- *Corresponding Author:
- Y.W. Li
Tianjin Key Laboratory of Brain Science and Neural Engineering, Tianjin University, Tianjin 300072, China
E-mail: liyanwei127@hotmail.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “28-35” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The non-small cell lung cancer is a common malignancy with high metastasis capacity. The brain is the primary site for non-small cell lung cancer patients with unclear mechanism. Here, in this study, we hypothesized an innovative signaling cascade for the disorder and systematically investigated the underlying mechanism. Their differentially gene expression analysis was based on 13 conventional nonsmall cell lung cancer and 15 non-small cell lung cancer brain metastasis patients respectively. The nonsmall cell lung cancer cell line, adenocarcinomic human alveolar basal epithelial cells-A549 and human bronchial epithelial cell line-BEAS-2B were established for in vitro study. The syngeneic mouse tumor models injected with 5×105 cells of control, C-C motif ligand 21 overexpressing, C-C motif chemokine receptor 7 overexpressing A549 cells were generated for in vivo analysis. Compared with conventional non-small cell lung cancer patients, the non-small cell lung cancer brain metastasis patients showed 352 differentially expressed genes, as 212 down-regulated ones and 140 up-regulated ones. Moreover, the C-C motif ligand 21 and C-C motif chemokine receptor 7 were prominently inhibited in non-small cell lung cancer brain metastasis patients. In A549 cells, C-C motif ligand 21 overexpressing could inhibit epithelial-mesenchymal transition progression. C-C motif ligand 21 modulated epithelial-mesenchymal transition and migration plus invasion ability but not proliferation capacity of non-small cell lung cancer cells through C-C motif chemokine receptor 7 dependent signaling pathway, as Janus kinase 2/signal transducer and activator of transcription 3 was a potential downstream signaling pathway. Moreover, C-C motif ligand 21/C-C motif chemokine receptor 7 signaling axis could modulate tumor growth and survival time in vivo. Collectively, these data initiated a C-C motif ligand 21/C-C motif chemokine receptor 7-Janus kinase 2/signal transducer and activator of transcription 3 signaling for non-small cell lung cancer patients with brain metastasis, which shed various beneficial insights for future study.
Keywords
Lung cancer, non-small cell lung cancer, C-C motif ligand 21, C-C motif chemokine receptor 7
Currently, lung cancer has become a global problem and public health issue, which bring severe economic burden for various countries. Lung cancer is considered as the third most abundant cancer, just behind breast and prostate cancer. However, lung cancer represents the largest proportion of all cancer-related deaths, which is 22 % estimated[1]. As reported, the worldwide incidence and mortality for lung cancer is about 1.82 and 1.6 million cases annually[2]. As a single etiologic agent, smoking cessation so far is still the primary risk factor for lung cancer progression[3], yet, a substantial minority of patients with lung cancer have never smoked before. In the United States (US) alone, there are about 17 000-26 000 lung cancer-related deaths annually are never smokers, which is suggested as the seventh leading cause for cancer mortality[4]. Clinically, lung cancer is classified into different histologic subtypes, including adenocarcinoma, squamous carcinoma, small cell lung cancer and large cell carcinoma (commonly referred to as Non-Small Cell Lung Cancer, NSCLC) as a major form which contribute to more than 80 % of all lung cancer cases[5]. Even with decades of efforts, the incidence rate did not start declining and the managements of NSCLC are still restricted. As reported, majority of lung cancer-associated deaths were caused by metastasis[6]. With unclear underlying mechanism, the brain is approved to be the primary site for NSCLC metastasis, which is responsible for approximately 50 % of all brain metastases[7,8]. The 5 y survival rate of NSCLC patients with brain metastasis is far from satisfaction, with a median survival rate less than 6 mo[9]. So far, the molecular mechanism behind NSCLC patients with brain metastasis is still poorly understood. One of the central explanation for this is the existence of Blood-Brain Barrier (BBB), which greatly hampers the accessibility of chemical therapeutic agents to the brain parenchyma[10].
Metastasis is an extremely complicated process, which might be the key point in solving the stubborn disease. An intact metastasis requires the following progressions-Evading of local tumor immunobiology host defense and spreading of tumor cells from primary sites to surrounding structures; invasion into vessels as well as circulatory system; extravasation into the appropriate secondary site[11]. For the starting and ending point, the communications between tumor cells and surrounding tumor microenvironment play key roles in establishment of the tumor metastasis. Accumulating evidences have suggested that the tumor microenvironment is maintained by multiple hormones, paracrine secreted soluble factors, cytokines and chemokines[12].
