- *Corresponding Author:
- Nian Zhu
Department of Emergency Medicine, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, Pudong, Shanghai Province 201399, China
E-mail: zhunian1984@126.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “159-166” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Reports about the increase in aggressive infections caused by highly virulent Klebsiella pneumoniae have risen in recent times. Nevertheless, the impact of its virulence on the progress and consequences of pneumonia patients has yet to be studied. Our research intended to evaluate and relate the clinical characteristics of highly virulent Klebsiella pneumoniae and classical Klebsiella pneumoniae strains obtained from intensive care unit patients who had pneumonia induced by Klebsiella pneumoniae. In this retrospective analysis, we included 173 individuals diagnosed with pneumonia induced by Klebsiella pneumoniae. We collected relevant demographic and clinical information from medical records. The study involved an examination of genetic markers associated with K1 and K2, antimicrobial sensitivity, and the presence of virulence genes, including regulator of mucoid phenotype A, isoform usage two-step analysis, enteroBactin, yersiniabactin, Klebsiella ferric iron uptake, fimbrial adhesin gene, and allantoin metabolism. Strains that showed both regulator of mucoid phenotype A and isoform usage two-step analysis were classified as hyper virulent Klebsiella pneumoniae (n=66), while the rest were designated as classic Klebsiella pneumoniae (n=107). The prevalence of patients with highly virulent Klebsiella pneumoniae strains was noticeably higher for bacteraemia (24.2 % vs. 8.4 %, p=0.004), metastatic spread (25.8 % vs. 8.4 %, p=0.002), liver abscess (24.2 % vs. 3.7 %, p<0.001), serum creatinine (63.6 % vs. 42.1 %, p=0.006), and 30 d mortality (25.8 % vs. 8.4 %, p=0.002). All 173 Klebsiella pneumoniae strains showed consistent sensitivity to the drug ampicillin. Multivariate regression analysis demonstrated that metastatic spread (odd ratio=3.596, 95 % confidence interval=1.193-10.838; p=0.023), liver abscess (odd ratio=7.537, 95 % confidence interval=1.850-30.715; p=0.005), acute physiology and chronic health evaluation II score (odd ratio=1.616, 95 % confidence interval=1.365-1.914; p<0.001), and serum creatinine (odd ratio=2.506, 95 % confidence interval=1.125-5.587; p=0.025) were all associated with highly virulent Klebsiella pneumoniae infection in patients with pneumonia induced by Klebsiella pneumoniae. Several clinical risk factors linked to highly virulent Klebsiella pneumoniae infection were identified in pneumonia patients with Klebsiella pneumoniae.
Keywords
Hypervirulence, Klebsiella pneumoniae, pneumonia, risk factor, intensive care unit
Klebsiella pneumoniae (K. pneumoniae), a prominent etiological agent of healthcare-associated infections, is a pathogen characterized by its opportunistic nature, targeting individuals with weakened immune systems and those undergoing hospitalization[1]. The prevalence of K. pneumoniae as a causative agent of Community-Acquired Pneumonia (CAP) ranks 2nd in Asia, standing out as the primary Gram-negative pathogen in this context[2]. Within the spectrum of Hospital-Acquired Pneumonia (HAP), which includes cases of Ventilator-Associated Pneumonia (VAP), K. pneumoniae emerges as the secondary utmost prevalent Gram-negative pathogen[3].
The invasive syndromes known as hyper virulent K. pneumoniae (hvKP) infections have been the subject of a thorough investigation of their clinical and bacterial characteristics[4-6]. The distinguishing features of hvKP infections can be briefly outlined as follows; they are mostly documented in Taiwan and Southeast Asia, contrasting with the global prevalence of classic K. pneumoniae (cKP) infections; prevalent forms of hvKP infections typically encompass pyogenic liver abscesses and, on occasion, other metastatic infections; in contrast to cKP, which primarily causes nosocomial infections in immunocompromised patients, hvKP infections usually present as community-onset infections in both immunocompetent and immunocompromised individuals; strains derived from hvKP infections exhibit the hypermucoviscosity phenotype, indicating an augmented production of capsules, which can be readily identified through the string test and a prevalence of capsular serotypes K1 and K2[7,8].
