- *Corresponding Author:
- Yujia Feng
Department of Internal Medicine, Qiqihar Medical University, Qiqihar, Heilongjiang 161000, China
E-mail: fyj@qmu.edu.cn
| This article was originally published in a special issue, “Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(2) Spl Issue “37-40” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To clarify clinical significance of high mobility group box 1 level in inflammation of asthma-chronic obstructive pulmonary disease overlap syndrome. A total of 124 asthma patients in acute attack (asthma group), 124 chronic obstructive pulmonary disease patients in acute exacerbation (chronic obstructive pulmonary disease group) and 124 asthma-chronic obstructive pulmonary disease overlap syndrome patients in acute exacerbation (asthma-chronic obstructive pulmonary disease overlap syndrome group) hospitalized in the department of geriatric respiratory medicine and the second department of respiratory medicine of our hospital from January 2020 to June 2022 were enrolled. The C-reactive protein level, white blood cell counts and high mobility group box 1 concentration were measured and compared in three groups. The relation of serum high mobility group box 1 level to C-reactive protein level or white blood cell counts was assessed through Pearson correlation. The relation of serum high mobility group box 1 level to forced expiratory volume 1 % predicted or forced expiratory volume 1/forced vital capacity was assessed through Pearson correlation. The general data presented no difference among three groups (p>0.05). The C-reactive protein level, white blood cell counts and high mobility group box 1 concentration of asthma-chronic obstructive pulmonary disease overlap syndrome patients presented elevation relative to those of asthma patients and chronic obstructive pulmonary disease patients (p<0.05). The serum high mobility group box 1 level presented a positive association with C-reactive protein level or white blood cell counts in asthma-chronic obstructive pulmonary disease overlap syndrome group (p<0.05). The serum high mobility group box 1 level presented a negative association with forced expiratory volume 1 % predicted or forced expiratory volume 1/forced vital capacity in asthma-chronic obstructive pulmonary disease overlap syndrome group (p<0.05). High mobility group box 1 level in blood specimens of asthma-chronic obstructive pulmonary disease overlap syndrome patient’s presents a marked elevation, suggesting that high mobility group box 1 has crucial clinical significance in diagnosis and therapy of asthma-chronic obstructive pulmonary disease overlap syndrome.
Keywords
Asthma-chronic obstructive pulmonary disease overlap syndrome, high mobility group box 1, C-reactive protein, white blood cell, inflammation
High Mobility Group Box 1 (HMGB1), a member of the HMG protein superfamily, has the functions of Deoxyribonucleic Acid (DNA) recombination, replication, repair, transcription and maintaining the stability of nucleosomes. HMGB1 can be released from activated immune cells such as monocytes, macrophages and pituitary cells or passively released from damaged and necrotic cells to stimulate "necrosis-induced inflammation"[1,2]. Currently, it has been demonstrated that cutting off HMGB1 signaling can block its pathological process and HMGB1 widely gets involvement in inflammatory response as a cytokine[3,4]. Currently, Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome (ACOS) patients are not rare and accounts for approximately 13 %-20 % of Chronic Obstructive Pulmonary Diseases (COPD) patients, consistent with the clinical manifestations of ACOS, and ACOS is more common in elderly patients over 80 y old[5,6]. The article on ACOS jointly drafted by Global Initiative for Asthma (GINA) and GOLD mentioned how to distinguish asthma, COPD and ACOS, and the guidelines only mentioned the combined situation of COPD and asthma, but did not mention its correct definition, accurate diagnostic criteria, treatment plan and prognosis. Our research clarified the possible role of HMGB1 in ACOS through analyzing differences of serum HMGB1 level in asthma in acute attack, COPD in acute exacerbation and ACOS in acute exacerbation.
From January 2020 to June 2022, a total of 124 asthma patients in acute attack (asthma group), 124 COPD patients in acute exacerbation (COPD group) and 124 ACOS patients in acute exacerbation (ACOS group) hospitalized in the department of geriatric respiratory medicine and the second department of respiratory medicine of our hospital received enrollment, with 372 subjects in total. The diagnosis criteria of asthma was in accordance with the Guidelines for the Prevention and Treatment of Bronchial Asthma (2008 edition) and the diagnosis criteria of COPD was in accordance with the guidelines for the diagnosis and treatment of COPD (2013 revision)[3]. ACOS was based on the consensus of ACOS diagnostic criteria published in 2012.
The main criteria includes the strong positive in Bronchial Dilation Test (BDT) in COPD patients (Forced Expiratory Volume 1 (FEV1) improvement value >400 ml, improvement rate >15 %) and previously diagnosed as bronchial asthma.
Minor criteria includes the total serum Immunoglobulin E (IgE) in COPD patients presented elevation; with a history of allergy; positive in BDT at least twice (FEV1 improvement value >200 ml, improvement rate >12 %). ACOS could be diagnosed if it met two main criteria, or one main criteria+two minor criteria based on the diagnosis of COPD. All subjects excluded diabetes, hypertension, connective tissue diseases, tumor, etc.