Chemokines represent a large protein family of chemotactic cytokines, which is functional as secreted extracellular ligands (CCL, short for C-C motif Ligand). Upon binding to the C-C motif Chemokine Receptor (CCR, also a classical G-protein coupled receptor), CCL could stimulate substantial downstream signaling cascades including Janus Kinase 2/Signal Transducer and Activator of Transcription 3 (JAK/STAT) pathways, Mitogen-Activated Protein Kinase (MAPK), etc[13]. The CCL dependent signaling axis has been demonstrated to be closely connected to diverse progressions of tumorgenesis, which acts as a central regulatory factor in tumor immunobiology via autocrine, paracrine or endocrine pattern[14]. As a fundamental member of CCL family, C-C motif Ligand 21 (CCL21) recruits C-C motif Chemokine Receptor 7 (CCR7) Dendritic Cells (DC) and T cells to enhance local immune defense. CCL21/CCR7 signaling was deeply studied in the patients of Rheumatoid Arthritis (RA) based on the facts that the synovial tissue of RA patients generate excessive amounts of CCL21 from fibroblasts and macrophages to further initiate angiogenesis[15]. In clinical trials, some innovative strategies start to deliver CCL21 in cancer patients and aim to reactivate the downregulated immune defense in the tumor microenvironment. However, the connections between CCL21/CCR7 and NSCLC especially the patients with brain metastasis remain unclear. Here, using Differentially Expressed Gene (DEG) analysis from clinical specimen as a starting point, we systematically investigated the functions of CCL21/CCR7 for NSCLC patients with brain metastasis. It could be found that the NSCLC patients with low expression level of CCL21 as well as CCR7. In lung cancer cell line, this signaling axis inhibited cell invasion, migration and Epithelial-Mesenchymal Transition (EMT) progression via JAK2/STAT3 signaling pathway, which was also verified in a syngeneic mouse tumor model. Collectively, all the outcomes will aim in the development of effective immunotherapy for NSCLC patients.
Materials and Methods
Data source:
The DEG analysis was based on the chip obtained from Gene Expression Omnibus (GEO) database, numbering Genomic Spatial Event (GSE) 161116. The chip contained 28 tissues from primary and brain metastases of NSCLC (13 from conventional NSCLC and 15 from NSCLC brain metastasis patients respectively). The whole genome expression profiles of the chip were performed by GeneSeed company (Guangdong, China). The expression analysis was conducted using the Affymetrix GeneChip Pico kit and hybridized to Affymetrix Clariom S arrays as described by the manufacturer (Affymetrix, USA). The Robust Multi-array Average (RMA) method was used to normalize the original data measured by the chip. Meanwhile, the edgeR package in R language was utilized to analyze DEGs. Finally, the values of Log2 Fold Change (FC)>1 and p-value<0.05 were considered as a significant different expression.
Cell lines:
The NSCLC cell lines, like Adenocarcinomic Human Alveolar Basal Epithelial Cells (A549) were purchased from Tongpai Biotechnology Co., Ltd (Shanghai, China). The Kaighn's Modification of Ham's F-12K Medium (Hyclone, United States of America (USA)) supplemented with 10 % Fetal Bovine Serum (FBS, Gibco, USA) was used for the cell culturing. The regular lung epithelial cell line like Human Bronchial Epithelial cell line (BEAS- 2B) was purchased from Tongpai Biotechnology Co., Ltd (Shanghai, China), culturing in Bronchial Epithelial Cell Growth Medium (BEGM) (Hyclone, USA) supplemented with 10 % FBS (Gibco, USA). The CCL21, CCR7 small interfering Ribonucleic Acids (siRNAs) and corresponding Negative Control siRNAs (NC siRNA) were constructed by Genewiz Corporation Co. Ltd. (Tianjin, China). The LipofectamineTM 3000 was used for plasmid transfection.
Functional experiments of A549 cells:
The functional experiments of A549 cells followed the procedures previously established[16]. The A549 cells were treated with F-12K medium with 100 ng/ ml CCL21 (PeproTech, Rocky Hill, NJ) to achieve CCL21 Over-Expression (CCL21-OE). For the migration capacity of tumor cells, a scratch was made after the cells became confluent monolayer. The wound healing areas were measured by Image J software. For the matrigel invasion assay, the basement matrigel membrane was purchased from BD Biosciences and established to precoat the transwell chamber with a filter of 8 μm pores (Corning, New York). 2×105 A549 cells, suspending in an FBS-free medium, were seeded on the upper of the chamber. The medium in the lower chamber contained 10 % FBS. After 48 h of incubation, the medium was aspirated and the cells on the upper chamber were wiped away. The remaining cells on the lower surface were then examined based on the crystal violet staining. For the cell proliferation assay, the experiments were established according to the instruction of Cell Counting Kit-8 (CCK-8) purchased from MCE company. At the same time, the Annexin V-Fluorescein Isothiocyanate (FITC)/ Propidium Iodide (PI) double staining of flow cytometry was performed to measure the apoptotic cells.
