- *Corresponding Author:
- Zhuangyan Mai
Department of Medical Oncology, The First Affiliated Hospital of Hainan Medical University, Hainan 570102, China
E-mail: hainanmzy@163.com
| This article was originally published in a special issue, “Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(2) Spl Issue “47-52” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To probe the clinical efficacy of rituximab combined with cyclophosphamide, adriamycin, vincristine together with prednisone regimen sequential or non-sequential local radiotherapy in patients with stage III-IV diffuse large B-cell lymphoma. The clinical data of 126 patients with high-risk diffuse large B-cell lymphoma in the First Affiliated Hospital of Hainan Medical University from January 2018 to December 2021 were retrospectively analyzed. Among them, the patients in the cyclophosphamide, adriamycin, vincristine together with prednisone group were adopted cyclophosphamide, adriamycin, vincristine together with prednisone regimen, the patients in the radiotherapy group were adopted radiotherapy in addition to cyclophosphamide, adriamycin, vincristine together with prednisone regimen and the patients in the combined group were treated with rituximab in addition to the radiotherapy group. The short-term and long-term efficacy, adverse reactions and immunoglobulin levels were compared in these three groups. In comparison with cyclophosphamide, adriamycin, vincristine together with prednisone group, the total effective rate of combined group and radiotherapy group was elevated and the whole effective rate of combined group was increased relative to radiotherapy group. Spearman correlation analysis unveiled that the clinical efficacy of the radiotherapy group together with the combined group was negatively correlated with the serum immunoglobulin M level after 4 courses and 6 courses of treatment. Rituximab combined with radiotherapy is effective in diffuse large B-cell lymphoma therapy, which can significantly promote the short-term efficacy of patients with high safety.
Keywords
Lymphoma, rituximab, cyclophosphamide, adriamycin, vincristine, prednisone
Diffuse Large B-Cell Lymphoma (DLBCL) belongs to the most frequent pathological type of Non- Hodgkin’s Lymphoma (NHL) with highly aggressive, which can destroy normal lymph nodes or extra nodal tissues. It accounts for 30 %-40 % of adult NHL[1]. DLBCL can occur in all ages, but it is more common in middle aged along with elderly people and most of them are male. The main feature is lymph node enlargement and some patients may show typical B symptoms (night sweat, fever, weight loss, etc.)[2]. DLBCL has rapid progression, high malignancy and poor prognosis[3]. Cyclophosphamide, Adriamycin, Vincristine together with Prednisone (CHOP) regimen is the primary chemotherapy choice for DLBCL patients and is of great significance in improving tumor burden and clinical cure rate of DLBCL patients, but there are still some patients who are difficult to benefit from it and the prognosis is not ideal[4]. Rituximab is a monoclonal antibody that specifically combines with Cluster of Differentiate (CD) 20 antigen on the surface of B lymphocytes, which can kill normal or abnormal B lymphocytes through a series of effects to achieve the purpose of killing tumor cells[3]. Previous studies have shown that rituximab combined with other chemotherapy regimens can significantly prolong the remission period of DLBCL patients and improve the clinical efficacy. Due to the emergence of targeted immunochemotherapy combined regimens, the efficacy of DLBCL patients has been continuously improved. The status of radiotherapy, which represents local treatment, is gradually declining[5]. However, due to the prevalence of comorbidities, the decline of organ function, poor drug metabolism ability and poor tolerance to systemic drug therapy in elderly patients, the overall efficacy is affected and even some elderly patients who cannot receive chemotherapy at all. In addition, most of the elderly DLBCL patients are at stage III-IV at the time of onset. Therefore, even after receiving chemotherapy, this population has a higher rate of subsequent recurrence and chemotherapy insensitivity than young patients[6]. Therefore, compared with young patients, elderly patients with DLBCL may need more radiotherapy intervention. The intention of this work was to probe the clinical effect of rituximab combined with CHOP regimen sequential or no sequential local radiotherapy in patients with stage III-IV DLBCL.
