- *Corresponding Author:
- F. Tian
Department of Alergy, Yuhuangding Hospital, No.20 Yuhuangding East Road, Yantai, Shandong, 264000, China
E-mail: tianfengying121306@126.com
| This article was originally published in a special issue: Special issue on “Drug Development and Human Health in China” |
| Indian J Pharm Sci 2020:82(1)spl issue2;97-101 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to study the changes of CD4+CD25+Foxp3+Treg cells and Th1Th2 cells in subcutaneous specific immunotherapy for allergic asthma, patients with allergic asthma in Yuhuangding Hospital were selected as the research subjects and either routine drug treatment or subcutaneous specific immunotherapy were given. Serum house dust mite sIgE, allergen skin prick test, pulmonary ventilation function test www.ijpsonline.com 98 Indian Journal of Pharmaceutical Sciences Special Issue 2, 2020 and bronchial provocation test were performed, respectively, so as to test the basic condition of the patients. Then changes in CD4+CD25+Foxp3+Treg cells and Th1Th2 cells in the control group (routine drug treatment) and specific immunotherapy group were determined by isolating and culturing the human peripheral mononuclear cells followed by flow cytometry before and after treatment. The results showed that percent of nTreg cells in CD4+T cells and the percentage of Th1/Th2 cells in the control group increases to some extent after treatment, but the difference was not statistically significant. After subcutaneous specific immunotherapy, the percentage of CD4+CD25+Foxp3+Treg cells in CD4+T cells and the percentage of Th1/Th2 cells in the specific immunotherapy group increased significantly. The percentage of Th1 cells in CD4+T cells was positively correlated with that of CD4+CD25+Foxp3+Treg cells in CD4+T cells. Therefore, subcutaneous specific immunotherapy has a significant effect on allergic asthma. Its mechanism of action appears to be through increasing the percentage of CD4+CD25+Foxp3+Treg cells and Th1 cells to CD4+T cells, thereby increasing the proportion of Th1/Th2 cells. Despite some shortcomings in this investigation, it still provided a basis and ideas for further research.
Keywords
Allergic asthma, subcutaneous specific immunotherapy, CD4+CD25+Foxp3+Treg cells, Th1/Th2 cells, house dust mites
Allergic asthma is a chronic airway inflammation involving mast cells, eosinophils and T lymphocytes [1]. This is a relatively stubborn disease, mostly occurring in infancy, if not paid attention to, might become a chronic lifelong condition [2]. Allergic asthma usually begins with sneezing, nasal itching, runny nose, cough, itching eyes, tears, chest tightness and other symptoms and then aggravated to bronchial obstruction, leading to asthma [3].
Patients with serious morbidity can be forced to sit breathing, dry cough or cough up a large amount of white foam sputum, or even appear cyanotic [4]. Asthma mostly occurs at night or in the early morning. Many stimulants can increase airway responsiveness. The common allergens are pollen, house dust, mites, animal dander, fungi, insect excrement, cold air and animal food [5]. Symptoms subside by themselves or by taking antiasthmatic drugs. Some patients would relapse after several hours of remission and even cause asthma persistence [6].
Specific immunity, also known as acquired immunity, is a treatment for allergic substances that cause allergic diseases but only target one allergen [7]. It is the ability of the human body to resist infection after acquired infection (recovery or asymptomatic infection) or artificial vaccination [8]. It is usually formed after stimulation by an antigen and can react specifically with that antigen [9]. Specific immunotherapy is to find specific antigens, inject these into the body to obtain antibodies, or manufacture antibodies in vitro and then inject into the body, so that the body resist those antigens [10].
In summary, most of the studies on the pathological mechanism of allergic asthma in China were in children, but only a few studies on the subcutaneous specific immunotherapy mechanism of adult allergic asthma have been reported. In order to understand the mechanism of subcutaneous specific immunotherapy in allergic asthma in more detail, adult allergic asthma patients were selected as the research subjects of this study. Through subcutaneous specific immunotherapy, the changes of CD4+CD25+Foxp3+Treg (nTreg) cells and Th1Th2 cells in subcutaneous specific immunotherapy for allergic asthma were investigated, so as to provide reference for clinical treatment of allergic asthma.
One hundred patients with allergic asthma in Yuhuangding Hospital (February 2017-January 2018) were randomly divided into two groups, the control group (50 cases) and the specific immunotherapy group (SIT group, 50 cases). The control group consisted of 26 males and 24 females, aged 15-50 y, with an average age of 27.78 y. The SIT group had 24 males and 26 females, aged 16-49 y, with an average age of 27.15 y. There is no statistical difference between the 2 groups with respect to age, gender, occupation, duration of illness or other basic data. Selection criteria were when all clinical symptoms met the diagnostic criteria of bronchial asthma prevention and treatment guidelines (definition, diagnosis, treatment, education and management of bronchial asthma) formulated by the Asthma Group of the Respiratory Diseases Society of the Chinese Medical Association in 2008. All patients were confirmed to be allergic to house dust mites by allergen skin prick test and serum house dust mite sIgE test. The informed consent signed by the patients or their family members was obtained and this study was approved by the medical ethics committee of Yuhuangding Hospital.