Western blotting:
The total proteins of A549 cells were collected and concentration was determined using the Bicinchoninic Acid (BCA) method. 50 μg proteins each was subjected to Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE). The membranes were transferred on semidry transfer apparatus, blocked with 5 % nonfat dry milk powder at 37° for 1.5 h. The corresponding primary antibodies (1:1000 dilution) were added, the membrane was then incubated overnight at 4°, following was washed extensively with Phosphate Buffered Saline (PBS) containing 0.1 % Tween-20 three times for 10 min each. The horseradish peroxidase labeled secondary antibodies (1:10 000 dilution) were incubated at room temperature for 1 h, the membrane was washed extensively, then developed by adding Enhanced Chemiluminescence (ECL) developer and photographed by ultrasensitive multifunctional imager (Carl Zeiss meditec, Hilden, Germany). The N-cadherin, E-cadherin, vimentin, Glyceraldehyde- 3-Phosphate Dehydrogenase (GAPDH), JAK2, phosphorylated-JAK (p-JAK), STAT3, as well as p-STAT3 antibodies were obtained from Jackson Laboratory.
Mouse tumor model and tumor harvest:
The mouse model was established as previously reported by Kang et al.[17]. 5×105 cells of control, CCL21-OE, CCL21 Low Expressing (CCL21-LE) A549 cells in PBS were injected subcutaneously into age-matched (6-8 w) B6 mice. The tumor sizes were measured at the indicated time points and calculated using the following formula: 1/2 (Length×Width2). When the tumors were larger than 2000 mm3 in size, the mice was considered as dead.
Statistical analysis:
The Statistical Analysis Software (SAS) 11.0 was developed for data collection and analysis in this study. The student t-test was used to compare the differences between groups, as p-value less than 0.05 was considered statistically significant. The data presented in the figures were mean±Standard deviation (SD), *p<0.05; **p<0.01; ***p<0.001; NS: Not Statistically Significant.
Results and Discussion
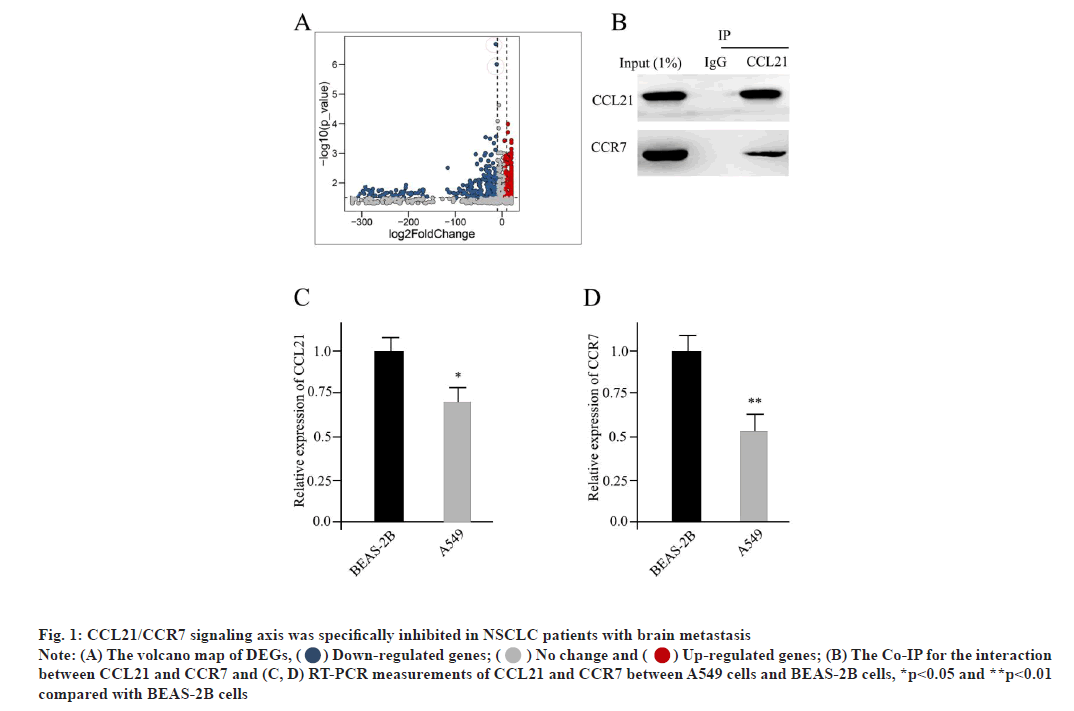
CCL21/CCR7 signaling axis was specifically inhibited in NSCLC patients with brain metastasis as shown in fig. 1. The specimens we analyzed contained whole genome information for 13 conventional NSCLC patients and 15 NSCLC brain metastasis patients respectively. Compared with conventional NSCLC patients, the NSCLC brain metastasis patients showed 352 DEGs, as 212 down-regulated ones and 140 up-regulated ones. The volcano map of DEGs was shown in fig. 1A. The horizontal axis indicates the multiple of differential expression (Log2FC), the vertical axis indicates -log10 ((False Discovery Rate (FDR)), while the blue dot represents down-regulated genes and the red dot represents upregulated genes respectively (fig. 1A). Moreover, the CCL21 and CCR7 were prominently inhibited in NSCLC brain metastasis patients (indicated in red dots of fig. 1A). The CCR7 was suggested as a potential ligand receptor for CCL21. Here, by using the method of Co-Immunoprecipitation (Co-IP), we could confirm the direct interaction between CCL21 and CCR7 (fig. 1B). Taken the NSCLC cell line A549 as well as the regular lung epithelial cell line BEAS-2B as read-out, compared with it in BEAS- 2B cells, the expression level of CCL21 in A549 cells was significantly repressed, based on Reverse Transcription-Polymerase Chain Reaction (RT-PCR) measurement, which was consistent with the DEG analysis (fig. 1C). Similarly, the expression level of CCR7 was also dramatically reduced in A549 cells compared to BEAS-2B cells (fig. 1D). All of these indicated that CCL21/CCR7 was a potential antitumor signaling cascade.
