- *Corresponding Author:
- X. He
Department of General Surgery, First People’s Hospital Affiliated to Huzhou Normal College, Huzhou, Zhejiang Province 313000, China
E-mail: hxw_hzyy@zjhu.edu.cn
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “262-266” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To study the effectiveness and safety of targeted therapy in combination with immunotherapy for neoadjuvant treatment of advanced hepatocellular carcinoma. 65 advanced hepatocellular carcinoma patients who underwent hepatectomy in our hospital from January 2019 to December 2021 were retrospectively analyzed. Among them, 45 patients in the control group underwent conventional surgery and 20 patients in the study group got neoadjuvant therapy. The short-term curative effect of the patients in the study group was assessed. The intraoperative blood loss, intraoperative blood transfusion volume, postoperative hospital stays, postoperative blood transfusion volume and operation time were collected from the two groups of patients and the postoperative complications were correlated between the two groups. The overall survival and postoperative recurrence of the patients were obtained through followup. The 3 mo overall response rate of patients in the study group who underwent apatinib combined with camrelizumab was 35.00 % and the disease control rate was 75.00 %. There were no notable variations between the two groups in operation time, intraoperative blood loss, postoperative blood transfusion volume, intraoperative blood transfusion volume and postoperative hospital stays (all p>0.05). The rate of postoperative complications did not significantly differ between the two groups (p>0.05). The follow-up results showed that the recurrence time was remarkably later than that of the control group (p=0.023) and the survival time of the control group (p=0.041) was remarkably shorter than that of the study group. Immune checkpoint inhibitors expressed by programmed cell death 1/programmed cell death ligand 1 monoclonal antibody and anti-antigenic targeted drugs combined with neoadjuvant therapy have good safety for patients with resectable hepatocellular carcinoma, can improve the prognosis of patients, prolong the patient's survival time, toxic and side effects are acceptable.
Keywords
Targeted therapy, immunotherapy, hepatocellular carcinoma, oncology
Hepatocellular Carcinoma (HCC) constitutes approximately 75 %-90 % of primary liver cancers, seriously threatening human life and health[1,2]. Surgical treatments such as liver transplantation and liver resection are recognized as cure methods that can obtain outstanding long-term benefits for HCC patients. However, many HCC patients lose the opportunity for surgical treatment because they are already in the middle or advanced stages when they are diagnosed or their underlying liver diseases and general condition, and other reasons forbid them from accepting surgical treatment. Even though the 5 y survival rate of liver cancer patients after surgical treatment can be increased to 60 %, the 1 y recurrence rate of patients with resectable liver cancer in stage IIb and IIIa of the Chinese liver cancer staging scheme (China Liver Cancer (CNLC) staging) exceeds 55 %[3]. Therefore, how to allow more HCC patients to obtain surgical treatment opportunities, reduce the postoperative recurrence rate and finally obtain a better prognosis has become an urgent problem. At present, targeted therapy and immunotherapy have made some progress in the treatment of HCC patients in the middle and advanced stage (CNLC stage IIb, IIIa, IIIb), which provides a better way for HCC patients to undergo radical resection, reduce postoperative recurrence rate and improve prognosis. This provide other possibilities and more choices for HCC perioperative treatment strategies[4,5]. Neoadjuvant therapy in the perioperative period of HCC refers to systemic anti-tumor therapy, radiotherapy or interventional therapy that be used to shrink the tumor or transform liver cancer from poor oncological characteristics to better oncological characteristics, so as to minimize postoperative recurrence rate and prolong survival time. In recent years, combined therapy of immune checkpoint inhibitors represented by Programmed Cell Death 1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1) monoclonal antibody and anti-angiogenic targeted drugs became the first-line systemic treatment options for cell carcinoma. This study retrospectively analyzed 80 patients with advanced HCC treated by liver resection in our hospital from January 2019 to December 2021 to examine the effectiveness and safety of targeted therapy combined with immunotherapy for patients with advanced HCC sex. The data of 65 patients with CNLC stage IIb and IIIa HCC who underwent hepatectomy in the Hepatobiliary Center of our hospital from January 2019 to December 2020 were collected retrospectively. 