Research has demonstrated risk factors associated with hvKP infections in individuals with K. pneumoniae bloodstream infections. According to Li et al.[9], diabetes mellitus and community-acquired Blood Stream Infections (BSIs) were recognized as autonomous risk elements for hvKP BSIs. In a study by Harada et al.[10], it was noted that patients affected by hvKP infection exhibited a higher prevalence of diabetes mellitus, with their infections displaying a notably increased tendency towards developing liver abscesses among Japanese individuals with K. pneumoniae BSIs. However, this study examined the risk factors associated with pneumonia caused by hvKP in Intensive Care Unit (ICU) patients.
Materials and Methods
General information:
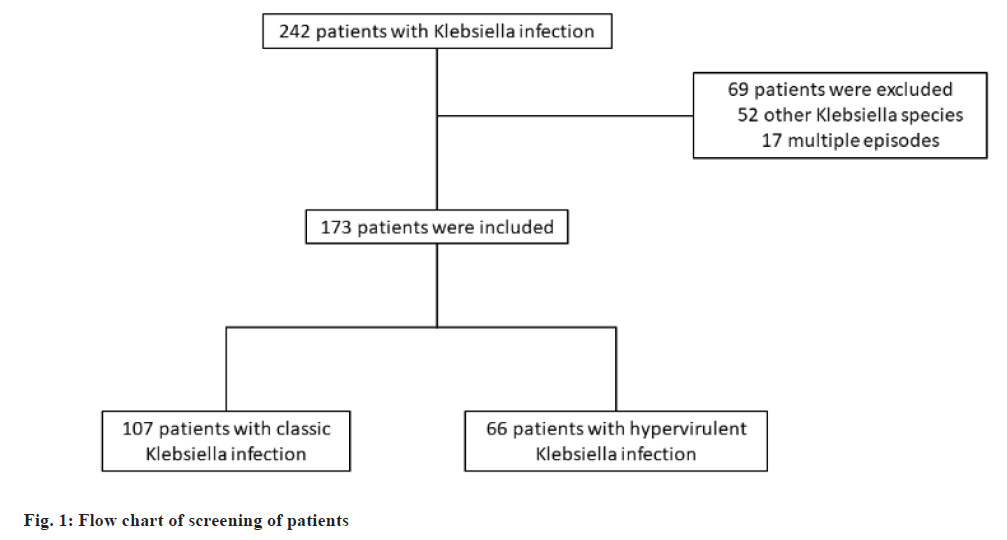
From January 2019 to May 2024, the ICU of Shanghai Pudong Hospital was the site of this retrospective case-control study. 415 patients were enrolled based on the inclusion criteria. In the course of the research, 173 patients with pneumonia due to Klebsiella infection were identified and included in this research, and 180 patients were excluded (fig. 1). The medical records of all patients were collected as follows; age, sex, type of pneumonia, underlying diseases, site of infection, Acute Physiology and Chronic Health Evaluation (APACHE) II, sepsis, shock, laboratory findings, antimicrobial treatment of pneumonia, and Antimicrobial Susceptibility Tests (AST) results. The principal clinical endpoint was mortality observed within a 30 d timeframe. Pneumonia was categorized into three distinct types; CAP, Healthcare-Associated Pneumonia (HCAP), and HAP. CAP was characterized as pneumonia that manifested outside of a hospital, long-term care opportunity, or nursing home environment, which did not fulfill the criteria for HCAP[11]. HCAP was characterized as pneumonia present in patients exhibiting a minimum of two of the following risk factors; hospitalization for a duration of two or more days within the preceding 90 d; living in a long- term care facility or nursing home; administration of antimicrobial treatment or chemotherapy within the last 30 d; undergoing hemodialysis and receiving home wound care[12]. HAP was defined as pneumonia that developed 48 h or more subsequent to hospital admission. hvKP was identified based on positivity for both regulator of mucoid phenotype A (rmpA) and isoform usage two-step Analysis (iutA)[13,14]. The institutional review board at Shanghai Pudong Hospital allowed the study.
