- *Corresponding Author:
- Chunhua Shi
Department of Nephrology, Shiyan Renmin Hospital, Hubei Medical University, Shiyan, Hubei Province 442000, China
E-mail: sidu72487@163.com
| This article was originally published in a special issue,“Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “270-278” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the effect of roxadustat on blood pressure and micro-inflammatory response in patients with hemodialysis anemia and to provide a viable technique for the treatment of hemodialysis in anemia patients. A total of 100 hemodialysis anemia patients admitted to the nephrology department of our hospital from July 2020 to July 2021 were enrolled and randomly divided into an observation group (roxadustat) and a control group (human recombinant erythropoietin). The clinical efficacy, blood lipid metabolism, inflammatory factor levels, iron metabolism-related indicators, blood biochemical indicators, anemia indicators, adverse reactions and, blood pressure were recorded and compared. There was no significant difference in baseline data and serum indicators (p>0.05). The study group demonstrated superior performance to the control group in terms of clinical efficacy, blood lipid metabolism, inflammatory factor levels, iron metabolism-related indicators, blood biochemical indicators, anemia indicators, adverse reactions and blood pressure (p<0.05). Compared with human recombinant erythropoietin treatment, roxadustat exhibits a promising outcome in treating anemia and has slight impact on blood pressure of patients.
Keywords
Roxadustat, human recombinant erythropoietin, blood pressure, micro-inflammatory state, lipid metabolism
Renal anemia is one of the common complications in patients with Chronic Kidney Disease (CKD), and has a correlation with the severity of CKD. Investigations show that more than 90 % of uremic maintenance hemodialysis patients have renal anemia, which seriously threatens the life safety of patients[1]. Renal anemia demonstrates a considerable incidence in patients with uremia, because the patient’s renal function is impaired, the glomerular filtration is less than 30 ml/min and the Serum Creatinine (SCR) exceeds 300 μmol/l and the hemoglobin shows a significant decrease. It presents with normochromic normocytic, hypo proliferative anemia that is associated with adverse reactions such as chills, lethargy, palpitations, shortness of breath, muscle weakness, loss of appetite, inability to concentrate, etc.,[2]. Additionally, it has a negative impact on the patient's intelligence and memory, showing a gradual decrease. Worse still, renal anemia can degrade the patient’s mobility, thereby hindering their normal life and compromising the quality of life[3,4].
There are multiple contributors to renal anemia, but it is majorly associated with iron metabolism disorders in the body and absolute or relative insufficiency of iron and Erythropoietin (EPO) caused by renal dysfunction, as well as insufficient dialysis, malnutrition, invisible blood loss and inflammation[5]. At this stage, there are three commonly used methods for the treatment of renal anemia.
Supplemention of erythrocyte production stimulators; the more commonly used drugs in clinical are recombinant Human Erythropoietin (rHuEPO), daepetin-alpha (α), etc.; iron supplementation method is a relatively basic treatment method that mainly corrects the patient's anemia by means of iron supplementation, and is aimed at the unsatisfactory effect of oral iron; targeted treatment of factors that affect or promote anemia, such as plasma clearance toxic substances, use of anti-EPO antibodies, correction of malnutrition, etc.,[6,7]. However, studies have shown that high-dose application of exogenous EPO cannot completely correct the anemia[8].
Roxadustat is an oral small-molecule hypoxiainducible factor propyl hydroxylase inhibitor drug, which can comprehensively regulate various factors that cause anemia and can promote the level of endogenous EPO and the absorption of iron, and the drug has a good corrective effect on the inhibition of erythropoiesis affected by inflammatory factors[9]. Roxadustat can significantly increase hemoglobin levels in the treatment of anemia in non-dialysisdependent CKD patients, which is more effective than placebo[10]. Inflammatory factors in the patient’s body are linked to the level of hepcidin and it causes iron retention and aggravates the disease when it increases. Roxadustat can play a role in reducing the level of inflammatory factors in patients, thereby reducing its impact on anemia patients[11].