Fig. 1: CCL21/CCR7 signaling axis was specifically inhibited in NSCLC patients with brain metastasis
Note: (A) The volcano map of DEGs, ( ) Down-regulated genes; (
) Down-regulated genes; (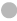 ) No change and (
) No change and ( ) Up-regulated genes; (B) The Co-IP for the interaction
between CCL21 and CCR7 and (C, D) RT-PCR measurements of CCL21 and CCR7 between A549 cells and BEAS-2B cells, *p<0.05 and **p<0.01
compared with BEAS-2B cells
) Up-regulated genes; (B) The Co-IP for the interaction
between CCL21 and CCR7 and (C, D) RT-PCR measurements of CCL21 and CCR7 between A549 cells and BEAS-2B cells, *p<0.05 and **p<0.01
compared with BEAS-2B cells
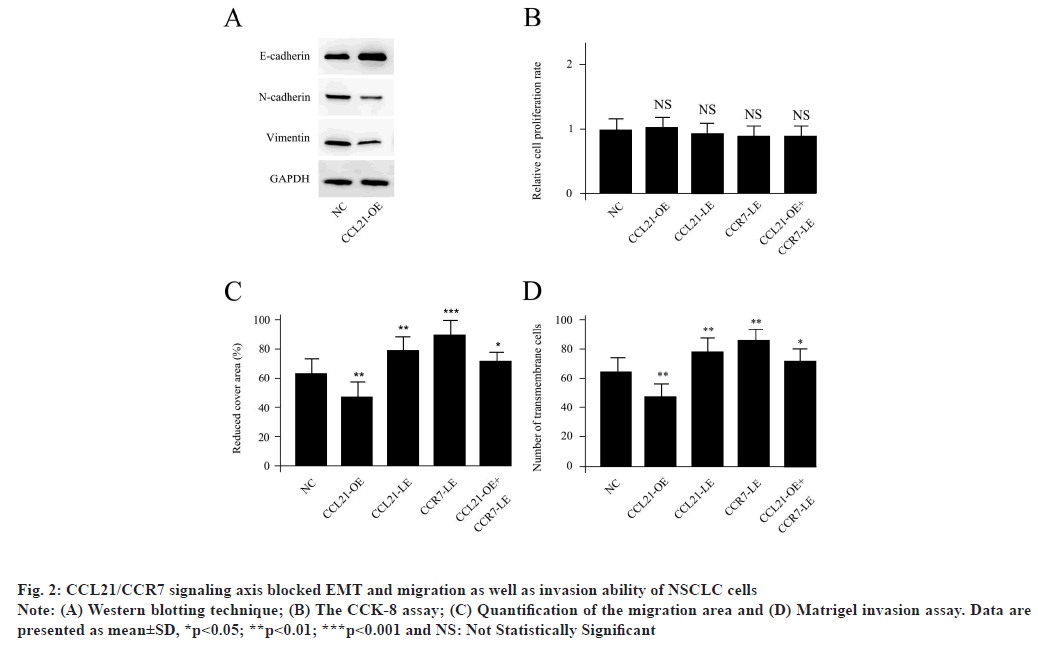
CCL21/CCR7 signaling axis blocked EMT and migration as well as invasion ability of NSCLC cells as shown in fig. 2. The A549 cells were treated with 100 ng/ml CCL21 for 24 h to obtain CCL21- OE. The Western blotting data demonstrated that the EMT biomarkers, such as loss of E-cadherin, gain of vimentin and N-cadherin, were significantly reduced in CCL21-OE, suggesting that the CCL21 treatment blocked EMT in NSCLC cells (fig. 2A). For the functional in vivo experiments, neither CCL21-OE nor CCL21-LE, (CCL21 siRNA transfected), CCR7- LE (CCR7 siRNA transfected) could change the relative proliferation rate compared with NC. The CCK-8 assay demonstrated the relative proliferation rate of A549 cells with different treatments (fig. 2B). However, CCL21-OE could significantly repress the cell migration ability while, both CCL21-LE and CCR7-LE treatments enhanced the cell migration capacities. The decreased migration level initiated by CCL21-OE could be abolished by co-transfection of CCR7 siRNA (fig. 2C). The matrigel invasion assay was performed to examine invasion ability of A549 cells with different treatments. At the same time, CCL21-OE could obviously reduce the cell invasion, while, both CCL21-LE and CCR7-LE treatments accelerated the cell invasion capacities. The decreased invasion activity caused by CCL21- OE could be abolished by co-transfection of CCR7 siRNA. The Student's t test was conducted for the differences between different treatments and NC (fig. 2D). Results are representative of three independent experiments. All of these suggested that CCL21 modulated EMT and migration as well as invasion ability but not proliferation capacity of NSCLC cells via CCR7 dependent signaling pathway.
