- *Corresponding Author:
- Huiyu He
Department of Prosthodontics, The First Affiliated Hospital of Xinjiang Medical University, Xinshi Urumqi 830028, China
E-mail: hehuiyu02@163.com
| This article was originally published in a special issue, “Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(2) Spl Issue “1-10” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This work aimed to investigate the effect of low-concentration cobalt chloride on the in vitro and in vivo proliferation of rat bone mesenchymal stem cells, the expression of hypoxia-inducing fraction-1 alpha and the related osteogenic genes. The bone mesenchymal stem cells were isolated from bone marrow of rats, and the surface biomarkers were identified by immunofluorescence staining and flow cytometry. The differentiation of bone mesenchymal stem cells was measured by alizarin red and alkaline phosphatase staining. The bone mesenchymal stem cells were treated with cobalt chloride at different concentrations and cell viability was assessed by cell counting kit 8. Cell migration was detected by Transwell and wound healing assay. The expression of collagen type 1, bone morphogenetic protein-2, runt-related transcription factor 2, alkaline phosphatase and hypoxia-inducing fraction-1 alpha were measured by quantitative real time polymerase chain reaction assay. The large segmental defect model was made to mimic the in vivo bone defect. The bone mesenchymal stem cells-collagen sponge scaffold was adopted for in vivo treatment and histological analysis of femur structure was performed to assess the effects of cobalt chloride-treated bone mesenchymal stem cells in promoting bone formation. X-ray and micro computed tomography were performed to observe bone structure. The cluster of differentiation 90 and cluster of differentiation 44 were highly expressed and cluster of differentiation 45 expression was relatively low in isolated bone mesenchymal stem cells. The cobalt chloride at 300 and 400 μmol/l could effectively suppress cell proliferation, compared with the control group. The low dose of cobalt chloride (50 μmol/l and 100 μmol/l) could promote cell proliferation. The cobalt chloride at 100 μmol/l could increase the positive staining of alkaline phosphatase and alizarin red. The expression of collagen type 1, bone morphogenetic protein-2, runt-related transcription factor 2, alkaline phosphatase and hypoxiainducing fraction-1 alpha were statistically up-regulated by cobalt chloride treatment. The cobalt chloridebone mesenchymal stem cells-collagen scaffold effectively accelerated the bone regeneration, increased new bone deposition and bone morphogenetic protein-2 expression in femur tissues. Appropriate concentration of cobalt chloride can promote the proliferation of bone mesenchymal stem cells and up-regulate the expression of hypoxia-inducing fraction-1 alpha and osteogenic genes in vitro, simultaneously promoted bone regeneration in vivo.
Keywords
Bone marrow mesenchymal stem cells, hypoxia, cobalt chloride, hypoxia-inducible factor-1 alpha, bone regeneration
Tissue engineering combines biomaterial scaffolds, seed cells and bioactive factors, which bring hopes for the treatment of bone damages[1,2]. At present, tissue engineering that based on stem cells is a feasible and sustainable strategy to restore structure and function of damaged bones[3]. The osteogenic differentiation of stem cells is the key process of the stem cell-based bone tissue repairment[4]. Studies have suggested that induction of osteogenic differentiation of stem cells may be a potential way to promote bone regeneration[5]. Mesenchymal stem cells have profound immunomodulatory and regenerative properties, which fits the need of multiple clinical symptoms[6].
The marrow Mesenchymal Stem Cells (MSCs) usually derived from bone marrow, adipose tissue, umbilical cord and placenta, among which the Bone Marrow Mesenchymal Stem Cells (BMSCs) are regarded as the most promising manner for bone regeneration[5], which can differentiate into osteoblasts and secrete angiogenic factors to promote bone regeneration and have the potential to differentiate into mesenchymal tissue lineages[7,8]. Therefore, bone marrow mesenchymal stem cells have a broad prospect in repairing bone defects.
During bone growth and regeneration, angiogenesis is closely related to bone regeneration[9]. The vascular system provides oxygen for bone growth and regeneration, transmits critical information to the matrix and promotes the differentiation of stromal cells, thus accelerating bone formation[10]. It has been well-recognized that Hypoxia-Inducible Factor-1 alpha (HIF-1α) is a key mediator in the adaptive cellular response to hypoxia and is widely expressed in most human tissues[11,12]. HIF-1α modulates the activity of a variety of genes, regulates stem cell proliferation, differentiation and pluripotency, and is essential for initiating the angiogenesisosteogenesis cascade[13]. Cobalt Chloride (CoCl2) is the most common hypoxic chemical inducer and proline hydroxylase inhibitor, the cobalt ion in which could replace the iron ions in the oxygen receptor hemoglobin porphyrin ring to impede the combination of hemoglobin with oxygen, inducing a series of reactions similar to hypoxia, thus imitating hypoxia state, simultaneously induces the expression of intracellular HIF-1α[14,15].
In this study, different doses of CoCl2 were used for treatment of BMSCs to select the optimal dose and observe the effects of the optimal dose of CoCl2 on in vitro bone formation of BMSCs. The BMSCscollagen sponge scaffold was then administrated for in vivo bone repair using rat model. Our work potentially provides new insight and experimental basis for the optimization of tissue engineering materials and provides a new strategy for treatment in prosthodontics.
Materials and Methods
Animals:
Male Sprague–Dawley (SD) rats that aged 4 w old and weighed (100-120) g were provided by Animal Laboratory Center of Xinjiang Medical University. All animal experiments were approved (IACUC-2021902-02) and conducted following the guidelines for the care and use of laboratory animals of the First Affiliated Hospital of Xinjiang Medical University.