Materials and Methods
General data:
The clinical data of 126 patients with high-risk DLBCL in The First Affiliated Hospital of Hainan Medical University from January 2018 to December 2021 were retrospectively analyzed. Among them, 42 patients were adopted CHOP regimen alone as the CHOP group, 38 patients were adopted CHOP regimen sequential or no sequential local radiotherapy as the radiotherapy group, and 46 patients were adopted rituximab combined with CHOP regimen plus sequential or no sequential local radiotherapy as the combined group. In CHOP group, 27 males together with 15 females were contained; aged from 19 y to 76 y, the mean age was (58.26±12.45) y. There were 19 cases of clinical stage III together with 23 cases of clinical stage IV. There were 26 cases with B symptoms together with 34 cases with extra nodal organ involvement. In radiotherapy group, 25 males together with 13 females were contained; aged from 19 y to 73 y, the mean age was (57.63±12.02) y. There were 18 cases of clinical stage III as well as 20 cases of clinical stage IV. There were 22 cases with B symptoms along with 30 cases with extra nodal organ involvement. In combined group, 32 males together with 14 females were contained; aged from 19 y to 75 y, the mean age was (56.31±10.68) y. There were 21 cases of clinical stage III together with 25 cases of clinical stage IV. There were 34 cases with B symptoms as well as 39 cases with extra nodal organ involvement. No significant difference was observed in general data among the three groups (p>0.05).
Inclusion criteria: DLBCL was confirmed by pathological biopsy and international prognostic index score >3; patients who were newly treated and ≥18 y old and the clinical data were complete.
Exclusion criteria: Combined with central nervous system lymphoma; patients with cardiac contraindications could not tolerate related treatment; combined with other active malignant tumors; previous infection with hepatitis B virus and human immunodeficiency virus. This study was approved by the Medical Ethics Committee of our hospital.
Methods:
CHOP regimen: Intravenous infusion of cyclophosphamide (750 mg/m2) on the 1st d; intravenous infusion of adriamycin (50 mg/m2) on the 1st d; vincristine (1.4 mg/m2) IV bolus on the 1st d; prednisone (60-100 mg) orally, from d 1 to d 5; 21 d was a course of treatment and the treatment was given for 6 consecutive courses.
Radiotherapy group: Patients in the radiotherapy group were adopted radiotherapy based on CHOP group: The specific operation was as follows; the patient was fixed in an appropriate position, Computed Tomography (CT) simulation was performed, and the target volume as well as organs at risk was delineated by experienced clinicians. The target volume was the involved field and the Gross Tumor Volume (GTV) was defined as the clinically known tumor area. Clinical Target Volume (CTV) was GTV and its adjacent anatomical structures. The Planning Target Volume (PTV) was generated by 5-8 mm margin on the basis of the primary CTV. Three Dimensional Conformal Therapy (3DCRT) or Intensity-Modulated Radiotherapy (IMRT) was used for radiotherapy. The dose volume histograms of the patients were collected and the target coverage was analyzed by physical dosimetry, including the minimum dose (Dmin), maximum dose (Dmax), mean dose (Dmean), V95 %, V90 %, V105 %, V110 % (percentage of the volume receiving X % of the prescribed dose), Conformity Index (CI) and Homogeneity Index (HI).
The patients in the combined group were adopted rituximab on the basis of the patients in the radiotherapy group. The specific scheme was given to the patients 1 d before CHOP chemotherapy and the dose of rituximab was 375 mg/m2 mixed with the same volume of normal saline and then intravenously infused. In addition, radiotherapy was given at the same time, 21 d as a course and 6 courses of treatment were given continuously.
Observation indicators
Long-term efficacy: Overall Survival (OS) together with Progression-Free Survival (PFS) were observed to evaluate the survival of patients in each group. OS was the time from diagnosis to death or the last follow-up. PFS was the time from treatment to disease progression, recurrence, death or the last follow-up. The deadline for follow-up was March 1st, 2022. The 126 patients were followed up for 2 mo-24 mo, with a median of 16 mo.