45 patients who did not get neoadjuvant therapy and met the surgical resection criteria were included in the control group. They included 14 females and 31 males; aged 45 y-79 y, with an average age of (60.25±12.31) y; 8 cases of CNLC stage IIb and 37 cases of IIIa. 20 patients with HCC who underwent neoadjuvant therapy with apatinib combined with camrelizumab were included in the study group. They included 6 females and 14 males; aged 46 y-75 y, with an average age of (62.14±10.51) y; Stage IIb cases were 4, whereas stage IIIa cases were 11. The baseline information for the two patient groups did not significantly differ from one another and they were comparable. Inclusion criteria for patients have CNLC stage IIb and IIIa HCC diagnosed by histological or cytological examination; all lesions were resectable; eastern cooperative oncology group physical status score was 0-1 and immunization and targeted drug therapy were not performed before enrollment. Exclusion criteria have history of apatinib or other PD-1/PD-L1 inhibitor drug application; combined with other primary tumors, autoimmune liver disease; intrahepatic cholangiocarcinoma, mixed HCC, sarcomatoid liver carcinoma or fibro lamellar carcinoma of the liver. Patients took apatinib mesylate tablets (Jiangsu Hengrui Medicine Co., Ltd., China) orally after meals, with an initial dose of 500 mg/time, once a day; (Hengrui Medicine Co., Ltd., China) intravenous infusion, 200 mg/time, 1 time/3 w. The clinical curative effect of patients was evaluated based on new criteria for tumor response evaluation (modified Response Evaluation Criteria in Solid Tumors (mRECIST)) and the curative effect was classified into Partial Remission (PR), Stable Disease (SD), Complete Remission (CR) and Overall Response Rate (ORR), Progression of Disease (PD) and Disease Control Rate (DCR) were computed. Where ORR=CR+PR, DCR=CR+PR+SD. The operation conditions of the two patient groups were collected and compared, including operation time, operation type, intraoperative blood transfusion, postoperative hospital stay and intraoperative blood loss. Postoperative complications were evaluated utilizing the Clavien-Dindo complication grading system and the postoperative liver failure grading criteria of the international hepatic surgery research group[6]. Survival follow-up within 2 y after surgery was mainly carried out by means of outpatient service, hospitalization and telephone. Follow-up was conducted every 3 mo in the 1 y and outpatient re-examination or telephone follow-up was performed every 6 mo after 1 y. The patient’s survival status, tumor recurrence and post-relapse treatment were recorded. Time to tumor recurrence was explained as the interval from the date of surgery to tumor recurrence or recurrence-free at the last follow-up. Overall Survival (OS) was explained as the interval from surgery date to death from any cause or alive at the previous follow-up. The deadline for follow-up is June 2022. Statistical Package for Social Sciences (SPSS) 23.0 software was employed for performing the statistical analysis. The measurement results conforming to the normal distribution were marked as (x±s), the count data were written as relative numbers, the Kaplan-Meier method was utilized to build the survival curve and the OS of the patients was counted; the graphics were drawn using GraphPad Prism 8 software. To examine the short-term curative effect of 20 HCC patients treated with apatinib combined with camrelizumab, it was found that the 3 mo ORR was 35.00 % (CR-0 cases, PR-7 cases) and the DCR was 75.00 % (CR-0 cases, PR-7 cases) example, SD-8 cases). There were no significant differences between the groups in intraoperative conditions such as intraoperative blood loss, postoperative hospital stay, operation time, postoperative blood transfusion volume and intraoperative blood transfusion volume (all p>0.05) as shown in Table 1. In the study group, postoperative grade I-II complications included 8 cases of grade A liver failure and 2 cases of the acute coronary syndrome; postoperative grade III-IV complications included biliary fistula in 3 cases, all of which were cured after puncture and drainage. In the control group, postoperative grade I-II complications included 20 cases of grade A liver failure, 3 cases of pleural effusion or ascites and 1 case of poor incision healing; III-IV grade complications included 1 case of grade C liver failure, 2 cases of severe pulmonary infection, 2 cases of biliary fistula, 3 cases of wound dehiscence with wound infection. The occurrence of postoperative complications did not significantly differ among the two groups (p>0.05) as shown in Table 2. The patients were examined for 2 y, the survival time of the study group was 22.37±0.58 mo (95 % CI: 21.24-23.51) and the survival time of the control group was 19.08±0.83 mo (95 % CI: 18.45-21.71), the difference in survival time between the groups was significant (p=0.041). The recurrence time of the study group was 17.32±1.60 mo (95 % CI: 14.20-20.45) and the recurrence time of the control group was 13.24±1.56 mo (95 % CI: 10.20- 16.30). The difference between the groups was significant (p=0.023) as shown in fig. 1. PD-1 can lead to phosphorylation of phosphatidylinositol-3-kinase and activation of protein kinase by binding to its ligand PD-L1, which further stimulates T cell activation, leading to downstream glucose metabolism, interferon secretion and anti-tumor immunity inhibition of PD-1/ PD-L1 can activate the body’s endogenous anti-tumor response, thereby achieving the purpose of treating tumors[7,8].