Hematocrit (HCT) is an index to measure the ratio of Red Blood Cells (RBC) after centrifugal anticoagulation. Anemia, hemoconcentration or dilution is closely related to its measured value, so the change of its value is often used as the standard for evaluating the blood volume change of patients[12]. Determination of Serum Ferritin (SF) contributes to diagnose iron deficiency anemia in patients. Transferrin Saturation (TSAT) refers to the iron transport and the availability of iron to the bone marrow. A reasonable TSAT in dialysis patients can avoid the fluctuation of Hemoglobin (HB) and maintain at the standard level, which serves as the main diagnostic basis for iron bioavailability[13]. In addition, the application of high-dose rHuEPO may lead to the risk of hypertension, thromboembolism and cardiovascular events. In clinical trials of roxadustat, a small number of patients experienced elevated blood pressure, but animal experiments indicated no increase of blood pressure[14]. In light of this, this study observed the effect of roxadustat on blood pressure and micro-inflammatory response in patients with hemodialysis anemia.
Materials and Methods
General information:
This study included 100 hemodialysis anemia patients, all of whom were hospitalized in the Department of Nephrology of our hospital. A random number table was generated using a computer-based tool and the patients were randomized into two groups. Before enrollment, all participants provided signed informed consent. The study protocol was approved by the hospital ethics committee, and all procedures were in compliance with the ethical guidelines in the Declaration of Helsinki as shown in Table 1.
| Study group (n=50) | Control group (n=50) | p | |
|---|---|---|---|
| Age (years) | 46.3±11.7 | 50.0±11.2 | 0.11 |
| Male, n (%) | 27 (54) | 24 (48) | 0.689 |
| Dialysis duration (months) | 11.5 (0, 27.5) | 9.5 (0, 25) | 0.232 |
| MAP (mmHg) | 110.3±12.9 | 107.5±12.0 | 0.276 |
| Hypertension, n (%) | 46 (92) | 44 (88) | 0.741 |
| Type 2 diabetes, n (%) | 5 (10) | 7 (14) | 0.76 |
| Number of blood pressure medication (n) | 1.48±0.9 | 1.5±1.0 | 0.913 |
| rHuEPO, n (%) | 23 (46) | 26 (52) | 0.689 |
| Iron supplementation, n (%) | 42 (84) | 43 (86) | 1 |
| Initial dialysis, n (%) | 20 (40) | 21 (42) | 1 |
| Kt/V | 1.82±0.5 | 1.96±0.5 | 0.128 |
| CCR (l/1.73m2) | 68.6±27.1 | 77.6±29.5 | 0.114 |
| HB (g/l) | 88.8±12.6 | 90.0±13.8 | 0.656 |
| TC (mmol/l) | 4.4±1.1 | 4.7±1.1 | 0.216 |
| Triacylglycerol (mmol/l) | 1.4 (1.2, 1.9) | 1.4 (1.1, 2.2) | 0.897 |
| HDL-C (mmol/l) | 1.0±0.3 | 1.2±0.3 | 0.13 |
| LDL-C (mmol/l) | 2.5±0.8 | 2.6±0.8 | 0.624 |
| Albumin (g/l) | 37.4±4.1 | 38.2±4.6 | 0.323 |
| Iron metabolism, n (%) | |||
| Ferritin=3100 ng/ml and TSAT ≥20 % | 26 (52) | 24 (48) | 0.842 |
| Ferritin<100 ng/ml or TSAT <20 % | 24 (48) | 26 (52) | 0.842 |
| Hs-CRP (mg/l) | 1.1 (0.6, 2.1) | 1.2 (0.5, 2.6) | 0.956 |
Note: MAP: Mean arterial pressure; Kt/V: Urea clearance index; CCR: Creatinine clearance; Hb: Hemoglobin; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol and hs-CRP: high-sensitivity C-reactive protein
Table 1: Baseline Data of Two Groups of Hemodialysis Anemia Patients
Inclusion and exclusion criteria:
Inclusion criteria: Met the diagnostic criteria for renal anemia; all patients were on maintenance hemodialysis; hemodialysis time was more than 3 mo and all signed informed consent and be able to strictly follow the doctor's orders to take medicine.