Cell isolation and culture:
The SD rats were sacrificed by cervical dislocation method and were immediately immersed in 75 % ethanol solution for 5 min. The femur and humerus of the rats were carefully separated with ophthalmic scissors and tweezers, and placed in pre-cooled Phosphate-Buffered Saline (PBS) solution on ice. After that, the metaphysical part was carefully removed and the bone marrow cavity was repeatedly rinsed by pure Alpha-Minimum Essential Medium (α-MEM) (Gibco, Untied States of America (USA)) using 5 ml syringe until the bone partially turned white. The BMSCs suspension was blown repeatedly with a 5 ml syringe and then a 1 ml syringe. After centrifuge at 1000 rpm at 22° for 5 min, the supernatant was abandoned and α-MEM containing 1 % streptomycin/penicillin (Gibco, USA), 1 % glutamine (Gibco, USA), and 15 % Fetal Bovine Serum (FBS); Gibco, USA) was used for re-suspension of cells, which was then cultured in an 37° incubator. After 2 d, the cells were washed with PBS and cultured in medium containing 10 % FBS, and the medium was changed every 2 to 3 d. The cells were used within three generations. The purity of the cells was detected by flow cytometry and immunofluorescence identification.
Hypoxia treatment:
BMSCs of the third generation were inoculated in the six-well plate at the concentration of 1×105 cells per well. After 24 h of cell adhesion, the cells were stimulated by the complete culture containing 0, 50, 100, 200, 300 and 400 μmol/l CoCl2 at 12 h, 24 h, 48 h and 72 h, respectively.
Preparation and identification of cell-collagen sponge scaffold complex:
The collagen sponge scaffold (Beijing Pashion Bio, China) was cut into a cube of 1×1×1 mm and was placed in a 24-well plate with 3/hole. For experimental group, the CoCl2 treated BMSCs were suspended in complete medium with 10 % FBS at a density of 2×106 cells/ml. A total of 100 μl cell suspensions was evenly added to the collagen sponge and incubated at 37° incubator for 4 h for adhesion, after which 1 ml medium was added for incubation. The medium was changed every 2 d. The structure of scaffold was observed under Scanning Electron Microscope (SEM); (SEM XL-30, Philips).
Cell proliferation:
Cell proliferation was measure by Cell Counting Kit 8 (CCK-8); Dojindo Laboratories, Japan. BMSCs of the third generation were seeded in 96-well plates at a concentration of 5×103 cells per well. After incubating at 37° for 24 h, complete medium containing 0, 50, 100, 200, 300, 400 μmol/l CoCl2 was replaced and cultured for 12 h, 24 h, 48 h and 72 h, respectively. To evaluate the pro-proliferative effect of cell-collagen sponge scaffold, the scaffold (3×3×2 mm) was seeded into 96-well plate and BMSCs suspended in complete medium containing 0 μmol/l or 100 μmol/l CoCl2 were added to scaffold in plate and incubated at 37° for 1, 3, 5 and 7 d respectively. Complete medium containing 10 % CCK-8 solution was then added and the absorbance at 450 nm was detected after incubation for 2 h in an incubator at 37°.
Transwell assay:
BMSCs of the third generation were inoculated in 24-well plates of Transwell system at a density of 5×104 cells per well. The experimental group and the control group were cultured with 100 μmol/l CoCl2 and 0 μmol/l CoCl2 for 24 h, respectively. The cells were inoculated with 100 μl serum-free medium in the upper chamber. A complete medium containing 10 % FBS of 600 μl was added to the lower chamber and cultured in a 37° incubator for 24 h. After the chamber was removed, the culture medium was removed and the attached cells on the inside of the upper chamber were carefully wiped with cotton swabs. The cells were repeatedly washed with PBS for 2-3 times, and then fixed with tissue fixating solution for 30 min. Each well was stained with 600 μl 0.1 % crystal violet solution for 20 min. After washing with PBS, the upper chamber captured under the microscope. After the photo was taken, the upper chamber of the experimental group and the control group was placed into the small hole of a new 24-well plate. Each well was decolorized with 600 μl 33 % acetic acid and the decolorization solution was put into the 96-well plate for full shock for 30 min. The decolorization solution was 100 μl/well into the 96-well plate.
Wound healing assay:
Three parallel lines were marked on the back of the 6-well plate before cell inoculation and then the BMSCs were seeded into the 6-well plate at a cell density of 1×105 cells per well. After 24 h, for cell adherence and growth, the sterile 200 μl pipette tip was scratched perpendicular to the three standard lines. Complete medium containing 0 and 100 μmol/l CoCl2 was added and cultured for 24 h. The scratch widths at 0 h and 24 h were calculated by Image-pro Plus software.
Oil red staining:
BMSCs were inoculated into 6-well plates at density of 1×105 cells per well. After 24 h of cell adhesion and the confluent degree reached 70 %-80 %, the cells were divided into 100 μmol/l CoCl2 group and control group. The two groups were added with lipid induction medium (α-MEM+5 % FBS+10 mg/l insulin+1 μM dexamethasone+500 μM 3-isobutyl-1- methylxanthine+0.2 mM indomethacin) containing 100 μmol/l and 0 μmol/l CoCl2. The medium was changed every 3 d. On the 7 d and 14 d, the mesenchymal stem cells in the 6-well plate were washed with PBS, fixed with 4 % neutral formaldehyde fixing solution for 30 min, and stained with Oil red O working solution for 30 min. After washing with PBS, cells were observed and photographed under an inverted microscope, and the stained area was analyzed by Image J software.