The C-Reactive Protein (CRP) level was measured with an Image specific protein analyzer (Beckman Coulter, United States of America (USA)). The White Blood Cell (WBC) counts were measured with a LH-750 blood analyzer (Beckman Coulter, USA). The serum HMGB1 level received determination through Enzyme-Linked Immunosorbent Assay (ELISA) with a HMGB1 ELISA kit (Research and Development, USA). None of the subjects received any treatment. At the time of enrollment, 2 ml of elbow veinous blood was drawn and received centrifugation at 3000 r/min for 20 min within 3 h. Serum received collection after centrifugation and storage in -80° refrigerator after sub package for determination of HMGB1 concentration.
The database of patient’s medical records was established and data received analysis and processing through Statistical Package for the Social Sciences (SPSS) 17.0 software. The measurement data were expressed as x̄±s, with comparison between groups through one-way Analysis of Variance (ANOVA). Pearson correlation method was utilized for correlation analysis. The counting data were expressed as constituent ratio and Chi square (χ²) test for counting data was utilized for comparison between groups. p<0.05 meant that difference was statistically significant.
There were 124 patients in COPD group, including 21 females and 103 males, aged (64.23±16.05) y. There were 124 patients in asthma group, with 30 females and 94 males, aged (65.11±15.21) y. There were 124 patients in ACOS group, with 27 females and 97 males, aged (65.69±15.06) y. The gender and age presented no difference among three groups (p>0.05) as shown in Table 1, which shows comparability.
| Groups | Gender | Age | |
|---|---|---|---|
| Male | Female | ||
| ACOS group | 97 | 27 | 65.69±15.06 |
| COPD group | 103 | 21 | 64.23±16.05 |
| Asthma group | 94 | 30 | 65.11±15.21 |
| χ²/F | 2.044 | 0.577 | |
| p | 0.36 | 0.562 | |
Table 1: General data in three groups.
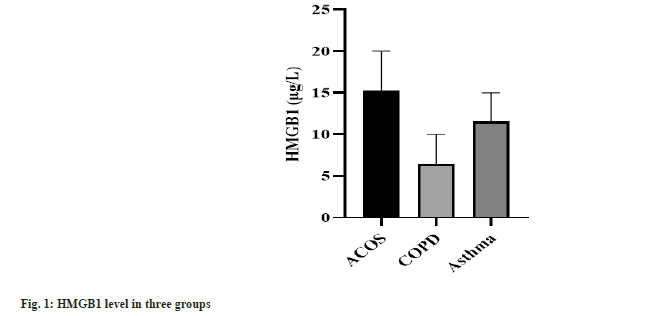
The CRP level, WBC count and HMGB1 concentration expressed the highest in ACOS group, the second in asthma group and the lowest in COPD group. CRP, WBC counts and HMGB1 concentration presented statistical significance among three groups (p<0.001) as shown in Table 2 and fig. 1.
| Groups | CRP (mg/l) | WBC counts (×109/l) | HMGB1 (μg/l) |
|---|---|---|---|
| ACOS group | 85.63±20.68 | 13.23±3.12 | 15.35±4.75 |
| COPD group | 24.18±7.59 | 8.22±1.41 | 6.44±4.03 |
| Asthma group | 50.43±14.61 | 12.79±2.18 | 11.85±3.24 |
| F | 604.528 | 157.147 | 155.99 |
| p | <0.001 | <0.001 | <0.001 |
Table 2: CRP level, WBC count and HMGB1 concentration in three groups.
Pearson correlation analysis demonstrated that serum HMGB1 level presented a positive association with CRP level or WBC counts in ACOS group (r=0.475, p<0.001; r=0.465, p<0.001) as shown in Table 3 and Table 4.
| Groups | HMGB1 (μg/l) | CRP (mg/l) |
|---|---|---|
| ACOS group | 15.35±4.75 | 85.63±20.68 |
| r | 0.465 | |
| p | <0.001 | |
Table 3: Association of CRP level with HMGB1 level in ACOS group.
| Groups | HMGB1 (μg/l) | WBC count (×109/l) |
|---|---|---|
| ACOS group | 15.35±4.75 | 13.23±3.12 |
| r | 0.475 | |
| p | <0.001 | |
Table 4: Association of WBC count with HMGB1 level in ACOS group.
Pearson correlation analysis demonstrated that serum HMGB1 level presented a negative association with FEV1 % predicted or FEV1/Forced Vital Capacity (FVC) in ACOS group (r=-0.542, p<0.001; r=-0.752, p<0.001) as shown in Table 5 and Table 6.
| Groups | HMGB1 (μg/l) | FEV1 % predicted (%) |
|---|---|---|
| ACOS group | 15.35±4.75 | 56.39±19.59 |
| r | -0.542 | |
| p | <0.001 | |
Table 5: Association of FEV1 % predicted with HMGB1 level in ACOS group.
| Groups | HMGB1 (μg/l) | FEV1/FVC (%) |
|---|---|---|
| ACOS group | 15.35±4.75 | 60.49±10.49 |
| r | -0.752 | |
| p | <0.001 | |
Table 6: Association of FEV1/FVC with HMGB1 level in ACOS group.