Three days before the allergen skin prick test, all patients were asked to stop using antihistamines and glucocorticoids. Using hormone topical drugs were avoided at the skin prick site (i.e., flexion of forearm). Standardized allergen skin prick solution (ALK-Abell, Denmark) was used as SPT reagent. Allergens included house dust mite, dust mite, tropical mite, cockroach, American cockroach, dog hair, cat hair, Artemisia argyi, ragweed, birch and olive oil.
The positive control solution was 10 mg/ml histamine, while the negative control solution was saline. Alcohol (75 %) was used to disinfect the flexion side of the left forearm twice. The allergens, positive control solution and negative control solution were pricked on the skin from bottom to top at intervals of 1 cm. After 20 min, the reaction of each prick site was observed and the diameter of the wind circle was recorded and measured on the skin. The diameter of the wind mass = (minimum diameter+maximum diameter)/2. If the diameter of the wind mass ≥3 mm, it is positive, indicating that the patient is allergic to the allergen.
Before and after treatment, 3 ml venous blood was collected and centrifuged at 2000 rpm for 10 min. Serum sIgE of house dust mite was measured by UniCAP 100 automatic allergen detector (Famasia, Sweden). SIgE was graded as following, less than 0.35 kU/l is grade 0; 0.35-0.7 kU/l is grade 1; 0.7-3.5 kU/l is grade 2; 3.5-17.5 kU/l is grade 3; 17.5-50 kU/l is grade 4; 50 -100 kU/l is grade 5 and more than 100 kU/l is grade 6.
All patients were examined for pulmonary ventilation function with a lung function detector (Shanghai Yuexin Tonghe Medical Devices Co., Ltd., China). The difference between the first second expiratory volume (FEV1) and any two forced vital capacity (FVC) was less than 150 ml. All patients were advised to stop using antihistamines, long-acting β agonists and glucocorticoids 7 d before the bronchial provocation test. Inhalation concentrations of histamine phosphate were 0.3, 0.6, 2.5 and 5 % (Beijing Baioleibo Technology Co., Ltd., China). When FEV1 % decreases more than 20 % of the baseline value, the drug is positive and stopped. Airway hyperreactivity (AHR) was graded according to cumulative dose of histamine phosphate inhalation, extremely mild- 3.3-7.8 μmol, mild- 0.9 -3.2 μmol, moderate- 0.1-0.8 μmol and severe, less than 0.1 μmol.
In the SIT group, standardized house dust mite allergen vaccine (ALK-Abellò, Denmark) was injected subcutaneously at 1/3 lateral part of the distal upper arm with an initial dose of 20SQU, which was increased weekly according to the following protocol, 20, 40, 80, 200, 400, 800, 2000, 4000, 8000, 10 000, 20 000, 40 000, 60 000, 80 000, 100 000. 100 000 SQU was maintained and injected once every 2, 4, 6 w. Next, they were injected once every 6 w and the treatment lasted for o1 y. During the period, patients with symptoms could take symptomatic drugs such as montelukast sodium tablets and budesonide aerosol according to doctor’s advice. The patients in the control group received only routine medication.
Both groups were received 7 ml of intravenous blood and diluted with sterile phosphate buffer solution (Beijing Quanjin Biotechnology Co., Ltd., China; dilution ratio 3:2). Three millilitres PBMC was added into a separating tube and centrifuged at 1000×g for 30 s. Diluted venous blood was added to the separating tube and centrifuged at 800×g for 15 min. The suspended cloud-like substances were aspirated in to a capillary tube and transferred to a centrifugal tube. Eight millilitres of RPMI 1640 medium (Shanghai Bosheng Biotechnology Co., Ltd., China) was added, and centrifuged at 250×g for 10 min. The supernatant was discarded and the operation was repeated. One milliliter of cell culture medium (1 % streptomycin, 1 % penicillin and 10 % calf serum) was added. It was mixed well and 10 ml was taken. Twenty microlitres of RPMI 1640 and 30 μl trypan blue (Beijing Soleboard Technology Co., Ltd., China) was added. After mixing evenly, 10 μl was aspirated and added to the cell counting board for counting. Cells were cultured in a concentrated medium with a dilution of 2×106/ml. The cells were cultured in 2 wells on a 24-well cell culture plate, one without stimulants and the other with 25 μg/ml stimulants. The cells were cultured in a cell culture chamber (5 % CO2, 37°). Seventy two hours later, the cell suspension was transferred into the centrifugal tube, centrifuged at 1900 rpm for 5 min, the supernatant was separated and frozen in the deep freezer (-40°). The concentration of cytokines in the supernatant was determined.