Osteogenesis induction:
BMSCs were inoculated into 6-well plates at a density of 1×105 cells per well. After 24 h of cell adhesion and cell confluence to 70 %-80 %, the cells were treated with 0 or 100 μmol/l CoCl2. At the same time, osteogenic induction solution (α-MEM+10 % FBS+10 mmol/l beta (β)-sodium glycerate+0.1 μM dexamethasone+50 μM ascorbic acid) were added to each group, and the solution was changed every 3 d. The growth and morphology of the cells were observed and photographed using an inverted microscope.
Alizarin red staining:
At the 21st d of osteogenic induction, the medium was removed, washed with PBS, fixed with fixating solution, and alizarin red solution was added to each well for 3 to 4 times and stood for 5 min. After washing with PBS, cells were observed and photographed under an inverted microscope and the stained area was analyzed by Image J software.
Alkaline Phosphatase (ALP) staining:
On the 7th d of osteogenic induction, the medium was removed, washed with PBS, fixed with fixating solution, incubated with 5-Bromo-4-Chloro-3- Indolyl Phosphate (BCIP)/Nitro Blue Tetrazolium (NBT) staining solution at room temperature for 30 min. After washing with distilled water, cells were observed and photographed under an inverted microscope and the stained area was analyzed by Image J software.
Quantitative real time Polymerase Chain Reaction (PCR):
BMSCs were inoculated in 6-well plates with 1×105 cells per well. After 24 h incubation, cells were cultured in complete medium with 100 μmol/l CoCl2 for 24 h. Total Ribonucleic Acid (RNA) was extracted from the cells using Trizol reagent (Invitrogen, USA) and reverse-transcribed into complementary Deoxyribonucleic Acid (cDNA) using PrimeScript RT kit (TaKaRa, Japan) under the reaction conditions of 42° for 15 min and 85° for 5 s. Finally, quantitative detection of the expression levels of osteogenic genes Collagen Type 1 (COL- 1), Bone Morphogenetic Protein (BMP)-2, Runt- Related Transcription Factor 2 (RUNX2), ALP and HIF-1α was performed by qPCR apparatus. The standard procedure of PCR was as follows; predenaturation 95° for 30 s, PCR reaction 95° for 5 s and PCR reaction 60° for 34 s. The expression level of genes was analyzed by Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) for standardization. Primers were designed by Shanghai Sangong as shown in Table 1.
| Gene | Sense (5’-3’) | Anti-sense (5’-3’) |
|---|---|---|
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| COL-1 | GCCTCCCAGAACATCACCTA | GCAGGGACTTCTTGAGGTTG |
| BMP-2 | AAGCGTCAAGCCAAACACAACAG | CCCCACATCACTGAAGTCCACATAC |
| RUNX2 | CCATAACGGTCTTCACAAATCCT | TCTGTCTGTGCCTTCTTGGTTC |
| ALP | AACGTGGCCAAGAACATCATCA | TGTCCATCTCCAGCCGTGTC |
| HIF-1α | CACCGCAACTGCCACCACTG | TGAGGCTGTCCGACTGTGAGTAC |
Table 1: Sequence of Primers for qPCR
Large Segmental Defect (LSD) model:
All experimental procedures followed the Guidelines for the care and use of laboratory animals and were authorized by the Animal Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Approval number: IACUC-2021902-02). SD rats aged 4 w-old and weighed 200 g to 300 g was bought from Animal Laboratory Center of Xinjiang Medical University. The rats were randomly divided into 3 groups (CoCl2+BMSCs+collagen sponge, BMSCs+collagen sponge and collagen sponge) with 8 in each group. The in vivo bone defect was mimicked using LSD model. In brief, the rats were fasted without water for 12 h before the operation and were anesthetized by intraperitoneal injection of 1 % sodium pentobarbital at a dose of 6 ml/kg. An anterolateral skin incision was made in the leg and a 3×3×2 mm defect in the middle of the femur was carefully made with a stomatological sander. After that, the 3×3×2 mm collagen sponge was grafted and pressed fit into the defect space. The wound was then closed. The rats were fasted for 12 h after the operation and return to normal diet. 8 w after operation, the rats were sacrificed and the femur tissues were collected for following study.
Radiography and micro Computed Tomography (μCT):
To determine the bone regeneration, in vivo radiographs were taken at 8 w after operation using X-ray machine (Kodak DR7500, USA). The radiographs were taken at 30 kV voltage and 20 mA current. The scanning angle is 2θ=10°-80° and the scanning rate is 10°/min. For μCT, the collected tissues were fixed in 4 % Paraformaldehyde (PFA) overnight and scanned
Histological analysis:
After the μCT scanning, the femur tissue was decalcified and embedded vertically in paraffin. The tissues were then cut into 5 μm slices for following HE staining, Masson's trichrome staining, and Immunohistochemistry (IHC) staining.
Statistical analysis:
Data were shown as mean±Standard Deviation (SD) and analyzed by Statistical Package for the Social Sciences (SPSS) 24.0 software and GraphPad Prism software. Comparison between multiple groups was conducted by one-way Analysis of Variance (ANOVA) analysis, and LSD-t test was used for intra-group data comparison. p<0.05 was considered as significant difference. All statistical graphs were drawn by GraphPad Prism software.