Microbiology:
The microorganisms isolated from blood samples were characterized as K. pneumoniae by applying sophisticated conventional methodologies and the VITEK® II diagnostic system (bioMerieux, Japan). All strains were systematically gathered, with only the initial strain subsequently incorporated into the study. The isolates were preserved at -80° until they were subjected to analytical procedures. Stringent testing protocols were executed on all obtained isolates. Deoxyribonucleic Acid (DNA) was extracted from the preserved strains of K. pneumoniae. The sequence type was elucidated through the sequencing of Polymerase Chain Reaction (PCR) amplified products (Sangon Biotech, Shanghai, China), with comparative analysis conducted utilizing an online Comparative Genomics Environment (CGE) tool (https://cge.cbs.dtu.dk). A multiplex PCR assay was employed to identify the K1, K2, and non-K1/K2 capsular serotypes and associated virulence genes. The virulence genes assessed included rmpA, iutA, enterobactin (entb), yersiniabactin (ybtS), Klebsiella ferric iron uptake (Kfu), fimbrial adhesin gene (mrkD), and allantoin metabolism (allS), which have been documented in prior research. AST was carried out utilizing the VITEK® system (amikacin, ampicillin-sulbactam, aztreonam, cefazolin, cefepime, cefotaxime, cefoxitin, ceftazidime, ertapenem, gentamicin, levofloxacin, meropenem, piperacillin-tazobactam, tigecycline), with the interpretation of results conducted by the 2017 Clinical and Laboratory Standards Institute (CLSI) guidelines.
Statistical analysis:
All statistical evaluations were performed utilizing the Statistical Package for the Social Sciences (SPSS) 20.0 statistical software package (IBM, Chicago, Illinois, USA). Categorical variables were represented through frequency analysis (expressed as percentages), while continuous variables were articulated as the mean with standard deviation. The Mann-Whitney U-test or student’s t-test were utilized to analyze continuous variables, while the Chi-square (χ2) or Fisher’s exact test was done for categorical data. Logistic regression analyses, both univariate and multivariate, were used to identify the risk factors linked to hvKP infection. All statistical evaluations were executed using the SPSS 20.0 statistical software package (IBM, Chicago, Illinois, USA). A p<0.05 was deemed a threshold for statistical significance, and all probability values were assessed in a two-tailed manner.
Results and Discussion
The patient demographics at baseline are shown in Table 1. cKP strains were identified in 107 (61.85 %) patients, while hvKP strains were found in 66 (38.15 %) patients. In all patients, CAP and serum creatinine (n=87, 50.29 %) were the most common underlying diseases, followed by HAP and diabetes mellitus (n=54, 31.21 %). Chronic lung disease, chronic kidney disease, cerebrovascular disease, other intra-abdominal infections, and chronic heart disease accounted for 29.48 % (n=51), 27.17 % (n=47), 24.28 % (n=42), 22.54 % (n=39), and 21.97 % (n=38), respectively. The frequency of patients with hvKP strains was noticeably higher for diabetes mellitus, bacteremia, metastatic spread, liver abscess, serum creatinine, shock, and 30 d mortality than those harboring cKP strains (42.4 % vs. 24.3 %, p=0.012; 24.2 % vs. 8.4 %, p=0.004; 25.8 % vs. 8.4 %, p=0.002; 24.2 % vs. 3.7 %, p<0.001; 63.6 % vs. 42.1 %, p=0.006; 24.2 % vs. 10.3 %, p=0.014 and 25.8 % vs. 8.4 %, p=0.002).
