drugs is one of the research directions for the treatment of pancreatic cancer. Gemcitabine is incorporated into DNA and leads to DNA strand termination, followed by DNA damage and cell death[26]. Here, CAD showed the role of inducing DNA damage and apoptosis and also has certain inhibitory effect on the proliferation of pancreatic cancer cells. And the undergoing mechanism of the effect from CAD in pancreatic cancer need further study.
Author’s contributions:
Conception and design of the research was done by Qirong Hu, Xiangwei Xu; acquisition of data: Jujia Zheng, Dongsheng Gao; analysis and interpretation of data: Qirong Hu; statistical analysis: Jujia Zheng; drafting the manuscript: Qirong Hu, Xiangwei Xu; revision of manuscript for important intellectual content: Xiangwei Xu. All authors read and approved the final manuscript.
Acknowledgements:
We would like to thank all experimenters who have participated in this research. The authors declare that they have no competing interests and all authors should confirm its accuracy.
Funding:
This work was supported by the Zhejiang TCM Science and Technology Program (2021ZB324).
Conflict of interests:
The authors declared no conflict of interest.
References
- Leinonen MK, Miettinen J, Heikkinen S, Pitkäniemi J, Malila N. Quality measures of the population-based Finnish Cancer Registry indicates sound data quality for solid malignant tumours. Eur J Cancer 2017;77:31-9.
[Crossref] [Google Scholar] [PubMed]
- Moffat GT, Epstein AS, O'Reilly EM. Pancreatic cancer-A disease in need: Optimizing and integrating supportive care. Cancer 2019;125(22):3927-35.
[Crossref] [Google Scholar] [PubMed]
- Lai E, Puzzoni M, Ziranu P, Pretta A, Impera V, Mariani S, et al. New therapeutic targets in pancreatic cancer. Cancer Treat Rev 2019;81:101926.
[Crossref] [Google Scholar] [PubMed]
- Jiang Y, Meng Q, Chen B, Shen H, Yan B, Sun B. The small-molecule IAP antagonist AT406 inhibits pancreatic cancer cells in vitro and in vivo. Biochem Biophys Res Commun 2016;478(1):293-9.
[Crossref] [Google Scholar] [PubMed]
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378(9791):607-20.
[Crossref] [Google Scholar] [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362(17):1605-17.
- Patil VM, Masand N. Natural product databases and tools for anti-cancer drug discovery. Mini Rev Med Chem 2021;21(18):2764-77.
[Crossref] [Google Scholar] [PubMed]
- Hermawan A, Putri H. Current report of natural product development against breast cancer stem cells. Int J Biochem Cell Biol 2018;104:114-32.
[Crossref] [Google Scholar] [PubMed]
- Spradlin JN, Hu X, Ward CC, Brittain SM, Jones MD, Ou L, et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat Chem Bio 2019;15(7):747-55.
[Crossref] [Google Scholar] [PubMed]
- Elkhalifa D, Siddique AB, Qusa M, Cyprian FS, El Sayed K, Alali F, et al. Design, synthesis, and validation of novel nitrogen-based chalcone analogs against triple negative breast cancer. Eur J Med Chem 2020;187:111954.
[Crossref] [Google Scholar] [PubMed]
- Zhu M, Wang J, Xie J, Chen L, Wei X, Jiang X, et al. Design, synthesis, and evaluation of chalcone analogues incorporate α, β-unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis. Eur J Med Chem 2018;157:1395-405.
[Crossref] [Google Scholar] [PubMed]
- Chow YL, Lee KH, Vidyadaran S, Lajis NH, Akhtar MN, Israf DA, et al. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int Immunopharmacol 2012;12(4):657-65.
[Crossref] [Google Scholar] [PubMed]
- Wang K, Lv Q, Miao YM, Qiao SM, Dai Y, Wei ZF. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol 2018;155:494-509.
[Crossref] [Google Scholar] [PubMed]
- Park S, Gwak J, Han SJ, Oh S. Cardamonin suppresses the proliferation of colon cancer cells by promoting β-catenin degradation. Biol Pharm Bull 2013;36 (6):1040-4.
[Crossref] [Google Scholar] [PubMed]
- Hou S, Yuan Q, Yu N, Liu B, Huang G, Yuan X. Cardamonin attenuates chronic inflammation and tumorigenesis in colon. Cell Cycle 2019;18(23):3275-87.
[Crossref] [Google Scholar] [PubMed]
- Ren G, Sun A, Deng C, Zhang J, Wu X, Wei X, et al. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am J Physiol Gastrointest Liver Physiol 2015;309(7):517-27.
[Crossref] [Google Scholar] [PubMed]
- Niu P, Shi D, Zhang S, Zhu Y, Zhou J. Cardamonin enhances the anti-proliferative effect of cisplatin on ovarian cancer. Oncol Lett 2018;15(3):3991-7.