Results and Discussion
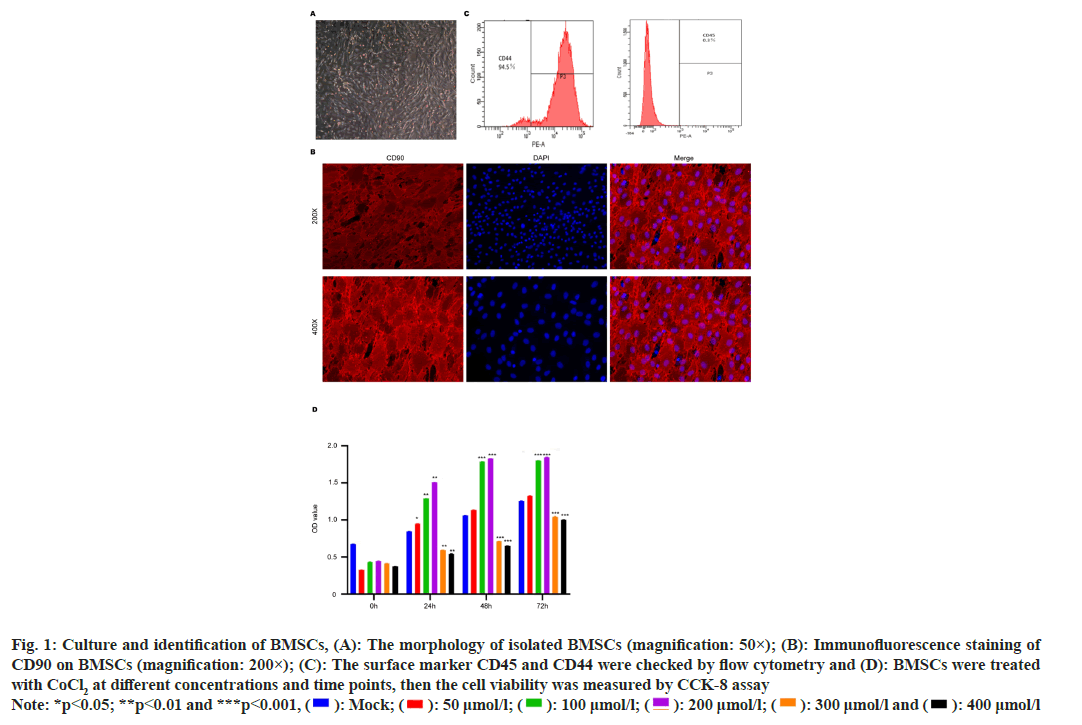
The morphology of isolated primary cells was observed under microscope 24 h after the operation. The shape of isolated cells was initially irregular and present the form of round, spindle, and polygonal. After replacing the medium and culture for 3 d, the cells rapidly grow and present spindle shape (fig. 1A). Subsequently, BMSC surface biomarker was checked. The stem cell marker Cluster of Differentiation (CD) 90 and CD44 was highly expressed in cells (>90 %) (fig. 1B), while the CD45 expression was relatively low (<1 %) (fig. 1C), suggesting the phenotype of MSCs. The CD90 and CD44 were highly expressed and CD45 expression was relatively low in isolated BMSCs. We next treated the BMSCs with CoCl2 at different doses to screen the appropriate dose for following experiments. We observed that the CoCl2 at 300 and 400 μmol/l could effectively suppress cell proliferation (p<0.0001), compared with the control group, whereas the low dose of CoCl2 (100 μmol/l and 200 μmol/l) could promote cell proliferation (p<0.0001) (fig. 1D). And according to the relevant literature, 100 μmol/l CoCl2 is the common experimental concentration. Therefore, we selected CoCl2 at 100 μmol/l for subsequent experiments.
Fig. 1: Culture and identification of BMSCs, (A): The morphology of isolated BMSCs (magnification: 50×); (B): Immunofluorescence staining of
CD90 on BMSCs (magnification: 200×); (C): The surface marker CD45 and CD44 were checked by flow cytometry and (D): BMSCs were treated
with CoCl2 at different concentrations and time points, then the cell viability was measured by CCK-8 assay
Note: *p<0.05; **p<0.01 and ***p<0.001, ( ): Mock; (
): Mock; ( ): 50 μmol/l; (
): 50 μmol/l; ( ): 100 μmol/l; (
): 100 μmol/l; ( ): 200 μmol/l; (
): 200 μmol/l; (  ): 300 μmol/l and (
): 300 μmol/l and ( ): 400 μmol/l
): 400 μmol/l
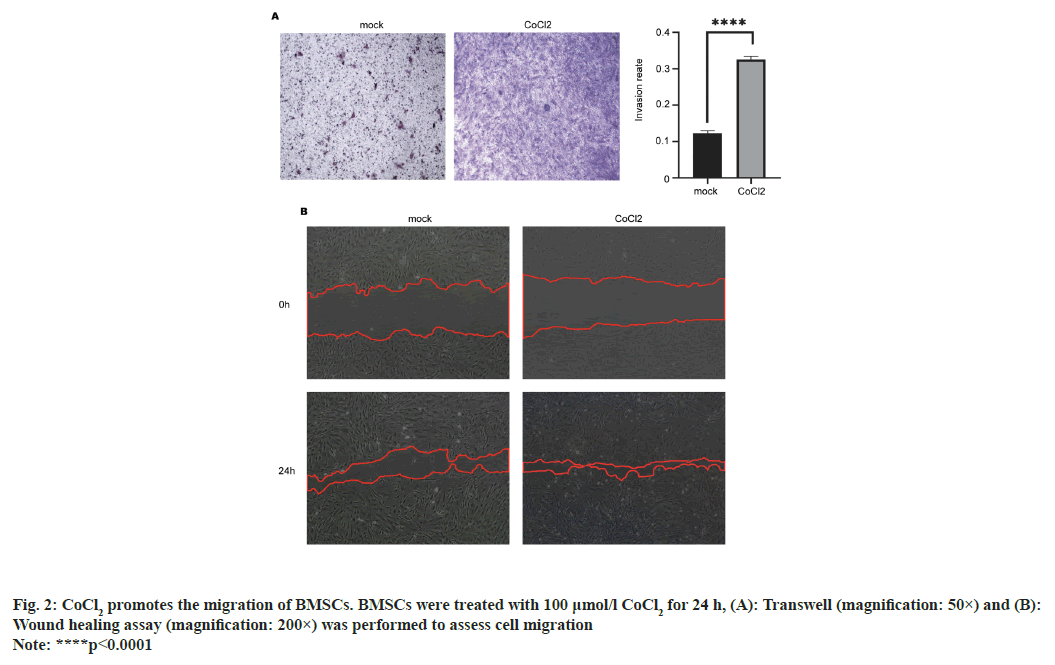
We next determined whether CoCl2 affects the migration of BMSCs. The results from Transwell experiment showed that the number of BMSCs that penetrated through the membrane was significantly increased at 24 h after incubation with CoCl2 (p<0.0001) (fig. 2A). The results from wound healing assay showed that after 24 h, the healing ability of the experimental group was significantly better than that of the control group and the distance between cell scratch was significantly shortened (p<0.01) (fig. 2B), indicating that 100 μmol/l CoCl2 could promote cell healing. These data indicated that CoCl2 can promote the migration of BMSCs.