In the 1960s, Johns had discovered HMGB. HMGB was named because of its small molecular weight and high migratory ability in polyacrylamide gel electrophoresis. There are three members in HMG family, which are HMGB1, HMGB2 and HMGB3. HMGB1 presents distribution in the heart, lung, brain, liver, spleen, kidney, lymph and other tissues and cells, which is the most abundant HMG protein. HMGB1 is secreted from the cells through damaged or necrotic cells, or some incentives actively stimulate immune cells; additionally, extracellular HMGB1 can stimulate other cells to produce adaptive biological effects through combining with receptors[7,8]. It has been demonstrated that HMGB1 also exerts a vital role in disease progression including end toxemia, sepsis, inflammation, autoimmune diseases, tumors, etc.[9], whereas its specific mechanism remains elusive. It has been revealed that HMGB1 is a newly discovered inflammatory cytokine at advanced stages[10] and changes in serum HMGB1 get involvement in occurrence and development of COPD and asthma[11,12]. Herein, HMGB1 concentration of ACOS patients presented elevation relative to that of asthma patients and COPD patients, and serum HMGB1 level presented a positive association with CRP level or WBC counts in ACOS group, which suggests that serum HMGB1 may also participate in occurrence and development of ACOS, is one of the inflammatory mechanisms underlying ACOS, and also indicates that ACOS has inflammatory responses similar to COPD and asthma. Additionally, CRP level and WBC counts are commonly used laboratory indicators to reflect inflammation severity of the body. Herein, CRP level, WBC count and HMGB1 concentration of ACOS patients presented elevation relative to those of asthma patients and COPD patients, suggesting that inflammation severity in ACOS patients with ACOS presents elevation relative to that in asthma patients and COPD patients. Furthermore, serum HMGB1 level presented a negative association with FEV1 % predicted or FEV1/FVC in ACOS group, suggesting that the worse the pulmonary function, the more severe the inflammation in ACOS patients.
In conclusion, HMGB1 level in blood specimens of ACOS patient’s presents a marked elevation, suggesting that HMGB1 has crucial clinical significance in diagnosis and therapy of ACOS. Nevertheless, due to relatively small sample size in our research, its clinical application value needs to be further clarified through expanding sample size.
Acknowledgement:
This work was supported by the Qiqihar Science and Technology Plan Joint Guidance Project (No. LHYD-202029).
Author’s contributions:
Yujia Feng and Qinghua Zhou have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol 2020;13(1):116.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, Huang G, Hu B, Qian GS, Song Y. Recombinant HMGB1 A box protein inhibits Th17 responses in mice with neutrophilic asthma by suppressing dendritic cell-mediated Th17 polarization. Int Immunopharmacol 2015;24(1):110-8.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 2018;15(1):116.
[Crossref] [Google Scholar] [PubMed]
- Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM. Indirect regulation of HMGB1 release by gasdermin D. Nat Commun 2020;11(1):4561.
[Crossref] [Google Scholar] [PubMed]
- Albertson TE, Chenoweth JA, Pearson SJ, Murin S. The pharmacological management of asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS). Expert Opin Pharmacother 2020;21(2):213-31.
[Crossref] [Google Scholar] [PubMed]
- Poh TY, Mac Aogáin M, Chan AK, Yii AC, Yong VF, Tiew PY, et al. Understanding COPD-overlap syndromes. Expert Rev Respir Med 2017;11(4):285-98.
[Crossref] [Google Scholar] [PubMed]
- Merianda TT, Coleman J, Kim HH, Sahoo PK, Gomes C, Brito-Vargas P, et al. Axonal amphoterin mRNA is regulated by translational control and enhances axon outgrowth. J Neurosci 2015;35(14):5693-706.
[Crossref] [Google Scholar] [PubMed]
- Nomura S, Maeda Y, Ishii K, Katayama Y, Yagi H, Fujishima N, et al. Relationship between HMGB1 and PAI-1 after allogeneic hematopoietic stem cell transplantation. J Blood Med 2016;7:1-4.
[Crossref] [Google Scholar] [PubMed]
- Gao R, Zhang Y, Kang Y, Xu W, Jiang L, Guo T, et al. Glycyrrhizin inhibits PEDV infection and proinflammatory cytokine secretion via the HMGB1/TLR4-MAPK p38 pathway. Int J Mol Sci 2020;21(8):2961.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol 2020;177(22):5224-45.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Sun X, Zhong X. Role of RAGE and its ligand HMGB1 in the development of COPD. Postgrad Med 2022;134(8):763-75.
[Crossref] [Google Scholar] [PubMed]
- Imbalzano E, Quartuccio S, di Salvo E, Crea T, Casciaro M, Gangemi S. Association between HMGB1 and asthma: A literature review. Clin Mol Allergy 2017;15(1):1-9.
[Crossref] [Google Scholar] [PubMed]