Exclusion criteria: With aplastic anemia and other blood system diseases; with active ulcer; with malignant tumor; with allergy to the drugs in this study; severe insufficiency of organs such as liver and kidney; severe malnutrition (plasma Albumin (Alb) <25 g/l); pregnant or lactating women; insufficient dialysis; pure red blood cell aplastic anemia and thalassemia.
Intervention methods:
After admission, the patients were treated with basic drugs such as iron and lipid-lowering drugs according to their individual conditions. All patients underwent regular high-flux hemodialysis treatment and the treatment time was controlled at 4 h/time and 3 times for a week.
Control group: Patients in the control group were given rHuEPO injection. After maintenance hemodialysis, intravenous injection of human EPO injection was given, 3000 IU/time and 1-3 times for week.
Study group: Patients in the study group received roxadustat treatment, i.e., the patients took roxadustat capsules orally and the initial dose was determined according to the individual weight of the patients (body weight >60 kg, 120 mg/time, body weight 45-60 kg, 100 mg/time), 3 times/week. One course of treatment lasted for 1 w and the two groups were treated consecutively for 12 w.
Observation indicators:
Clinical efficacy: The clinical efficacy was assessed after 12 w of treatment. Markedly effective means the clinical symptoms such as pale complexion, fatigue, palpitations, etc., are significantly mitigated, hemoglobin ≥100 g/l, HCT increased ≥0.1 compared to baseline parameters; effective clinical symptoms are mitigated, hemoglobin increased ≥15 g/l, HCT ≥0.05; ineffective clinical symptoms, HCT and hemoglobin were basically the same as the corresponding values at baseline or even worsening.
Total effective rate=(Effective+markedly effective)×number of cases/total number of cases×100 %
Blood lipid metabolism: 4 ml of fasting cubital venous blood was collected from the two groups before treatment and after 12 w of treatment and the levels of Low Density Lipoprotein Cholesterol (LDL-C), Total Cholesterol (TC) and Triglyceride (TG) were detected by Roche 8000 automatic biochemical analyzer (made in Switzerland).
Levels of inflammatory factors: 4 ml of fasting cubital venous blood was collected from the two groups before and after treatment and serum was centrifuged by Baiyang BY-600A low-speed at a speed of 3000 r/min, and a radius of 13 cm, for 10 min and the serum C-Reactive Protein (CRP) levels of the two groups were detected by enzyme-linked immunosorbent assay.
Iron metabolism-related indicators: Before and after treatment, the above sera were collected to detect serum TSAT and ferritin levels in the two groups and serum hepcidin levels in the two groups were detected by enzyme-linked immunosorbent assay.
Blood biochemical indicators: Including blood calcium, blood phosphorus, Parathyroid Hormone (PTH), Alb and SCR were determined.
Anemia indicators: RBC, HB, HCT and Reticulocytes (RTC) were determined.
Adverse reactions: Adverse reactions in the two groups during the treatment period were recorded.
Mean arterial pressure before dialysis: The average value of the 4 blood pressure values was taken; for instance, the dialysis was performed on Monday, Wednesday and Friday, and then the 4 values of the mean arterial pressure before dialysis on Wednesday were averaged.
Before and after treatment, 5 ml of fasting venous blood was collected from patients and an automatic biochemical analyzer was used to detect serum calcium, blood phosphorus, Alb, TG, TC, SCR, serum iron, ferritin, total iron binding capacity and other indicators, calculate TSAT; RBC, HB, HCT and other indicators were detected by blood cell analyzer; CRP was detected by immunoturbidimetry method and PTH level was detected by immunochemical fluorescence method.
Statistical analysis:
All data analysis was performed using Statistical Package for the Social Sciences (SPSS) 23.0 statistical software. The enumeration data were expressed as n (%) and analyzed by Chi-square (χ2) test and the measurement data were expressed as the mean±Standard Deviation (SD) and the independent samples t test was used for comparison between groups and the paired samples t-test was used for comparison within groups. p<0.05 were considered statistically significant.