Evaluation of short-term therapeutic effect: After 6 cycles of treatment, according to the International Working Group response criteria, patients were classified as complete remission (there were no palpable lymph nodes or negative biopsies, lymph nodes 1.5 cm or less in diameter and bone marrow morphology or histology was normal and stable for at least 28 d), partial remission (measurable lesions were declined by >50 % relative to those before treatment), stable disease (measurable lesions were decreased by ≤50 % or lesions were elevated by ≤25 % compared with those before treatment), progressive disease (measurable lesions were increased by >25 % or new lesions appeared) and complete remission+partial remission were considered as the total effective rate.
Measurement of CD4+, CD8+, CD20+, Immunoglobulin M (IgM), Immunoglobulin G (IgG), and Beta-2 Macroglobulin (β2-MG): The expression levels of CD4+, CD8+ and CD20+ in peripheral blood were assessed using flow cytometry before treatment and after 2, 4 and 6 courses of treatment. The levels of serum IgM and IgG were examined by immunoturbidimetry.
Adverse reactions: These were evaluated according to the World Health Organization toxicity standard, including anemia, low platelet count, nausea and vomiting, fever, neurovirulence, cardiac injury and liver injury.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 22.0 software was adopted to analyze all the data and the measurement data were analyzed by means of standard deviation. The t test was used for comparison between groups. Count data were expressed as the number of cases and rate (0 %) and were expressed as the number of cases and rate (0 %). Rank sum test was used for comparison between groups. p<0.05 was considered statistically significant.
Results and Discussion
Compared with CHOP group, the whole effective rate of combined group and radiotherapy group was higher, and the total effective rate of combined group was increased in comparison with radiotherapy group (p<0.05; Table 1).
| Groups | n | Complete remission | Partial remission | Stable | Progressive | Total effective rate |
|---|---|---|---|---|---|---|
| CHOP group | 42 | 20 (47.62) | 6 (14.28) | 9 (21.43) | 7 (16.67) | 26 (61.90) |
| Radiotherapy group | 38 | 21 (52.63) | 8 (21.05) | 6 (15.79) | 3 (7.89) | 27 (73.68)* |
| Combined group | 46 | 26 (56.53) | 10 (21.73) | 5 (10.87) | 5 (10.87) | 36 (78.26)*# |
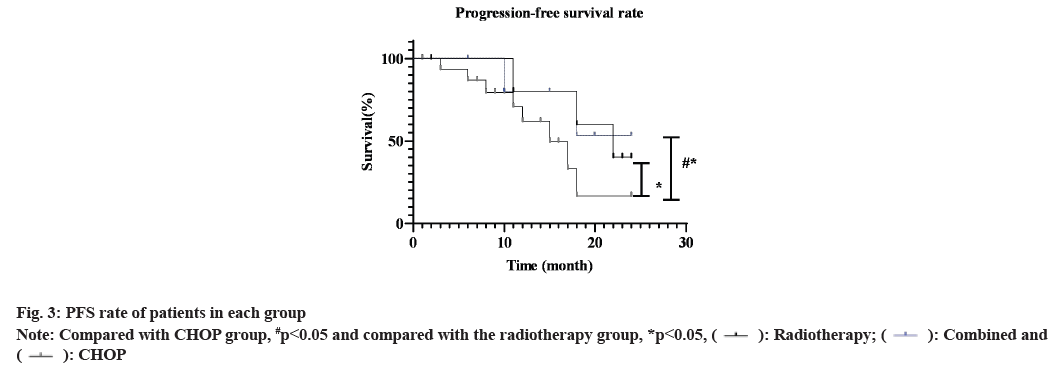
Note: Compared with CHOP group, #p<0.05 and compared with the radiotherapy group, *p<0.05
Table 1: Comparison of Short-Term Clinical Efficacy in Each Group
There was no patient who stopped treatment within 6 courses in the three groups, and no significant difference was discovered in the occurrence of adverse events among the three groups (p>0.05; Table 2).