Fig. 2: CCL21/CCR7 signaling axis blocked EMT and migration as well as invasion ability of NSCLC cells
Note: (A) Western blotting technique; (B) The CCK-8 assay; (C) Quantification of the migration area and (D) Matrigel invasion assay. Data are
presented as mean±SD, *p<0.05; **p<0.01; ***p<0.001 and NS: Not Statistically Significant
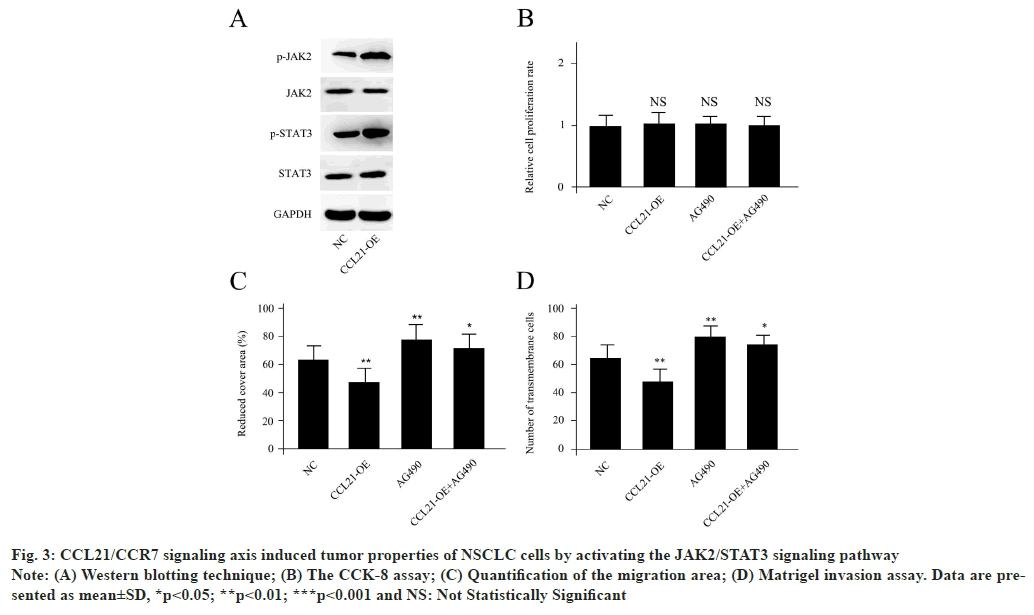
CCL21/CCR7 signaling axis induced tumor properties of NSCLC cells by activating the JAK2/STAT3 signaling pathway as shown in fig. 3. Based on the Western blotting analysis, it could be demonstrated that CCL21-OE cells displayed remarkably enhanced p-JAK2 and p-STAT3, while the expression levels of total JAK2 and STAT3 were unchanged (fig. 3A). The JAK2/STAT3 signaling pathway specific inhibitor AG490 was utilized to confirm if the activation of the p-JAK2 and p-STAT3 participated in tumor properties caused by CCL21/CCR7 signaling axis. The cell proliferation levels were unchanged for CCL21-OE, AG490 or a combination of CCL21-OE and AG490 (fig. 3B). The Matrigel invasion assay was performed to examine invasion ability of A549 cells with different treatments. AG490 co-treatment could abolish the effects of CCL21-OE for A549 cells migration inhibition as well as invasion capacity blocking, suggesting JAK2/STAT3 was a potential downstream signaling pathway for CCL21/CCR7 (fig. 3C and fig. 3D). Results are representative of three independent experiments.