Changes of nTreg cells and Th1 (IFN-γ+CD4+T)/Th2 (IL-4+CD4+T) cells were detected by flow cytometry. Cell suspension was added to the test tube and PBS was added to 2 ml. After centrifugation at 1900 rpm for 5 min, the liquid was discarded. Cell dyeing buffer was added to the concentration of culture and mixed evenly. Ten microlitres of antiCD4-FITC (Hangzhou Lianke Biotechnology Co., Ltd., China) and 5 μl antiCD25-PE-cy5 (Shanghai Hengfei Biotechnology Co., Ltd., China) was added. It was placed for 15 min at room temperature, away from light, 0.5 ml breaking solution (Wuhan Seville Biotechnology Co., Ltd., China) was added, and it was cultured for 30 min (4°, in the dark). After adding 2 ml membrane breaking lotion and centrifuged at 2100 rpm for 5 min, the lotion was discarded. Membrane breaking lotion was added again and the operation was repeated. Five millilitres of antiFoxp3-PE (Wuhan Boote Biotechnology Co., Ltd., China) was added and light was avoided for 30 min at 4°. Two millilitres of membrane breaking lotion was added. After centrifugation at 2100 rpm for 5 min, the liquid was discarded. 0.5 ml 1 % polyformaldehyde was added.
In the 24-well culture plate, 20 ng/ml PMA, 1 μg/ml ionomycin (Shanghai Hengfei Biotechnology Co., Ltd., China) and 10 μg/ml BFA (Shanghai Hongye Biotechnology Co., Ltd., China) were added. After stimulation for 4 h, cell suspension was added to the test tube for transformation. After adding PBS to 2 ml and centrifuged for 5 min (1900 rpm), the liquid was discarded. Cell dyeing buffer was added until it becomes the concentration of culture, and it was mixed evenly. Ten millilitres of antiCD4-PE-CY5 (Xiamen Huijia Biotechnology Co., Ltd., China) was added, and kept aside for 15 min at room temperature in the dark. Half a milliliter of breaking solution was added and cultured for 30 min at 4° in the dark. Two millilitres of membrane breaking lotion was added. After centrifugation at 2100 rpm for 5 min, the solution was discarded. Membrane breaking lotion was added again and the procedure was repeated.
AntiIFN-gamma-FITC (Shanghai Hengfei Biotechnology Co., Ltd., China) and antiIL-4-PE (Wuhan Boote Biotechnology Co., Ltd., China) was added. Light was avoided for 30 min at 4° and 2 ml membrane breaking lotion was added. After centrifugation at 2100 rpm for 5 min, the liquid was discarded and 0.5 ml of 1 % polyformaldehyde was added. Each test tube was equipped with homologous control. The stained cells were detected by flow cytometry (Epics XL/XL-MCL, Beckman Kurt Company, USA).
SPSS21.0 statistical software was used for data processing and analysis. Quantitative data conforming to normal distribution were described by mean ± standard deviation. Two independent samples to test or rank sum test were used to compare the two groups. Chi-square test was used to compare the rates of different groups.
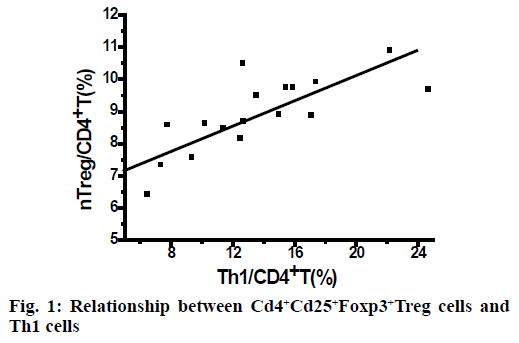
As shown in Table 1, the percentage of nTreg cells in CD4+T cells in the control group increased but not significantly after routine drug treatment. In the SIT group, the percentage of nTreg cells in CD4+T cells increased significantly (p<0.05) after subcutaneous specific immunotherapy. In the SIT group, after subcutaneous specific immunotherapy, the percentage of Th1 cells in CD4+T cells was positively correlated with that of nTreg cells in CD4+T cells (r = 0.785), as shown in Figure 1. The control group has no correlation.
| Group | Before treatment | After treatment |
|---|---|---|
| Control group | 8.09 ± 1.52 | 8.54 ± 1.67 |
| SIT group | 8.30 ± 1.64 | 9.52 ± 1.71 |
Table 1: Comparison of Percentage of CD4+CD25+FOXP3+TREG Cells to CD4+T Cells
As shown in Table 2, the percentage of Th1/Th2 cells in the control group increased but not significantly after general drug treatment. The percentage of Th1/Th2 cells in the SIT group increased significantly (p<0.05) after subcutaneous specific immunotherapy.