Results and Discussion
The clinical efficacy of the observation group was significantly better (p<0.05) as shown in Table 2. There was no significant difference in blood lipid metabolism-related indicators between the two groups before the intervention, and the improvement in the study group was greater after the intervention (p<0.05) as shown in Table 3.
| Group | n | Cured | Markedly effective | Effective | Ineffective | Total effective rate (%) |
|---|---|---|---|---|---|---|
| Study | 50 | 19 | 18 | 5 | 8 | 84 % |
| Control | 50 | 13 | 12 | 8 | 17 | 66 % |
Table 2: Perioperative Conditions of the Two Groups (X±S)
| Group | n | LDL | TG | TC | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| Control | 50 | 1.89+0.38 | 1.62+0.29* | 1.48+0.41 | 1.23+0.26* | 4.17±0.82 | 3.97±0.85 |
| Study | 50 | 1.83+0.36 | 1.41+0.23* | 1.42+0.35 | 1.04+0.18* | 3.74±1.57 | 3.75±0.75 |
| t | 0.79 | 3.843 | 0.759 | 4.021 | 0.896 | 3.976 | |
| p | 0.432 | <0.001 | 0.45 | <0.001 | 0.37 | <0.001 | |
Table 3: Comparison of Blood Lipid Metabolism-Related Indicators Between the Two Groups Before and After Intervention (X±S)
There was no significant difference in inflammatory indicators between the two groups before the intervention, and the parameters in the study group were mitigated significantly after the intervention (p<0.05) as shown in fig. 1.
There was no significant difference in iron metabolism-related indicators between the two groups before the intervention and the improvement in the study group was significant after the intervention (p<0.05) as shown in Table 4. There was no significant difference in blood biochemical indicators between the two groups before the intervention and the improvement in the study group was more notable after the intervention (p<0.05) as shown in Table 5.
| Group | n | Transferrin saturation (%) | Ferritin (mg/l) | Hepcidin (ug/l) | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| Control | 50 | 12.39+3.14 | 23.75+5.81* | 215.73+68.54 | 172.57+49.82* | 213.85+29.62 | 172.62+23.19* |
| Study | 50 | 12.15+3.23 | 42.89+7.65* | 212.96+67.61 | 134.26+44.35* | 210.76+28.53 | 153.25+18.72* |
| t | 0.37 | 14.199 | 0.199 | 3.933 | 0.519 | 4.409 | |
| p | 0.712 | <0.001 | 0.843 | <0.001 | 0.605 | <0.001 | |
Note: Compared with before intervention, *p<0.05
Table 4: Comparison of Iron Metabolism-Related Indicators Between the Two Groups Before and After Intervention (X±S)
| Group (n=50) | Intervention | Blood calcium (mmol/l) | Blood phosphorus (mmol/l) | PTH (pg/ml) | Alb (g/l) | SCr (mol/l) |
|---|---|---|---|---|---|---|
| Control | Before | 2.28±0.18 | 1.57±0.53 | 169.29±116.27 | 35.63±3.62 | 633.07±251.02 |
| After | 2.28±0.15 | 1.56±0.38 | 169.60±131.72 | 36.89±5.70 | 635.63±222.71 | |
| Study | Before | 2.23±0.12 | 1.78±0.57 | 210.79±161.75 | 36.69±4.28 | 640.63±273.41 |
| After | 2.23±0.12 | 1.62±0.29 | 200.41±144.52 | 35.59±4.44 | 566.77±211.40 |
Table 5: Comparison of Blood Biochemical Indexes Before and After Intervention in the Two Groups (X±S)
There was no significant difference in anemia index between the two groups before the intervention and significant improvement was observed in the study group after the intervention (p<0.05) as shown in Table 6. During the treatment period, there were 2 cases of abdominal pain, 4 cases of nausea, and 1 case of constipation in the control group, and the incidence of adverse reactions was 14 % (7/50). In the study group, there were 2 cases of mild acid reflux and 2 cases of nausea and the incidence of adverse reactions was 6.00 % (3/50). There was no significant difference in the incidence of adverse reactions between the two groups (χ2=0.068, p=0.794)