Fig. 3: CCL21/CCR7 signaling axis induced tumor properties of NSCLC cells by activating the JAK2/STAT3 signaling pathway
Note: (A) Western blotting technique; (B) The CCK-8 assay; (C) Quantification of the migration area; (D) Matrigel invasion assay. Data are presented
as mean±SD, *p<0.05; **p<0.01; ***p<0.001 and NS: Not Statistically Significant
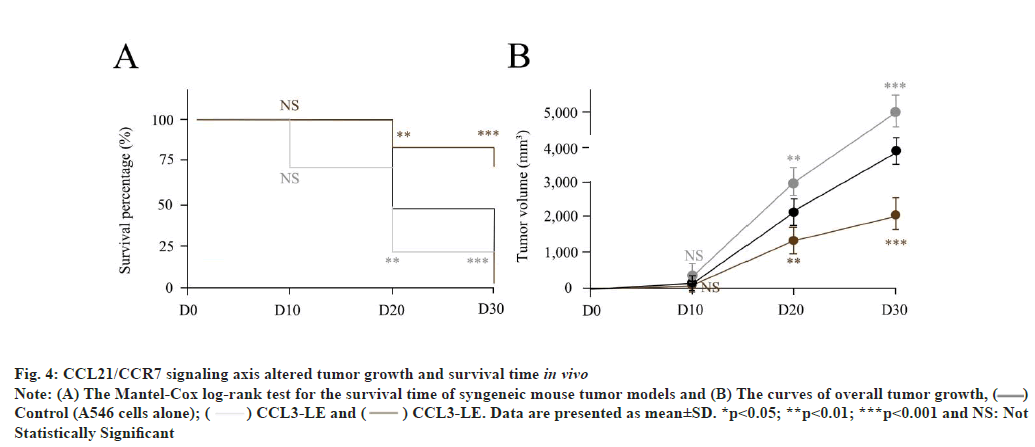
CCL21/CCR7 signaling axis altered tumor growth and survival time in vivo as shown in fig. 4. As previously reported[17], control, CCL21-OE as well as CCL21-LE syngeneic mouse tumor models were established age-matched (6-8 w) B6 mice. Compared with control (A549 cells injected alone), CCL21-OE mice demonstrated remarkably prolonged survival time and reduced tumor growth. On the other hand, CCL21-LE mice displayed short survival time and increased tumor growth (fig. 4A and fig. 4B). The Student’s t test was conducted for the differences between different treatments and NC. These results indicated that CCL21/CCR7 signaling axis could manipulate tumor growth and survival time in vivo. Results are representative of three independent experiments.
Fig. 4: CCL21/CCR7 signaling axis altered tumor growth and survival time in vivo
Note: (A) The Mantel-Cox log-rank test for the survival time of syngeneic mouse tumor models and (B) The curves of overall tumor growth, (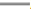 )
Control (A546 cells alone); (
)
Control (A546 cells alone); ( ) CCL3-LE and (
) CCL3-LE and (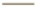 ) CCL3-LE. Data are presented as mean±SD. *p<0.05; **p<0.01; ***p<0.001 and NS: Not
Statistically Significant
) CCL3-LE. Data are presented as mean±SD. *p<0.05; **p<0.01; ***p<0.001 and NS: Not
Statistically Significant
Currently, the morbidity and mortality of lung cancer keep in a high level, resulted from the tumor metastasis. To this end, rebuilding the host immune defense of NSCLC patients has become an attractive approach. CCL21, also called Secondary Lymphoid Chemokine (SLC), was abundantly expressed in DC of lung cancer microenvironment and played a pivotal role in maintaining the communications between tumor cells and surrounding microenvironment. At the same time, CCL21 was also found to be constitutively expressed in lymph nodes and lymphatic vessels and stromal cells in various tissues[18]. Upon solely binding to CCR7, CCL21 was activated to modulate the recruitment and colocalization of naive lymphocytes and antigen stimulated DC into T cell zones, following the facilitation of T-cell activation. Based on its critical functions, intratumoral injection of CCL21 gene-modified autologous DC has been utilized in a phase I clinical trial for NSCLC patients. The patients with strategy demonstrated individual immune enhancement, tumor shrinkage as well as reveal tolerability. Like every coin has two sides, it could also be suggested that the immunetolerant tumor microenvironment stimulated local immune evasion. To this end, better understanding the downstream signaling axis and searching a combined approach might program durable antitumor immune responses. For instance, in the CCL21 clinical trial, Lee and his colleagues found that a combination treatment of CCL21 and Programmed Cell Death Protein 1 (PD-1, also known as Cluster of Differentiation 279 (CD279)) could effectively enhance antitumor immune responses and capture more NSCLC patients to be responsive to PD-1 checkpoint blockade therapy[19].