| Group | Intraoperative blood transfusion (n) | Postoperative blood transfusion (n) | Operation time (min) | Intraoperative blood loss (ml) | Postoperative hospital stay (d) | ||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Research group (n=20) | 9 | 11 | 15 | 5 | 241.81±66.58 | 642.11±95.36 | 11.97±2.94 |
| Control group (n=45) | 23 | 22 | 31 | 14 | 227.37±58.65 | 613.94±89.18 | 13.41±3.45 |
| p | 0.7893 | 0.7703 | 0.3829 | 0.2542 | 0.1099 | ||
Table 1: Comparison of Surgical Data among the Groups
| Adverse reaction | Research group (n=20) | Control group (n=45) | p |
|---|---|---|---|
| I-II | |||
| Grade A liver failure | 6 | 17 | - |
| Acute coronary syndrome | 1 | 0 | - |
| Pleural effusion or ascites | 0 | 3 | - |
| Poor incision healing | 0 | 2 | - |
| Total | 7 | 22 | 0.4185 |
| III-IV | |||
| Biliary fistula | 3 | 2 | - |
| Grade C liver failure | 0 | 1 | - |
| Severe pulmonary infection | 0 | 2 | - |
| Wound dehiscence with infection | 0 | 3 | - |
| Total | 3 | 8 | >0.9999 |
Table 2: Adverse Reactions among the Two Groups of Patients
In recent years, immune checkpoint inhibitors expressed by PD-1/PD-L1 monoclonal antibody have gradually become the most effective systemic treatment for liver cancer, but there are few studies on immunotherapy in the neoadjuvant treatment of liver cancer. Camrelizumab is currently the only domestic PD-1 monoclonal antibody drug approved for the cure of primary liver cancer, and its price has certain advantages compared with similar drugs[9]. Apatinib is a small molecule anti-angiogenic drug and its anti-tumor mechanism is mainly through highly selective binding to Vascular Endothelial Growth Factor Receptor-2 (VFGFR-2) to competitively inhibit the binding process of Vascular Endothelial Growth Factor (VEGF), play an anti-tumor effect. Previous studies have shown that immune checkpoint inhibitors combined with antiangiogenic drugs combined with adjuvant treatment of HCC patients can achieve a good curative effect and the side effects can be controlled. For example, Xu et al. found that the ORR of apatinib and camrelizumab in first-line and second-line treatment was 34.3 %[10]; the study by Kudo et al. described that the ORR of avelumab combined with axitinib in the first-line cure of HCC patients was 13.60 %[11]. Previous studies tended to study the effectiveness and safety of immune checkpoint inhibitors and anti-angiogenic drugs in the adjuvant treatment of HCC patients, but the research focus of this work is to evaluate the effectiveness and safety of immunotherapy joined with targeted therapy as neoadjuvant therapy on resettable HCC. The drugs selected in this study were camrelizumab and apatinib. The results showed that in patients with severe HCC before resection, the 3 mo ORR of camrelizumab with apatinib reached 35.00 % and the DCR reached 75.00 %. The results of a domestic single-arm phase II clinical trial showed that the perioperative application of camrelizumab combined in patients with resectable HCC with apatinib was effective. Based on the assessment criteria of RECIST V.1.1, the ORR reached 33.3 %. The ORR of this study was similar