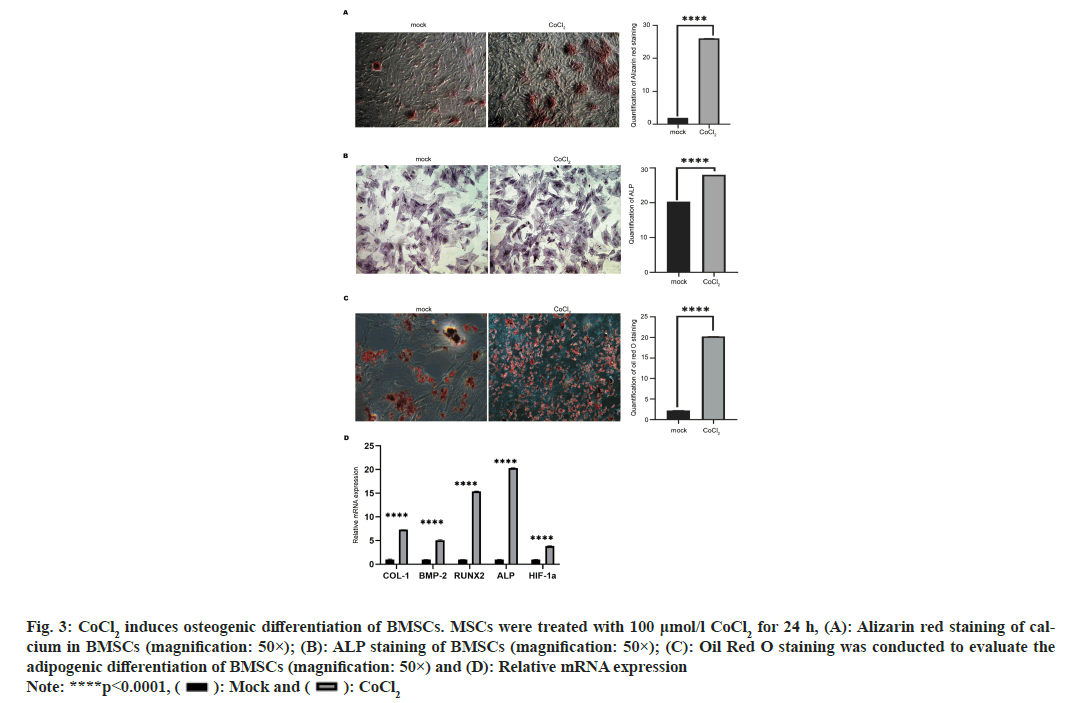
Subsequently, we evaluated the effects of CoCl2 on osteogenic differentiation of BMSCs. As shown in fig. 3A, the Alizarin red staining of calcium was notably enhanced by CoCl2 treatment (p<0.0001), indicating the elevated osteogenesis. Moreover, elevated secretion of ALP is an early biomarker of osteogenesis. Here, we found that CoCl2 treatment enhanced the level of ALP compared with the control group (fig. 3B), suggesting the enhanced osteogenesis. On the other hand, we measured the accumulation of lipid drops using Oil Red O reagent, to check the adipogenic differentiation of BMSCs. We observed more lipid droplets accumulate on the edge of cells in CoCl2 treatment group and the control group (fig. 3C). We also measured the expression of critical genes related to osteogenesis and angiogenesis in BMSCs. The expression of osteogenesis biomarkers (COL-1, BMP-2, RUNX2 and ALP) and the angiogenesis biomarker HIF-1α were significantly elevated by CoCl2 treatment. These results showed that 100 μmol/l CoCl2 could promote the osteogenic and adipogenic differentiation of BMSCs (p<0.001 ) (fig. 3D).