| Groups | n | Anemia | Low platelet count | Nausea and vomiting | Fever | Neurovirulence | Cardiac injury | Liver injury | Total effective rate |
|---|---|---|---|---|---|---|---|---|---|
| CHOP group | 42 | 7 (16.67) | 1(2.38) | 7 (16.67) | 1 (2.38) | 0 (0.00) | 1 (2.38) | 3 (7.14) | 20 (47.62) |
| Radiotherapy group | 38 | 6 (15.79) | 1 (2.63) | 6 (15.79) | 0 (0.00) | 1 (2.63) | 1 (2.63) | 4 (10.52) | 19 (50.00) |
| Combined group | 46 | 7 (15.22) | 2(4.35) | 8 (17.39) | 1 (2.17) | 1 (2.17) | 1 (2.17) | 4 (8.69) | 24 (52.17) |
Table 2: Occurrence of Adverse Reactions in Each Group
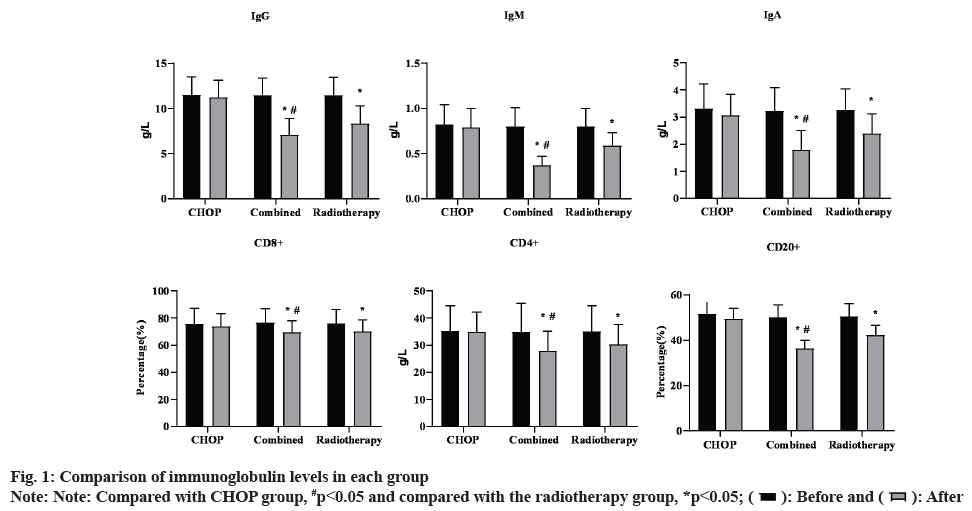
As shown in fig. 1, before treatment, no significant difference was observed in immunoglobulin levels among the three groups (p>0.05). After treatment, IgG, IgA and IgM levels in the radiotherapy group together with the combined group were reduced than those before treatment (p<0.05). IgG, IgA and IgM levels in CHOP group were not significantly different from those before treatment (p>0.05). After treatment, IgG, IgA and IgM levels in the combined group were decreased than those in CHOP group and the radiotherapy group (p<0.05). Similarly, before treatment, no significant difference was observed in CD8+, CD4+ and CD20+ among the three groups (p>0.05). After treatment, CD8+, CD4+ and CD20+ levels in the radiotherapy group together with the combined group were declined than those before treatment (p<0.05). CD8+, CD4+ and CD20+ levels in CHOP group were not significantly different from those before treatment (p>0.05). After treatment, CD8+, CD4+ and CD20+ levels in the combined group were declined compared to the CHOP group as well as the radiotherapy group (p<0.05).
The PFS rate of the combined group was 71.90 % and the radiotherapy group was 51.30 %, which was higher than 39.30 % of the CHOP group (χ2=7.643, p=0.006). The OS rate of the combined group and the radiotherapy group was 81.30 % and 54.80 %, respectively, which was higgher than 42.90 % of the CHOP group (χ2=10.510, p=0.001) (fig. 2 and fig. 3).
DLBCL originates from the cells of the human immune system and its precursor cells and the immune function of the body is linked to the development of DLBCL. Functioning as one of the most promising treatment strategies in cancer, immunotherapy also has a crucial role in DLBCL treatment. As a chimeric mouse anti-human monoclonal antibody, rituximab can specifically bind to CD20 antigen and induce B cell lysis through complement and cell-mediated cytotoxicity, thus playing an anti-tumor role[7]. An observational study on the efficacy of rituximab in DLBCL treatment[8] found that the application of rituximab could significantly improve the sensitivity of B lymphocytes to chemotherapy drugs, thereby prolonging the PFS of patients and improving their clinical efficacy. Since rituximab kills lymphoma cells is mainly by destroying abnormal or normal B cells, the effect of rituximab on the immune function of patients with DLBCL has become a current research hotspot.