| Characteristics | cKP (n=107) | hvKP (n=66) | p |
|---|---|---|---|
| Age | 62.76±11.73 | 62.97±11.88 | 0.908 |
| Gender (male) | 57 (53.3 %) | 38 (57.6 %) | 0.58 |
| Type of pneumonia | 0.577 | ||
| CAP | 54 (50.5 %) | 33 (50.0 %) | |
| Healthcare-associated pneumonia | 22 (20.6 %) | 10 (15.2 %) | |
| HAP | 31 (29.0 %) | 23 (34.8 %) | |
| Underlying diseases | |||
| Diabetes mellitus | 26 (24.3 %) | 28 (42.4 %) | 0.012 |
| Biliary tract disease | 17 (15.9 %) | 9 (13.6 %) | 0.687 |
| Malignancy | 20 (18.7 %) | 13 (19.7 %) | 0.87 |
| Chronic lung disease | 34 (31.8 %) | 17 (25.8 %) | 0.399 |
| Chronic kidney disease | 22 (20.6 %) | 20 (30.3 %) | 0.147 |
| Chronic liver disease | 13 (12.1 %) | 7 (10.6 %) | 0.758 |
| Chronic heart disease | 23 (21.5 %) | 15 (22.7 %) | 0.849 |
| Cerebrovascular disease | 28 (26.2 %) | 19 (28.8 %) | 0.707 |
| Site of infection | |||
| Bacteremia | 9 (8.4 %) | 16 (24.2 %) | 0.004 |
| Metastatic spread | 9 (8.4 %) | 17 (25.8 %) | 0.002 |
| Liver abscess | 4 (3.7 %) | 16 (24.2 %) | <0.001 |
| Biliary tract infection | 22 (20.6 %) | 12 (18.2 %) | 0.702 |
| Other intra-abdominal infection | 25 (23.4 %) | 14 (21.2 %) | 0.742 |
| Urinary tract infection | 22 (20.6 %) | 13 (19.7 %) | 0.891 |
| Lung abscess | 5 (4.7 %) | 5 (7.6 %) | 0.427 |
| Other/unknown | 19 (17.8 %) | 9 (13.6 %) | 0.475 |
| APACHE II | 13.19±2.23 | 16.56±3.13 | <0.001 |
| Serum creatinine (>94.69 μM) | 45 (42.1 %) | 42 (63.6 %) | 0.006 |
| Shock | 11 (10.3 %) | 16 (24.2 %) | 0.014 |
| 30 d mortality | 9 (8.4 %) | 17 (25.8 %) | 0.002 |
Note: Continuous variables are expressed as mean±Standard Deviation (SD), and analyzed using t-test or Wilcoxon-Mann-Whitney test. Categorical variables are expressed as frequency (%), and analyzed using the χ2 test
Table 1: Clinical and Bacterial Characteristics of Patients Infected with K. pneumoniae
A comparison of the virulence factors and capsular serotype frequencies between cKP and hvKP strains was shown in Table 2. Compared to cKP strains, hvKP strains showed a significantly higher prevalence of the ST23 sequence type (36.4 % vs. 0 %, p<0.001). Out of all K. pneumoniae strains, the capsular serotypes K1 and K2 accounted for 16.76 % (29/173) and 19.08 % (33/173) of the strains, respectively. Additionally, hvKP strains showed a potentially higher prevalence of the rmpA (100 % vs. 22.4 %, p<0.001), iutA (100 % vs. 16.8 %, p<0.001), entb (87.9 % vs. 57.0 %, p<0.001), ybtS (77.3 % vs. 37.4 %, p<0.001), and allS (21.2 % vs. 10.3 %, p=0.047) virulence factors compared to cKP strains.