Fig. 3: CoCl2 induces osteogenic differentiation of BMSCs. MSCs were treated with 100 μmol/l CoCl2 for 24 h, (A): Alizarin red staining of calcium
in BMSCs (magnification: 50×); (B): ALP staining of BMSCs (magnification: 50×); (C): Oil Red O staining was conducted to evaluate the
adipogenic differentiation of BMSCs (magnification: 50×) and (D): Relative mRNA expression
Note: ****p<0.0001, ( ): Mock and (
): Mock and ( ): CoCl2
): CoCl2
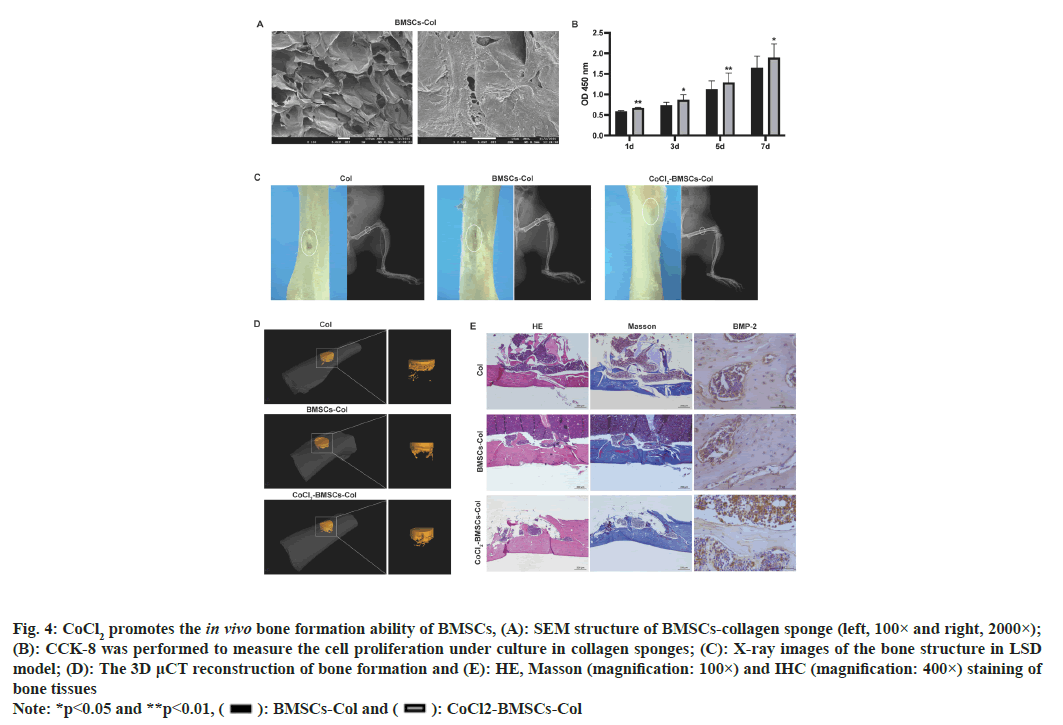
We next established an LSD rat model to assess the effects of CoCl2-treated BMSCs on bone formation. We prepared the collagen sponge, BMSCs-collagen sponge and CoCl2-BMSCs-collagen sponge for treatment of defected bone structure. The BMSCscollagen sponge scaffold was porous with pores ranging between 10 to 100 μm in diameter (fig. 4A). Results from CCK-8 assay demonstrated that the CoCl2 treatment significantly enhanced the pro-proliferative effect of BMSCs-collagen sponge scaffold (fig. 4B). The 2-Dimensional (2D) imaging of bone structure by X-ray showed that CoCl2-BMSCscollagen sponge scaffold largely repaired the bone defect of LSD model, compared with the BMSCscollagen sponge treatment and collagen sponge treatment further improved the therapeutic effect of BMSCs collagen sponge, which almost restored the integrity of damaged bone (fig. 4C). These results of 2D assessments of defect bone structure were further verified by 3-Dimensional (3D) μCT reconstructions (fig. 4D and Table 2). CoCl2 treatment notably elevated Bone Volume/Tissues Volume (BV/TV) and Bone Metastases (BM) at 8 w after treatment (Table 2; p<0.05). Moreover, the CoCl2 treatment facilitated the formation and deposition of new bone as was denoted by HE and Masson’s staining (fig. 4E). The enhanced expression of BMP-2 in bone tissues also determined the stronger bone formation upon CoCl2 treatment (fig. 4E).
Fig. 4: CoCl2 promotes the in vivo bone formation ability of BMSCs, (A): SEM structure of BMSCs-collagen sponge (left, 100× and right, 2000×);
(B): CCK-8 was performed to measure the cell proliferation under culture in collagen sponges; (C): X-ray images of the bone structure in LSD
model; (D): The 3D μCT reconstruction of bone formation and (E): HE, Masson (magnification: 100×) and IHC (magnification: 400×) staining of
bone tissues
Note: *p<0.05 and **p<0.01, ( ): BMSCs-Col and (
): BMSCs-Col and ( ): CoCl2-BMSCs-Col
): CoCl2-BMSCs-Col
| Group | BV/TV % | BMD g/cm3 |
|---|---|---|
| CoCl2+BMSCs+collagen sponge | 35.39±3.20* | 1066.47±113.00* |
| BMSCs+collagen sponge | 27.26±1.12 | 858.58±32.66 |
| Collagen sponge | 16.09±4.80 | 451.87±72.60 |
| p | <0.05 | <0.05 |
Note: *p<0.05
Table 2: Metrological Analysis of The Region of Interest of Bone Defect Areas In Each Group at 8 W After Implantation
Nowadays, a great number of patients who suffered from tissue or organ defects are desperate for emergency treatment every year[16]. Regenerative medicine is largely developed due to the insufficient supply of organ and tissue replacement, repair and regeneration of patients caused by donor shortage, graft rejection and other problems[17]. Transplantation of tissue and organ, surgery or mechanical devices are common treatments for regeneration, among which the synthetic and natural implants are principle tissue regeneration strategy[17]. However, these current strategies have significant limitations, thus increasing the need for novel and effective tissue engineering manners[1]. The selection of appropriate cell stimulation methods, scaffold synthesis and tissue transplantation play an important role in the successful tissue engineering[18].