Here, we focused on the NSCLC patients with brain metastasis. For the metastasis, there are two existing hypotheses, “the hemodynamic or mechanical hypothesis”, suggesting that a successful evading of tumor cells into the lymphatic system or circulating system is the central step for the complicate procedure[20]; “seed-and-soil hypothesis”, suggesting that primary lung tumor cells are considered as “seed” while permissive secondary tissues are characterized as a “soil”[21]. As reported, brain metastasis contributes to more than 50 % of NSCLC patients with metastasis. If the first hypothesis is true, other tissues except brain should demonstrate equal level of metastasis since they accept similar blood invasion, however, the case is not such. Switching to the second hypothesis, primary lung tumor cells (seeds), permissive secondary tissue (soil) as well as tumor microenvironment (may be called as fertilizer) composed of CCLs and other chemokines are the prerequisite for metastasis in NSCLC patients.
To date, various CCLs show both antitumor and pro-tumor behaviors which are context dependent highlighting the complexity of the underlying interrelated signaling cascades[22]. For example, CCL3, another key member of chemokine family, is upregulated in tumor cells-derived chemokinecontaining microvesicles to promote tumorpromoting microenvironment on one side[23], while on the other side, CCL3 is also found to enhance intratumoral antitumor immunity including infiltration of regulatory T cells (Tregs), Tumor- Associated Macrophages (TAMs), and Myeloid- Derived Suppressor Cells (MDSCs)[24,25]. Previously, Chen et al. claimed that CCL21 and its associated binding receptor CCR7 were abnormally abundant in Oral Squamous Cell Carcinoma (OSCC) tissues, and closely associated with the poor prognosis of disorder[16]. Furthermore, with exogenous CCL21 stimulation, EMT was enhanced in OSCC cells and cancer stem cell-related markers CD133, CD44, B cell-specific Moloney murine leukemia virus integration site 1 (BMI1), Aldehyde Dehydrogenase 1A1 (ALDH1A1) and Octamer-binding transcription factor 4 (Oct4) increased. The migration, invasion, tumorsphere formation and colony formation abilities of OSCC cells were also increased, indicating that the stemness of OSCC cells was also improved. All of these together suggested that CCL21/CCR7 signaling axis was functional as a pro-tumor in OSCC development, while in this study, we observed the opposite pattern. Both CCL21 and CCR7 were inhibited in NSCLC patients with brain metastasis. CCL21/CCR7 signaling cascade inhibited EMT of NSCLC cell line A549, blocked the cell migration and invasion. Moreover, the critical functions of CCL21 for tumor cell metastasis were achieved through JAK2/STAT3 signaling pathways. These outcomes suggested that the behaviors (antitumor or pro-tumor) of CCL21/CCR7 might be tissuedependent.
Some limitations have to be acknowledged in this study. It is universal for the functional redundancy of the chemokine family. For example, in an unbiased screen of chemokines, Hirth et al. claimed that CCL21 and CXCL10 worked as co-proteins to enhance migration of pancreatic cancer cells toward sensory neurons. While, inhibition of these chemokines or their receptors could effectively reduce hypersensitivity in mice with orthotopic tumors and patients with pancreatic ductal adenocarcinoma as well as increase frequency of cancer-associated pain[26].
In this study, CCL21 and CCR7 demonstrated prominent differential expression pattern for NSCLC patients with brain metastasis, so that we did not recruit other CCLs which might be interesting directions for future study. Except to direct targeting antitumor immunity, CCLs were also found to associate with other regulatory factors. In NSCLC, based on multiplex profiling of immune-related proteins in Fine-Needle Aspirate (FNA) samples of thoracic lesions from patients with NSCLC, Programmed Death-Ligand 1 (PD-L1) was implied to function with multiple key immune signaling components (including CD73, granzyme A and chemokines CCL3 as well as CCL21)[27]. At the same time, co-expression of cytokines Interleukin-7 (IL-7) and CCL21 were crucial to boost cell recruitment, survival and proliferation in Chimeric Antigen Receptor (CAR) T cells therapy for solid tumors approaches, serving as a promising therapy strategy[28].
To this end, it would be beneficial to enroll PD-L1 or other immune checkpoints for metastasis in next steps. Collectively, our study suggested CCL21/ CCR7 as a key signaling axis for NSCLC especially patients with brain metastasis and initiated a JAK2/ STAT3 dependent downstream signaling pathway of CCL for EMT as well as various tumor-associated behaviors, which shed numerous insights for future caner study, not limiting in metastasis of NSCLC.
Conflict of interests:
The authors declared no conflict of interest.
References
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet 2021;398(10299):535-54.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-86.
[Crossref] [Google scholar] [PubMed]
- Mao Y, Yang D, He J, Krasna MJ. Epidemiology of lung cancer. Surg Oncol Clin N Am 2016;25(3):439-45.
[Crossref] [Google scholar] [PubMed]
- Rivera GA, Wakelee H. Lung cancer in never smokers. Adv Exp Med Biol 2016;893:43-57.