| Characteristics | cKP (n=107) | hvKP (n=66) | p |
|---|---|---|---|
| Sequence types | |||
| ST23 | 0 (0 %) | 24 (36.4 %) | <0.001 |
| ST86 | 15 (14.0 %) | 10 (15.2 %) | 0.837 |
| ST65 | 4 (3.7 %) | 4 (6.1 %) | 0.48 |
| ST29 | 20 (18.7 %) | 4 (6.1 %) | 0.02 |
| ST15 | 22 (20.6 %) | 3 (4.5 %) | 0.004 |
| ST660 | 18 (16.8 %) | 1 (1.5 %) | 0.002 |
| ST11 | 19 (17.8 %) | 14 (21.2 %) | 0.574 |
| Others | 9 (8.4 %) | 3 (4.5 %) | 0.331 |
| Capsular serotype | |||
| K1 | 0 (0 %) | 29 (43.9 %) | <0.001 |
| K2 | 19 (17.8 %) | 14 (21.2 %) | 0.574 |
| Non K1/K2 | 88 (82.2 %) | 23 (34.8 %) | <0.001 |
| Virulence factor | |||
| rmpA | 24 (22.4 %) | 66 (100.0 %) | <0.001 |
| iutA | 18 (16.8 %) | 66 (100.0 %) | <0.001 |
| entB | 61 (57.0 %) | 58 (87.9 %) | <0.001 |
| ybtS | 40 (37.4 %) | 51 (77.3 %) | <0.001 |
| kfu | 15 (14.0 %) | 11 (16.7 %) | 0.636 |
| mrkD | 88 (82.2 %) | 57 (86.4 %) | 0.475 |
| allS | 11 (10.3 %) | 14 (21.2 %) | 0.047 |
Note: Categorical variables are expressed as frequency (%), and analysed using the χ2 test
Table 2: Frequencies of Sequence Types, Capsular Serotypes and Virulence Factors in Patients Infected with K. pneumoniae
All 173 K. pneumoniae strains showed consistent susceptibility to ampicillin. There were no remarkable differences in antimicrobial susceptibility rates, except for susceptibility to ampicillin-sulbactam, aztreonam, and levofloxacin (p<0.05) (Table 3).
| Characteristics | cKP (n=107) | hvKP (n=66) | p |
|---|---|---|---|
| Amikacin | 14 (13.1 %) | 5 (7.6 %) | 0.26 |
| Ampicillin-sulbactam | 38 (35.5 %) | 11 (16.7 %) | 0.008 |
| Aztreonam | 49 (45.8 %) | 18 (27.3 %) | 0.015 |
| Cefazolin | 45 (42.1 %) | 24 (36.4 %) | 0.458 |
| Cefepime | 41 (38.3 %) | 21 (31.8 %) | 0.386 |
| Cefotaxime | 48 (44.9 %) | 22 (33.3 %) | 0.134 |
| Cefoxitin | 45 (42.1 %) | 20 (30.3 %) | 0.121 |
| Ceftazidime | 43 (40.2 %) | 21 (31.8 %) | 0.268 |
| Ertapenem | 17 (15.9 %) | 7 (10.6 %) | 0.329 |
| Gentamicin | 22 (20.6 %) | 11 (16.7 %) | 0.527 |
| Levofloxacin | 27 (25.2 %) | 8 (12.1 %) | 0.037 |
| Meropenem | 13 (12.1 %) | 5 (7.6 %) | 0.339 |
| Piperacillin-tazobactam | 19 (17.8 %) | 6 (9.1 %) | 0.115 |
| Tigecycline | 16 (15.0 %) | 4 (6.1 %) | 0.076 |
Note: Categorical variables are expressed as frequency (%), and analysed using the χ2 test
Table 3: Frequencies of Antimicrobial Resistance in Patients Infected with K. pneumoniae: cKP vs. hvKP
The multivariate logistic regression analyses were done to assess the clinical risk factors for hvKp infection. The study results indicated that metastatic spread (Odds Ratio (OR)=3.596, 95 % Confidence Interval (CI)=1.193-10.838; p=0.023), liver abscess (OR=7.537, 95 % CI=1.850-30.715; p=0.005), APACHE II score (OR=1.616, 95 % CI=1.365-1.914; p<0.001), and serum creatinine (OR=2.506, 95 % CI=1.125-5.587; p=0.025) were all associated with hvKp infection in patients with pneumonia induced by K. pneumoniae (Table 4).