| Group (n=50) | HCT (%) | HB (g/l) | RTC (×109/l) | RBC (×1012/l) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Study | 20.92±3.35 | 56.61±4.54* | 71.87±9.80 | 119.21±9.24* | 51.36±8.45 | 88.43±9.66* | 1.97±0.23 | 5.48±0.61* |
| Control | 21.05±3.28 | 49.97±3.62* | 72.25±9.35 | 111.34±9.31* | 51.25±8.63 | 78.60±9.24* | 2.00±0.30 | 4.67±0.41* |
| t | 0.196 | 8.086 | 0.198 | 4.243 | 0.064 | 5.2 | 0.561 | 7.793 |
| p | 0.845 | <0.001 | 0.843 | <0.001 | 0.949 | <0.001 | 0.576 | <0.001 |
Table 6: Comparison of Anemia Indicators Between the Two Groups Before and After Intervention (X±S)
After the intervention, the blood pressure in the study group was superior to that of the control group (p<0.05) as shown in Table 7.
| 100 g/l≤HB<130 g/l | ||
|---|---|---|
| Study group (n=50) | Control group (n=50) | |
| Mean arterial pressure (mmHg) | 92.04±11.59* | 98.11±9.54 |
| Hypertension, n (%) | 3 | 5 |
| Antihypertensive drug points | 2.00 (1.00~5.25)* | 4.00 (2.00~6.00) |
Table 7: Comparison of Blood Pressure Before and After Intervention Between the Two Groups (X±S)
CKD is a long-term progressive disease characterized by progressive loss of renal function, which can eventually lead to end-stage renal disease. Renal anemia is a common complication and the incidence of anemia increases with the progression of CKD[15-18]. The mechanism of renal anemia mainly includes the following aspects; various types of kidney diseases cause insufficient production of EPO and anemia caused by toxic substances in the plasma of patients with uremia that interfere with the production and metabolism of erythrocytes; the reduction of EPO and the stimulation of inflammatory factors will cause the increase of hepcidin synthesis in the liver, which will eventually lead to the absorption and utilization of iron disorders; when renal failure occurs, renal blood flow is reduced, the renal tubulointerstitium is destroyed, the sensitivity of EPO-secreting cells to hypoxia is reduced, the production of EPO is reduced, resulting in absolute deficiency of EPO, resulting in a reduction in the rate of erythropoiesis and an inflammatory response state. The lack of hematopoietic raw materials, the inhibitory effect of PTH on the bone marrow, and acute and chronic blood loss will further aggravate the degree of anemia[19,20].
In recent years, EPO recombinant variants have been used clinically to induce the production of erythrocytes, but they lacked the desired effect. Studies have also shown that long-term high-dose use will increase the incidence of cardiovascular and cerebrovascular, hypertension and other adverse events[21]. Therefore, it is of great clinical significance to seek a safe and effective regimen for the treatment of renal anemia in uremic maintenance hemodialysis. Roxadustat belongs to a Hypoxia- Inducible Factor Prolyl Hydroxylase Inhibitor (HIF-PHI). When the partial pressure of oxygen is normal, the drug can inhibit the degradation of hypoxia-inducible factor and effectively maintain its stability and then promote the production of endogenous EPO, improve the utilization rate of iron and comprehensively regulate erythropoiesis[22]. Roxadustat can effectively regulate the level of EPO in the body after entering the patient's body. Among the substances that regulate and induce oxygen in the kidneys, hypoxia-inducible factor plays an important role, mainly composed of heterodimers and also plays an important role in the regulatory factor of EPO[23].