In this research, the short-term and long-term efficacy, adverse reactions and immunological indexes of patients with DLBCL treated with rituximab combined with radiotherapy were analyzed. The outcomes of the study demonstrated that after chemotherapy, the OS together with PFS of the combined group were elevated compared to the CHOP group as well as the radiotherapy group, suggesting that rituximab combined with radiotherapy could improve the clinical efficacy of chemotherapy, which was in line with the results of previous reports[9]. On the one hand, rituximab can eliminate malignant B cells by complementdependent tolerance and effectively inhibit tumor progression. On the other hand, it can inhibit the proliferation of tumor cells and new blood vessels, and play a synergistic effect with chemotherapy, thereby improving the short-term efficacy of DLBCL patients[10]. In this study, there were some adverse reactions in the three groups during the treatment, but no significant difference was observed in the occurrence of adverse reactions among the three groups, suggesting that the application of rituximab does not increase the toxic as well as side effects of chemotherapy and the patients are well tolerated and safe.
As tumor cells produce a large number of cytokines in the process of proliferation and metastasis, which affect the normal immunity of the body, most patients with DLBCL have abnormal immune function[11]. The outcomes of this research revealed after treatment, CD8+, CD4+ and CD20+ levels in the combined group together with radiotherapy group were declined compared with before treatment. The level of each T lymphocyte in the CHOP group did not change significantly compared with that before treatment, suggesting that rituximab could affect the overall cellular immune status of DLBCL patients. However, there was no significant effect on the stability of immune function. Recent studies[12] have analyzed the impacts of rituximab combined with chemotherapy in indolent NHL (iNHL) therapy and found that the application of rituximab can effectively control iNHL and has no significant effect on the body’s T lymphocyte subsets and the anti-tumor function of the body is not weakened. At the same time, it can more effectively enhance the inhibitory effect on tumor when combined with radiotherapy. In this work, immunoglobulin levels in the combined group together with radiotherapy group were reduced than those before treatment and IgG, IgA and IgM levels in the combined group were declined relative to the CHOP group and the radiotherapy group. IgG, IgA and IgM levels in the CHOP group were not significantly different from those before treatment. This suggested that rituximab could affect the immunoglobulin levels of patients. Rituximab kills abnormal B lymphocytes through complementmediated cytotoxicity, resulting in a decrease in the number of B lymphocytes in peripheral blood, leading to a reduction in the number of antibodyproducing cells and ultimately a decrease in the level of immunoglobulin[13,14].
However, studies by some scholars[15] have found that rituximab has no significant effect on the body's immunoglobulin, which may be related to the large differences in the ages of the subjects included in the study and the different treatment cycles of the study. Therefore, when rituximab is used in the clinical treatment of chemotherapy, the changes in serum immunoglobulin levels of patients should be closely monitored to reduce the incidence of immunoglobulinemia[16]. In addition, malignant tumor cells with active metabolism can synthesize a large amount of β2-MG and its serum expression level is closely related to tumor burden. The dynamic changes of β2-MG can assist in judging the prognosis of DLBCL[17]. Moreover, the clinical efficacy of combined group and radiotherapy group was significantly negatively correlated with serum β2-MG level after 4 and 6 courses of treatment, indicating that the dynamic change of β2-MG was helpful to determine the progression of high-risk DLBCL and β2-MG could be actively monitored in clinical practice, which had guiding significance for diagnosis and treatment.
In conclusion, rituximab combined with radiotherapy is effective in DLBCL treatment, which can significantly enhance the short-term efficacy of patients with high safety.
Author’s contributions:
Daorui Lin and Yang Wen have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ren YR, Jin YD, Zhang ZH, Li L, Wu P. Rituximab treatment strategy for patients with diffuse large B-cell lymphoma after first-line therapy: A systematic review and meta-analysis. Chin Med J 2015;128(3):378-83.