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p | OR | 95 % CI | p | |
| Age | 1.005 | 0.965-1.045 | 0.822 | |||
| Gender (male) | 1.073 | 0.435-2.650 | 0.878 | |||
| Diabetes mellitus | 2.458 | 0.959-6.298 | 0.061 | |||
| Biliary tract disease | 2.803 | 0.631-12.452 | 0.176 | |||
| Malignancy | 1.717 | 0.528-5.585 | 0.369 | |||
| Chronic lung disease | 1.434 | 0.518-3.972 | 0.488 | |||
| Chronic kidney disease | 1.822 | 0.614-5.407 | 0.28 | |||
| Chronic liver disease | 1.492 | 0.375-5.940 | 0.57 | |||
| Chronic heart disease | 0.946 | 0.302-2.960 | 0.924 | |||
| Cerebrovascular disease | 2.141 | 0.793-5.780 | 0.133 | |||
| Bacteremia | 0.884 | 0.223-3.502 | 0.861 | |||
| Metastatic spread | 4.011 | 0.966-16.650 | 0.056 | 3.596 | 1.193-10.838 | 0.023 |
| Liver abscess | 9.958 | 2.028-48.909 | 0.005 | 7.537 | 1.850-30.715 | 0.005 |
| Biliary tract infection | 0.456 | 0.091-2.283 | 0.34 | |||
| Other intra-abdominal infection | 1.570 | 0.412-5.973 | 0.509 | |||
| Urinary tract infection | 0.723 | 0.189-2.762 | 0.635 | |||
| Lung abscess | 0.551 | 0.065-4.705 | 0.586 | |||
| Other/unknown | 0.829 | 0.208-3.306 | 0.791 | |||
| APACHE II | 1.837 | 1.474-2.288 | <0.001 | 1.616 | 1.365-1.914 | <0.001 |
| Serum creatinine (>94.69 μM) | 3.437 | 1.302-9.073 | 0.013 | 2.506 | 1.125-5.587 | 0.025 |
| Shock | 0.364 | 0.093-1.424 | 0.146 | |||
Note: Univariate and multivariate logistic regression analysis were applied to assess the clinical risk factors for hvKp infection
Table 4: Univariate and Multivariate Analysis of hvKP Infection in the Patients with Pneumonia Caused by K. pneumoniae
The objective of this study was to compare and assess the clinical features of hvKP and cKP strains extracted from patients who had pneumonia brought on by K. pneumoniae in the ICU. It also sought to investigate the effects of virulence factors and the K1 and K2 serotypes on mortality. This investigation holds significance due to its unique approach in comparing hvKP and cKP strains in various forms of pneumonia caused by K. pneumoniae, encompassing CAP, healthcare-associated pneumonia, and HAP. Our findings revealed that the presence of virulence factors and the K1 and K2 serotypes did not have an impact on mortality. Conversely, factors such as metastatic spread, liver abscess, APACHE II score, and serum creatinine levels emerged as independent risk factors for mortality.
Several noteworthy risk factors were linked to hvKP infection compared to cKP infection. These include diabetes mellitus, bacteremia, metastatic dissemination, liver abscess, APACHE II score, serum creatinine levels, shock, and 30 d mortality, all of which were connected to a notably increased risk for hvKP infection. Nevertheless, our analysis did not reveal any significant disparity between hvKP and cKP among patients with CAP, healthcare- associated pneumonia, and HAP. Correspondingly, research conducted in China demonstrated a lack of distinction between hvKP and cKP in HAP induced by K. pneumoniae[15].