[Crossref] [Google scholar] [PubMed]
- Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin 2020;13(1):17-33.
[Crossref] [Google scholar] [PubMed]
- Dragoj M, Milosevic Z, Bankovic J, Tanic N, Pesic M, Stankovic T. Targeting CXCR4 and FAK reverses doxorubicin resistance and suppresses invasion in non-small cell lung carcinoma. Cell Oncol 2017;40(1):47-62.
[Crossref] [Google scholar] [PubMed]
- Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol 2005;75(1):5-14.
[Crossref] [Google scholar] [PubMed]
- Minchom A, Yu KC, Bhosle J, O'Brien M. The diagnosis and treatment of brain metastases in EGFR mutant lung cancer. CNS Oncol 2014;3(3):209-17.
[Crossref] [Google scholar] [PubMed]
- Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S. Brain metastasis: Opportunities in basic and translational research. Cancer Res 2009;69(15):6015-20.
[Crossref] [Google scholar] [PubMed]
- Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015;7(1):a020412.
[Crossref] [Google scholar] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7-30.
- Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The Osteosarcoma Microenvironment: A complex but targetable ecosystem. Cells 2020;9(4):976.
[Crossref] [Google scholar] [PubMed]
- Gibaldi D, Vilar-Pereira G, Pereira IR, Silva AA, Barrios LC, Ramos IP, et al. CCL3/macrophage inflammatory protein-1α is dually involved in parasite persistence and induction of a TNF-and IFNγ-enriched inflammatory milieu in Trypanosoma cruzi-induced chronic cardiomyopathy. Front Immunol 2020;11:306.
[Crossref] [Google scholar] [PubMed]
- Adekoya TO, Richardson RM. Cytokines and chemokines as mediators of prostate cancer metastasis. Int J Mol Sci 2020;21(12):4449.
[Crossref] [Google scholar] [PubMed]
- van Raemdonck K, Umar S, Palasiewicz K, Volkov S, Volin MV, Arami S, et al. CCL21/CCR7 signaling in macrophages promotes joint inflammation and Th17-mediated osteoclast formation in rheumatoid arthritis. Cell Mol Life Sci 2020;77(7):1387-99.
[Crossref] [Google scholar] [PubMed]
- Chen Y, Shao Z, Jiang E, Zhou X, Wang L, Wang H, et al. CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J Cell Physiol 2020;235(9):5995-6009.
[Crossref] [Google scholar] [PubMed]
- Kang TG, Park HJ, Moon J, Lee JH, Ha SJ. Enriching CCL3 in the tumor microenvironment facilitates T cell responses and improves the efficacy of anti-PD-1 therapy. Immune Netw 2021;21(3):e23.
[Crossref] [Google scholar] [PubMed]
- Nguyen T, Lagman C, Chung LK, Chen CH, Poon J, Ong V, et al. Insights into CCL21's roles in immunosurveillance and immunotherapy for gliomas. J Neuroimmunol 2017;305:29-34.
[Crossref] [Google scholar] [PubMed]
- Sharma S, Kadam P, Dubinett S. CCL21 programs immune activity in tumor microenvironment. Adv Exp Med Biol 2020;1231:67-78.
[Crossref] [Google scholar] [PubMed]
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer 2006;106(7):1624-33.
[Crossref] [Google scholar] [PubMed]
- Obenauf AC, Massagué J. Surviving at a distance: Organ-specific metastasis. Trends Cancer 2015;1(1):76-91.
[Crossref] [Google scholar] [PubMed]
- Ntanasis-Stathopoulos I, Fotiou D, Terpos E. CCL3 signaling in the tumor microenvironment. Adv Exp Med Biol 2020;1231:13-21.
[Crossref] [Google scholar] [PubMed]
- Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother Cancer 2002;25(3):207-17.
[Crossref] [Google scholar] [PubMed]
- Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell 2018;33(3):480-94.
[Crossref] [Google scholar] [PubMed]
- Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J Exp Med 2009;206(8):1717-25.
[Crossref] [Google scholar] [PubMed]
- Hirth M, Gandla J, Höper C, Gaida MM, Agarwal N, Simonetti M, et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology 2020;159(2):665-81.
[Crossref] [Google scholar] [PubMed]
- Franzén B, Viktorsson K, Kamali C, Darai‐Ramqvist E, Grozman V, Arapi V, et al. Multiplex immune protein profiling of fine‐needle aspirates from patients with non‐small‐cell lung cancer reveals signatures associated with PD‐L1 expression and tumor stage. Mol Oncol 2021;15(11):2941-57.
[Crossref] [Google scholar] [PubMed]
- Luo H, Su J, Sun R, Sun Y, Wang Y, Dong Y, et al. Coexpression of IL-7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin Cancer Res 2020;26(20):5494-505.
[Crossref] [Google scholar] [PubMed]