BMSCs are pluripotent stem cells that can differentiate into cell types of mesodermal origin, including osteocytes, adipocytes and chondrocytes[6,19]. In recent years, a great number of studies have verified that BMSCs can directly participate in tissue regeneration, as well as participating in tissue repair through directly migrating to the damaged tissue or paracrine mechanism[20]. In addition, BMSCs can trigger a variety of immunomodulatory signals that reduce inflammation and immune responses[21].
In some certain pathological conditions, such as trauma, tumors, stroke and arthritis, hypoxia is closely associated with the development of the disease[11,13]. Peterson et al.[22] found that moderate hypoxic preconditioning could preserve cell biological behavior and cell survival rate. CoCl2 is a well-known hypoxia inducer and frequently used to establish hypoxic microenvironment in vitro[23]. HIF- 1 is a representative protein during hypoxia, which consists of two subunits; HIF-1α and HIF-1β[24]. It has been reported that the HIF-specific propyl hydroxylase that promotes HIF-1α degradation has an iron-binding core which could be replaced by cobalt, thus suppressing the degradation of HIF- 1α[25].
In this work, we isolated BMSCs from rats and demonstrated that the proliferation of BMSCs treated with 100 μmol/l CoCl2 for 24 h was significantly higher than of the control group, and the proliferation capacity was proportional to the increase of CoCl2 concentration, while the high level of CoCl2 (400 μmol/l) suppressed growth of BMSCs, suggesting the pro-proliferative effect of low dose CoCl2. Moreover, the RNA level of HIF-1α was elevated by CoCl2, which is consistent with previous reports. We utilized the alizarin red staining and ALP staining to determine the osteogenic differentiation of BMSCs and found that CoCl2 significantly elevated formation of calcium nodules, manifesting the enhanced osteogenesis.
BMP is a member of the transforming growth factor-β family that commonly used to stimulate bone regeneration in clinics, as well as regulates the osteogenic function of type I and II serine/threonine kinase receptors via forming complexes[26]. BMP- 2 is the most studied growth factor in osteogenesis and exhibits great potential in bone formation and clinical application[27]. Lin et al.[28] introduced BMP- 2 overexpressed human BMSCs into hydrogel scaffolds and found the overexpression of BMP-2 promoted the viability of BMSCs and angiogenesis and induced osteogenesis within 14 d, which was consistent with our results from in vivo experiments that the CoCl2 treatment elevated the expression of BMP-2.
RUNX2 is the main transcriptional factor of osteoblast differentiation and bone development and induce the expression of many osteoblast markers[29]. The RUNX2 is regulated by BMP, Wnt, Notch and other signaling pathways[30]. Hu et al.[31] verified that BMSCs overexpressed with RUNX2 could improve the repair of defected rabbit knee cartilage in vivo, of which the cell morphology and arrangement were similar to the surrounding normal articular cartilage. It is reported that fragmentation of RUNX2 gene improves bone mass by promoting the differentiation of BMSCs into osteoblasts[32]. The ALP and COL- 1 are early biomarkers of osteogenic differentiation. ALP is expressed on the membrane of many tissues, including osteoblasts[33]. The function of ALP in osteoblasts is to produce phosphates that bind to hydroxyapatite, inorganic bone components and degrade pyro phosphatase to promote mineralization of osteoblasts extracellular matrix[34]. We found that the BMP-2, RUNX2, ALP and COL-I were up regulated by 100 μmol/l CoCl2 treatment, thus promoting osteogenic differentiation of BMSCs.
A great number of studies have indicated that collagen-sponges that contains BMP-2 is the clinical standard for treatment of large bone defects when the auto graft is insufficient, which has drawn great attention[35]. In this work, we used collagen sponge as scaffold of BMSCs to repair the bone defect in LSD model and observed that CoCl2 notably enhanced repair ability of BMSCs-collagen sponges using the LSD rat model.
The hypoxia environment caused by CoCl2 treatment could notably facilitate the osteogenic differentiation of BMSCs and expression of angiogenesis-related factors. We selected CoCl2 at 100 μmol/l as optimum culture condition and verified the enhanced in vivo therapeutic effects of CoCl2-BMSCs-collagen sponge scaffold. Our work may facilitate better application of BMSCs-collagen scaffold in clinical treatment of bone defect.
Conflict of interests:
The authors declared no conflict of interests.
Funding:
This work was supported by Xinjiang Uygur Autonomous Region Post-graduate Scientific Research Innovation Project (Grant No.CXCY202201); Xinjiang Uygur Autonomous Region Science and Technology Assistance Project (Grant No.2020E2133).
References
- Dosh RH, Jordan-Mahy N, Sammon C, Le Maitre CL. Tissue engineering laboratory models of the small intestine. Tissue Eng Part B Rev 2018;24(2):98-111.
[Crossref] [Google Scholar] [PubMed]
- Reddy LV, Murugan D, Mullick M, Begum Moghal ET, Sen D. Recent approaches for angiogenesis in search of successful tissue engineering and regeneration. Curr Stem Cell Res Ther 2020;15(2):111-34.
[Crossref] [Google Scholar] [PubMed]
- Lee YC, Chan YH, Hsieh SC, Lew WZ, Feng SW. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect model. Int J Mol Sci 2019;20(20):5015.
- Chrostek MR, Fellows EG, Crane AT, Grande AW, Low WC. Efficacy of stem cell-based therapies for stroke. Brain Res 2019;1722:146362.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, et al. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng Part A 2021;27(13-14):962-76.
[Crossref] [Google Scholar] [PubMed]
- Arthur A, Gronthos S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int J Mol Sci 2020;21(24):9759.
[Crossref] [Google Scholar] [PubMed]
- Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res 2015;14(3):243-57.
[Crossref] [Google Scholar] [PubMed]
- Shen Q, Huang Z, Yao J, Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J Adv Res 2022;37:221-33.