Studies conducted in Asia, Europe, and America have demonstrated a strong correlation between hvKP and serotypes K1 and K2[16]. Our research observed that 65.5 % of hvKP cases contained K1 and K2 serotypes, a proportion significantly more significant than that found in cKP. Recent investigations into VAP stemming from K. pneumoniae revealed that the prevalence rates of K1 and K2 serotypes in hvKP were 70.6 % and 64.3 %, respectively. Moreover, in cases of bacteremia CAP, the prevalence rate of K1 and K2 serotypes in hvKP stood at 53.1 %, surpassing that of cKP at 46.9 %[11,17]. The elevated prevalence of K1 and K2 in hvKP can be attributed to the heightened resistance of strains belonging to these serotypes against phagocytosis and intracellular elimination by macrophages and neutrophils compared to other serotypes[18,19]. Furthermore, a VAP study highlighted that virulence factors in hvKP were notably higher than in cKP[17].
In our study, hvKP strains demonstrated a notably higher prevalence of the ST23 sequence type (36.4 % vs. 0 %, p<0.001) in comparison to cKP strains. The capsular serotypes K1 and K2 accounted for 16.76 % (29/173) and 19.08 % (33/173) of all K. pneumoniae strains, respectively. Furthermore, hvKP strains exhibited a significantly elevated prevalence of the rmpA (100 % vs. 22.4 %, p<0.001), iutA (100 % vs. 16.8 %, p<0.001), entb (87.9 % vs. 57.0 %, p<0.001), ybtS (77.3 % vs. 37.4 %, p<0.001), and allS (21.2 % vs. 10.3 %, p=0.047) virulence determinants when compared to cKP strains.
All ASTs demonstrated no significant disparity between the hvKP and cKP strains, except susceptibility to ampicillin-sulbactam, aztreonam, and levofloxacin. Previously conducted research has indicated that the resistance to third-generation cephalosporins in hvKP-related VAP ranged from 0 % to 10 %[17], 14.3 %[20], and 30 % to 40 %[21]. Within the confines of this particular investigation, the resistance level of hvKP towards cefotaxime was measured at 29.2 %. Nonetheless, the escalation in the prevalence of antibiotic-resistant hvKP underscores the necessity for continuous surveillance of antibiotic resistance in such strains, given that prompt antibiotic intervention is crucial for managing pneumonia in affected patients[22-24].
Our research faced a number of drawbacks. First, it was a small sample-size retrospective study carried out at a single center. Second, we could only analyze some potential risk factors through an observational study. Third, there is no universally recognized description of hvKP. Finally, we only examined a subset of virulence factors and did not assess any of them in an animal study. It is also significant to note that many other research groups may have variations in virulence studies.
Our investigation is pivotal for elucidating the correlation between bacterial virulence and the clinical prognosis for pneumonia. Our findings indicate that a number of clinical risk factors were significantly correlated with hvKp infection in pneumonia patients afflicted by K. pneumoniae. Moreover, all K. pneumoniae strains exhibited uniform susceptibility to the antibiotic ampicillin. Additional research is required to validate the associations between the pertinent virulence factors and the impact of other virulence determinants on the clinical outcomes of pneumonia induced by K. pneumoniae.
Authors’ contributions:
Nian Zhu designed the project, supervised the project, and revised the manuscript. Bo Gao performed experiments and wrote the first draft of the manuscript. Huiqing Fan helped perform the experiments, collect data, analyzed the data and performed statistical analysis.
Ethical approval:
The Ethics Committee permitted (No: SPH- 2019-ZA2) the research conducted at Shanghai Pudong Hospital. All procedures involving human participants strictly followed ethical principles outlined by relevant institutional and/or national research committees, the Helsinki Declaration of 1964 and its subsequent amendments, or similar ethical frameworks. Each individual participant in the investigation, or their legal representatives, provided their informed consent.
Funding:
This work was supported by Pudong New Area Health Commission Health and Family Planning Research Project (No: PW2020A-72) supported this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 2018;8:4.