| Group | Before treatment | After treatment |
|---|---|---|
| Control group | 3.32 ± 1.02 | 3.80 ± 1.86 |
| SIT group | 3.45 ± 1.12 | 4.49 ± 1.37* |
Table 2: Comparison of TH1/TH2 Cell Proportion
To explore the changes of nTreg and Th1Th2 cells due to subcutaneous specific immunotherapy for allergic asthma, the patients with allergic asthma being treated in the Yuhuangding Hospital were enrolled as the research subjects. They were treated with either routine drugs or subcutaneous specific immunotherapy. Serum house dust mite sIgE, allergen skin prick test, pulmonary ventilation function test and bronchial provocation test were performed so as to test the basic condition of the patients. Then the changes in nTreg cells and Th1Th2 cells in the control group (routine drug treatment) and the SIT group (specific immunotherapy) were analyzed and compared by isolation and culturing of human peripheral mononuclear cells and flow cytometry before and after treatment. The results showed that the percentage of nTreg cells in CD4+T cells and the percentage of Th1/Th2 cells in the control group increased but not significantly. After subcutaneous specific immunotherapy, the percentage of nTreg cells in CD4+T cells and the percentage of Th1/Th2 cells in the SIT group increased significantly. The percentage of Th1 cells in CD4+T cells was positively correlated with that of nTreg cells in CD4+T cells.
Therefore, by studying the changes in nTreg and Th1Th2 cells after subcutaneous specific immunotherapy of allergic asthma, it was found that subcutaneous specific immunotherapy has a significant therapeutic effect on allergic asthma. Its mechanism may be realized through increasing the percentage of CD4+CD25+Foxp3+Treg cells and Th1 cells to CD4+T cells, thereby increasing the proportion of Th1/Th2 cells. However, some shortcomings in this study, such as smaller sample size could be overcome by increasing the sample size in future studies to obtain highly reliable results.
References
- Bui TT, Piao CH, Song CH. Bupleurum chinense extract ameliorates an OVA-induced murine allergic asthma through the reduction of the Th2 and Th17 cytokines production by inactivation of NFκB pathway. Biomed Pharmacother 2017; 91:1085.
- Villaseñor A, Rosace D, Obeso D. Allergic Asthma: An Overview of Metabolomic Strategies leading to the Identification of Biomarkers in the field. Clin Exp Allergy 2017;47(4): 442-456.
- Rodríguez DRP, Pitsios C, Tsoumani M. Physicians' experience and opinion on contraindications to allergen immunotherapy: The CONSIT survey. Ann Allergy Asthma Immunol 2017;118(5):621.
- Rajakulasingam RK, Farah N, Huber PAJ, Durham SR, Clark AT, Nasser SM, et al. Practice and Safety of Allergen Specific Immunotherapy for Allergic Rhinitis in the UK National Health Service: A report of 'real world' clinical practice. Clin Exp Allergy 2017; 48(1):89-92.
- Richter AK, Klimek L, Merk HF, Mülleneisen N, Renz H, Wehrmann W, et al. Impact of increasing treatment rates on cost-effectiveness of subcutaneous immunotherapy (SCIT) in respiratory allergy: a decision analytic modelling approach. Eur J Health Econ 2018;(3): 1-14.
- Tie X, Qu F, Liu P. Expression of CD105 and CD4 (+) CD25 (+) Foxp3 (+) Treg in peripheral blood of patients with non-small cell lung cancer undergoing Endostar plus chemotherapy and its significance. Int J Clin Exp Med 2017;10(6): 9723-9728.
- Jin Y, Jiang L, Guo J. Effects of acupuncture on the ratio of CD4+ CD25+ regulatory T cells and expression of transcription factor Foxp3 in patients with septic shock. Chin J Primary Med Pharm 2017;24(18):2784-7.
- Verma ND, Robinson CM, Tran GT. Human CD4+ CD25+ CD127loFOXP3+ Treg Activation by IL-4 and Alloantigen Induces Activated Treg that do not Express CD45RA and Express the IL-5 Receptor Alpha (CD125). Transplantation 2017;101(5S-3): S7.
- Xu FF, Yu SQ, Zhao CL. Effect of hydrogen-rich saline on the CD4 (+) CD25 (+) Foxp3 (+) Treg cells of allergic rhinitis guinea pigs model. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017; 52(7): 506-511.
- De la Cruz-Mosso U, García-Iglesias T, Bucala R, Estrada-García I, González-López L, Cerpa-Cruz S, et al. MIF promotes a differential Th1/Th2/Th17 inflammatory response in human primary cell cultures: Predominance of Th17 cytokine profile in PBMC from healthy subjects and increase of IL-6 and TNF-α in PBMC from active SLE patients. Cell Immunol 2018; 324: 42-49.