to that of this study[12]. Regarding safety, there was no notable change in multiple indicators reflecting the perioperative safety of HCC patients in the study group receiving neoadjuvant therapy (intraoperative blood loss, postoperative hospital stay, intraoperative blood transfusion, operation time and postoperative complications) matched with that in the control group, suggesting that the surgical safety of targeted+immune neoadjuvant therapy is good and the adverse reactions are tolerable. The high recurrence rate has seriously affected the long-term survival of liver cancer patients after surgical resection. In this study, the patients of the two groups were followed up and found that the recurrence time and OS of the study group were remarkably greater than that of the control group, indicating that targeting+immune neoadjuvant therapy significantly prolongs the survival time of patients with resectable HCC and reduces the recurrence rate. In summary, immune checkpoint inhibitors expressed by PD-1/PD-L1 monoclonal antibodies and antiangiogenic targeted drugs combined with neoadjuvant therapy have good safety for patients with resectable HCC and can enhance the prognosis of patient’s situation, prolong the survival time of patients and the toxic and side effects are acceptable. The study’s remaining drawbacks are its limited sample size and brief follow-up time. Follow-up studies still need to expand the sample size to improve the test and improve the reliability of the research data.
Conflict of interest:
The authors declared no conflict of interests.
References
- Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol 2020;21(7):e342-9.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. BioSci Trends 2021;15:138-41.
- Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: A review. Int J Mol Sci 2021;22(11):5801.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res 2019;38(1):447.
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149(5):713-24.
[Crossref] [Google Scholar] [PubMed]
- Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol 2019;234(2):1313-25.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Li J, Tang G, Tian Y, Su S, Li Y. Clinical outcomes and influencing factors of PD-1/PD-L1 in hepatocellular carcinoma. Oncol Lett 2021;21(4):279.
[Crossref] [Google Scholar] [PubMed]
- Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21(4):571-80.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trialcamrelizumab plus apatinib in advanced HCC. Clin Cancer Res 2021;27(4):1003-11.
[Crossref] [Google Scholar] [PubMed]
- Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, et al. Avelumab in combination with axitinib as first-line treatment in patients with advanced hepatocellular carcinoma: Results from the phase 1b VEGF liver 100 trials. Liver Cancer 2021;10(3):249-59.
[Crossref] [Google Scholar] [PubMed]
- Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: A single-arm, open label, phase II clinical trial. J Immunother Cancer 2022;10(4):e004656.
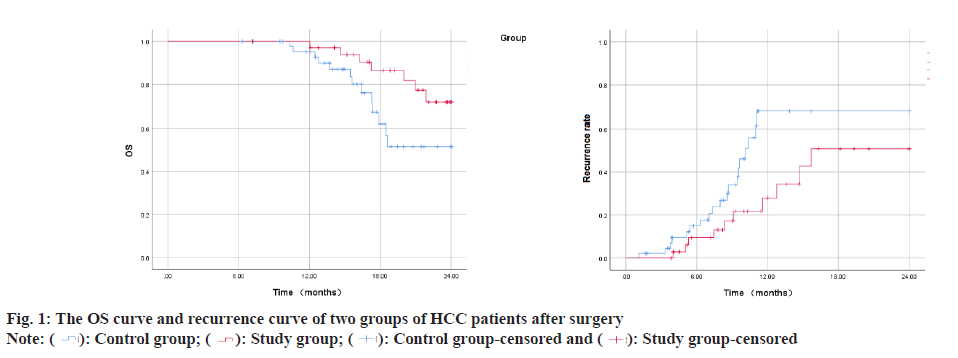
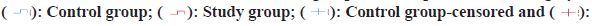 Study group-censored
Study group-censored