[Crossref] [Google Scholar] [PubMed]
- Li W, Wu X, Li M, Wang Z, Li B, Qu X, et al. Cardamonin alleviates pressure overload-induced cardiac remodeling and dysfunction through inhibition of oxidative stress. J Cardiovasc Pharmacol 2016;68(6):441-51.
[Crossref] [Google Scholar] [PubMed]
- Break MK, Chiang M, Wiart C, Chin CF, Khoo AS, Khoo TJ. Cytotoxic activity of Boesenbergia rotunda extracts against nasopharyngeal carcinoma cells (HK1). Cardamonin, a Boesenbergia rotunda constituent, inhibits growth and migration of HK1 cells by inducing caspase-dependent apoptosis and G2/M–phase arrest. Nutr Cancer 2021;73(3):473-83.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Sikka S, Siveen KS, Lee JH, Um JY, Kumar AP, et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017;22(1):158-68.
[Crossref] [Google Scholar] [PubMed]
- Wu N, Liu J, Zhao X, Yan Z, Jiang B, Wang L, et al. Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumor Biol 2015;36(12):9667-76.
[Crossref] [Google Scholar] [PubMed]
- Buck AC, Schirrmeister HH, Guhlmann CA, Diederichs CG, Shen C, Buchmann I, et al. Ki-67 immunostaining in pancreatic cancer and chronic active pancreatitis: Does in vivo FDG uptake correlate with proliferative activity? J Nucl Med 2001;42(5):721-5.
- Hruban RH, Gaida MM, Thompson E, Hong SM, Noe M, Brosens LA, et al. Why is pancreatic cancer so deadly? The pathologist's view. J Pathol 2019;248(2):131-41.
[Crossref] [Google Scholar] [PubMed]
- Jin J, Qiu S, Wang P, Liang X, Huang F, Wu H, et al. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J Exp Clin Cancer Res 2019;38(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Yue Y, Liu L, Liu P, Li Y, Lu H, Li Y, et al. Cardamonin as a potential treatment for melanoma induces human melanoma cell apoptosis. Oncol Lett 2020;19(2):1393-9.
[Crossref] [Google Scholar] [PubMed]
- Mini E, Nobili SF, Caciagli BF, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol 2006;17:7-12.
[Crossref] [Google Scholar] [PubMed]
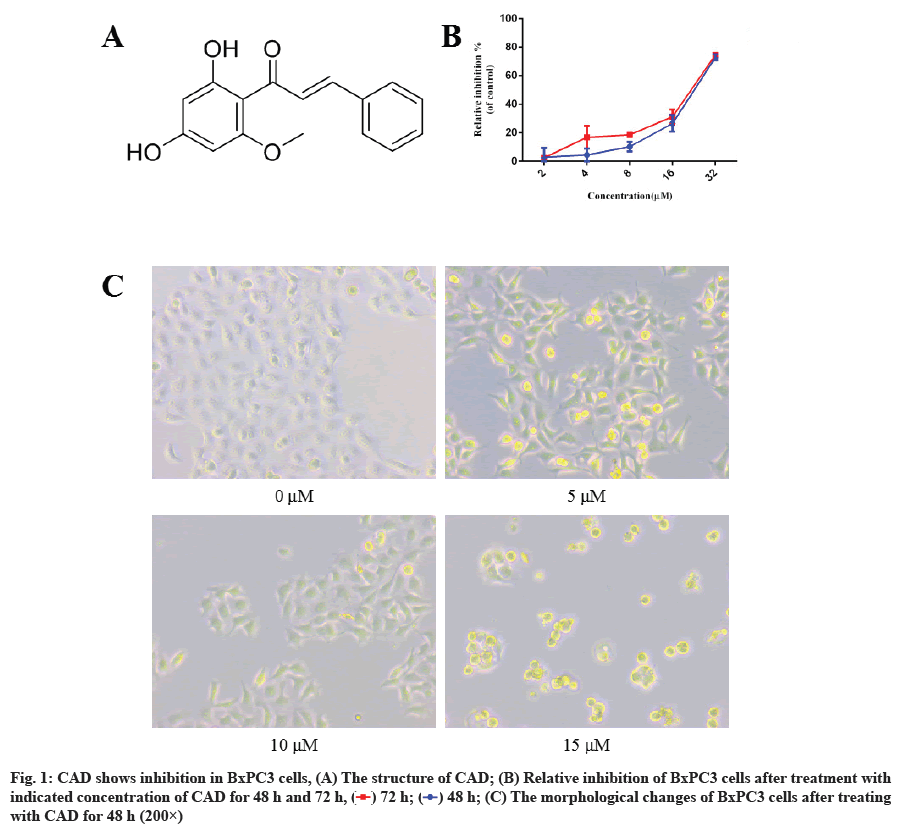
 ) 72 h; (
) 72 h; ( ) 48 h; (C) The morphological changes of BxPC3 cells after treating with CAD for 48 h (200×)
) 48 h; (C) The morphological changes of BxPC3 cells after treating with CAD for 48 h (200×)