[Crossref] [Google Scholar] [PubMed]
- Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017;20:291-302.
[Crossref] [Google Scholar] [PubMed]
- Wang F, Qian H, Kong L, Wang W, Wang X, Xu Z, et al. Accelerated bone regeneration by astragaloside IV through stimulating the coupling of osteogenesis and angiogenesis. Int J Biol Sci 2021;17(7):1821-36.
- Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: Role of HIF-1 as key regulator and therapeutic target. Int J Mol Sci 2021;22(11):5703.
[Crossref] [Google Scholar] [PubMed]
- Ding T, Kang W, Li J, Yu L, Ge S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J Nanobiotechnol 2021;19(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 2001;13(2):167-71.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Chen M, Tao Q, Chi Z. Cobalt chloride-stimulated hypoxia promotes the proliferation of cholesteatoma keratinocytes via the PI3K/Akt signaling pathway. Int J Med Sci 2021;18(15):3403-11.
[Crossref] [Google Scholar] [PubMed]
- Wagatsuma A, Arakawa M, Matsumoto H, Matsuda R, Hoshino T, Mabuchi K. Cobalt chloride, a chemical hypoxia-mimicking agent, suppresses myoblast differentiation by down regulating myogenin expression. Mol Cell Biochem 2020;470:199-214.
- Perić Kačarević Ž, Rider P, Alkildani S, Retnasingh S, Pejakić M, Schnettler R, et al. An introduction to bone tissue engineering. Int J Artif Organs 2020;43(2):69-86.
[Crossref] [Google Scholar] [PubMed]
- Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: History, progress and challenges. Ann Rev Chem Biomol Eng 2011;2:403-30.
[Crossref] [Google Scholar] [PubMed]
- Bakhshandeh B, Zarrintaj P, Oftadeh MO, Keramati F, Fouladiha H, Sohrabi-Jahromi S, et al. Tissue engineering; strategies, tissues and biomaterials. Biotechnol Genet Eng Rev 2017;33(2):144-72.
[Crossref] [Google Scholar] [PubMed]
- Jin J, Ou Q, Wang Z, Tian H, Xu JY, Gao F, et al. BMSC-derived extracellular vesicles intervened the pathogenic changes of scleroderma in mice through miRNAs. Stem Cell Res Ther 2021;12(1):327.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Zhang X, Shao J, Liu H, Liu X, Luo E. Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/β-catenin pathway. Sci Rep 2017;7(1):3652.
- Polymeri A, Giannobile WV, Kaigler D. Bone marrow stromal stem cells in tissue engineering and regenerative medicine. Horm Metab Res 2016;48(11):700-13.
[Crossref] [Google Scholar] [PubMed]
- Peterson KM, Aly A, Lerman A, Lerman LO, Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: Role of oxidative stress. Life Sci 2011;88(1-2):65-73.
[Crossref] [Google Scholar] [PubMed]
- Muñoz-Sánchez J, Chánez-Cárdenas ME. The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol 2019;39(4):556-70.
[Crossref] [Google Scholar] [PubMed]
- Tirpe AA, Gulei D, Ciortea SM, Crivii C, Berindan-Neagoe I. Hypoxia: Overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci 2019;20(24):6140.
[Crossref] [Google Scholar] [PubMed]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001;107(1):43-54.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Guo J, Zhou Y, Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev 2014;20(1):84-92.
[Crossref] [Google Scholar] [PubMed]
- Vanhatupa S, Ojansivu M, Autio R, Juntunen M, Miettinen S. Bone morphogenetic protein-2 induces donor-dependent osteogenic and adipogenic differentiation in human adipose stem cells. Stem Cells Transl Med 2015;4(12):1391-402.
[Crossref] [Google Scholar] [PubMed]
- Lin H, Tang Y, Lozito TP, Oyster N, Wang B, Tuan RS. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res Ther 2019;10(1):254.
[Crossref] [Google Scholar] [PubMed]
- van Meurs JB, Boer CG, Lopez-Delgado L, Riancho JA. Role of epigenomics in bone and cartilage disease. J Bone Miner Res 2019;34(2):215-30.
[Crossref] [Google Scholar] [PubMed]
- Tang CY, Wu M, Zhao D, Edwards D, McVicar A, Luo Y, et al. Runx1 is a central regulator of osteogenesis for bone homeostasis by orchestrating BMP and WNT signaling pathways. PLoS Genet 2021;17(1):e1009233.
[Crossref] [Google Scholar] [PubMed]
- Hu J, Zou WZ, Li L, Shi ZS, Liu XZ, Cai HT, et al. Overexpressing Runx2 of BMSCs improves the repairment of knee cartilage defects. Curr Gene Ther 2020;20(5):395-404.
[Crossref] [Google Scholar] [PubMed]
- Wei J, Song Y, Du Z, Yu F, Zhang Y, Jiang N, et al. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J Mol Histol 2020;51(4):455-66.
[Crossref] [Google Scholar] [PubMed]
- Jo S, Han J, Lee YL, Yoon S, Lee J, Wang SE, et al. Regulation of osteoblasts by alkaline phosphatase in ankylosing spondylitis. Int J Rheum Dis 2019;22(2):252-61.
[Crossref] [Google Scholar] [PubMed]
- Chen XJ, Shen YS, He MC, Yang F, Yang P, Pang FX, et al. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed Pharmacother 2019;112:108746.
[Crossref] [Google Scholar] [PubMed]
- Andrews S, Cheng A, Stevens H, Logun MT, Webb R, Jordan E, et al. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem Cells Transl Med 2019;8(6):575-85.
[Crossref] [Google Scholar] [PubMed]