[Crossref] [Google Scholar] [PubMed]
- Fleury I, Chevret S, Pfreundschuh M, Salles G, Coiffier B, van Oers MH, et al. Rituximab and risk of second primary malignancies in patients with non-Hodgkin lymphoma: A systematic review and meta-analysis. Ann Oncol 2016;27(3):390-7.
[Crossref] [Google Scholar] [PubMed]
- Lin RJ, Behera M, Diefenbach CS, Flowers CR. Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood 2017;130(20):2180-5.
[Crossref] [Google Scholar] [PubMed]
- Castillo-Trivino T, Braithwaite D, Bacchetti P, Waubant E. Rituximab in relapsing and progressive forms of multiple sclerosis: A systematic review. PloS One 2013;8(7):e66308.
[Crossref] [Google Scholar] [PubMed]
- Luo C, Wu G, Huang X, Ma Y, Zhang Y, Song Q, et al. Efficacy and safety of new anti-CD20 monoclonal antibodies vs. rituximab for induction therapy of CD20+ B-cell non-Hodgkin lymphomas: A systematic review and meta-analysis. Sci Rep 2021;11(1):3255.
[Crossref] [Google Scholar] [PubMed]
- Galaznik A, Huelin R, Stokes M, Guo Y, Hoog M, Bhagnani T, et al. Systematic review of therapy used in relapsed or refractory diffuse large B-cell lymphoma and follicular lymphoma. Future Sci OA 2018;4(7):FSO322.
[Crossref] [Google Scholar] [PubMed]
- Chigurupati SV, Shukla M, Pandey M. Primary sacral non-Hodgkin’s lymphoma: Report of a case and systematic review of literature. World J Surg Oncol 2021;19(1):61.
[Crossref] [Google Scholar] [PubMed]
- Bataillard EJ, Cheah CY, Maurer MJ, Khurana A, Eyre TA, El-Galaly TC. Impact of R-CHOP dose intensity on survival outcomes in diffuse large B-cell lymphoma: A systematic review. Blood Adv 2021;5(9):2426-37.
[Crossref] [Google Scholar] [PubMed]
- Iacoboni G, Zucca E, Ghielmini M, Stathis A. Methodology of clinical trials evaluating the incorporation of new drugs in the first-line treatment of patients with diffuse large B-cell lymphoma (DLBCL): A critical review. Ann Oncol 2018;29(5):1120-9.
[Crossref] [Google Scholar] [PubMed]
- Li L, Li Y, Que X, Gao X, Gao Q, Yu M, et al. Prognostic significances of overexpression MYC and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: A systematic review and meta-analysis. Sci Rep 2018;8(1):6267.
[Crossref] [Google Scholar] [PubMed]
- Read JA, Koff JL, Nastoupil LJ, Williams JN, Cohen JB, Flowers CR. Evaluating cell-of-origin subtype methods for predicting diffuse large B-cell lymphoma survival: A meta-analysis of gene expression profiling and immunohistochemistry algorithms. Clin Lymphoma Myeloma Leuk 2014;14(6):460-7.
[Crossref] [Google Scholar] [PubMed]
- Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346(4):235-42.
[Crossref] [Google Scholar] [PubMed]
- van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 intergroup trial. Blood 2006;108(10):3295-301.
[Crossref] [Google Scholar] [PubMed]
- Cabanillas F. Rituximab in DLBCL: 6 years on. Lancet Oncol 2011;12(11):984-5.
[Crossref] [Google Scholar] [PubMed]
- Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-cell lymphoma. J Clin Exp Hematopathol 2011;51(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Gisselbrecht C, Glass B, Mounier N, Gill DS, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28(27):4184.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zang J, Xu J, Bai C, Qin Y, Liu K, et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: A systematic review and meta-analysis. Chest 2011;140(1):117-26.
[Crossref] [Google Scholar] [PubMed]
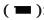 : Before and
: Before and  : After
: After
 : Radiotherapy;
: Radiotherapy;  : Combined and
: Combined and  : CHOP
: CHOP