[Crossref] [Google Scholar] [PubMed]
- Song JH, Oh WS, Kang CI, Chung DR, Peck KR, Ko KS, et al. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: A prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents 2008;31(2):107-14.
[Crossref] [Google Scholar] [PubMed]
- Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control 2008;36(4):S93-100.
[Crossref] [Google Scholar] [PubMed]
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: A new invasive syndrome. Lancet Infect Dis 2012;12(11):881-7.
[Crossref] [Google Scholar] [PubMed]
- Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013;4(2):107-18.
[Crossref] [Google Scholar] [PubMed]
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol Mol Biol Rev 2016;80(3):629-61.
[Crossref] [Google Scholar] [PubMed]
- Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 2004;199(5):697-705.
[Crossref] [Google Scholar] [PubMed]
- Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: An emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 2007;45(3):284-93.
[Crossref] [Google Scholar] [PubMed]
- Li J, Ren J, Wang W, Wang G, Gu G, Wu X, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis 2018;37:679-89.
[Crossref] [Google Scholar] [PubMed]
- Harada S, Aoki K, Yamamoto S, Ishii Y, Sekiya N, Kurai H, et al. Clinical and molecular characteristics of Klebsiella pneumoniae isolates causing bloodstream infections in Japan: Occurrence of hypervirulent infections in health care. J Clin Microbiol 2019;57(11):10-128.
[Crossref] [Google Scholar] [PubMed]
- Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: Clinical and microbiological characteristics in Taiwan, 2001-2008. BMC Infect Dis 2010;10:1-7.
[Crossref] [Google Scholar] [PubMed]
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171(4):388-416.
[Crossref] [Google Scholar] [PubMed]
- Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev 2019;32(3):10-128.
[Crossref] [Google Scholar] [PubMed]
- Compain F, Babosan A, Brisse S, Genel N, Audo J, Ailloud F, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol 2014;52(12):4377-80.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother 2016;60(10):6115-20.
[Crossref] [Google Scholar] [PubMed]
- Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, et al. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: Epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol 2017;7:483.
[Crossref] [Google Scholar] [PubMed]
- Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis 2016;35:387-96.
[Crossref] [Google Scholar] [PubMed]
- Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, Lin JC, et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol 2007;45(2):466-71.
[Crossref] [Google Scholar] [PubMed]
- Lee CH, Chang CC, Liu JW, Chen RF, Yang KD. Sialic acid involved in hypermucoviscosity phenotype of Klebsiella pneumoniae and associated with resistance to neutrophil phagocytosis. Virulence 2014;5(6):673-9.
[Crossref] [Google Scholar] [PubMed]
- Guo S, Xu J, Wei Y, Xu J, Li Y, Xue R. Clinical and molecular characteristics of Klebsiella pneumoniae ventilator-associated pneumonia in mainland China. BMC Infect Dis 2016;16:1-7.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Guo J. Characteristics of ventilator-associated pneumonia due to hypervirulent Klebsiella pneumoniae genotype in genetic background for the elderly in two tertiary hospitals in China. Antimicrob Resist Infect Control 2018;7:1-8.
[Crossref] [Google Scholar] [PubMed]
- Li W, Sun G, Yu Y, Li N, Chen M, Jin R, et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis 2014;58(2):225-32.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Gu Y, Li X, Liu Y, Ye Y, Guan S, et al. Identification and characterization of NDM-1-producing hypervirulent (hypermucoviscous) Klebsiella pneumoniae in China. Ann Lab Med 2019;39(2):167-75.
[Crossref] [Google Scholar] [PubMed]
- Palmieri M, Schicklin S, Pelegrin AC, Chatellier S, Franceschi C, Mirande C, et al. Phenotypic and genomic characterization of AmpC-producing Klebsiella pneumoniae from Korea. Ann Lab Med 2018;38(4):367-70.
[Crossref] [Google Scholar] [PubMed]