The results of this study showed that after treatment, the clinical efficacy and ferritin saturation of the study group were higher and the levels of LDL, TG, serum CRP, ferritin and hepcidin were lower in the study group, suggesting that roxadustat treatment is effective in maintaining blood circulation. Dialysis patients with renal anemia have exact curative effect, can improve the blood lipid metabolism level, reduce the body's inflammatory response, and can regulate iron metabolism. Roxadustat is a novel drug for the treatment of renal anemia. It can stabilize the hypoxia-inducible factor, inhibit the degradation of hypoxia-inducible factor, increase the concentration of EPO and increase the expression of EPO Receptor (EPOR). Sensitivity, promote the production of RBC, can increase the saturation of transferrin, reduce hepcidin, thereby promoting the body's utilization and absorption of iron[24]. Studies have shown that hepcidin is a peptide substance (containing 25 amino acids) that can sequester iron in organs. In addition, the availability of stored iron is limited, thereby reducing the body's iron utilization and iron transport, resulting in functional deficiency. Iron, thereby causing anemia of inflammation and it is regulated by hypoxia-inducible factor[25]. Relevant studies have shown that roxadustat can correct renal anemia in patients with CKD by stabilizing hypoxiainducible factor, thereby regulating EPO synthesis, and regulating iron metabolism (promoting iron absorption, promoting iron transport and reducing hepcidin)[26]. Roxadustat can correct the anemia in patients with uremic maintenance hemodialysis renal anemia through multiple ways, such as improving the body’s reabsorption of endogenous iron and the absorption of exogenous iron; simulating intracellular hypoxia to promote the body to produce endogenous EPO; regulating the body's inflammatory response by reducing the level of hepcidin[27,28].
In addition, roxadustat is also effective in improving blood biochemical indicators, anemia indicators and blood pressure. Because roxadustat can improve the level of hypoxia-inducible factor in the body after entering the patient's body, thereby promoting the level of EPO, providing favorable conditions for the patient's hematopoietic function, thereby improving the patient's anemia status[29]. In addition, roxadustat is the world's first oral HIF-PHI. After oral administration, the drug can inhibit the activity of Prolyl Hydroxylase Domain proteins (PHDs) in the human body and effectively maintain the stability of HIF, promoting EPO/EPOR expression. In addition, when the drug is used to treat renal anemia, it can also reduce the serum hepcidin level in the body through different ways and there is no need for additional intravenous iron supplementation. Therefore, roxadustat cannot only simulate the hypoxic environment in cells and then produce a large amount of EPO, but also promote the absorption of iron in the body without being affected by inflammation and finally achieve the purpose of effectively correcting renal anemia[30,31]. The results of this study showed that there was no significant difference in the incidence of adverse reactions between the two groups during the treatment period between the control group and the study group, and the adverse reactions of the two groups could be relieved spontaneously without special treatment, suggesting that roxadustat in the treatment of maintain hemodialysis kidney. It has a good safety profile in patients with anemia.
With the continuous advancement of medical research, in-depth research HIF in recent years has provided a new therapeutic direction for the treatment of renal anemia. HIF promotes EPO gene synthesis under hypoxic conditions under normal oxygen supply conditions, HIF is rapidly hydroxylated and degraded by PHD after synthesis, but the catalytic activity of PHD is inhibited under hypoxic conditions[32]. Roxadustat is a kind of HIFPHI, which inhibits the activity of PHD, so that the concentration of HIF remains stable, promotes the expression of EPO/EPOR, increases the utilization of iron and relieves the symptoms of anemia. Therefore, the diagnosis and treatment effect of this method is more prominent[33]. It can be seen that the clinical effect of oral roxadustat capsule in the treatment of patients with CKD and renal anemia is better than that of subcutaneous injection of rHuEPO for injection. Our preliminary study showed that roxadustat treatment of anemia had little effect on blood pressure in maintenance hemodialysis patients compared with rHuEPO treatment. It has less impact on blood pressure and cardiac workload, and has a lower incidence of cardiovascular and cerebrovascular complications, which is worthy of in-depth clinical research and discussion. Further research is needed on the effect of roxadustat on blood pressure in patients and its mechanism.
This trial performed roxadustat treatment for patients with hemodialysis anemia, which has certain guiding significance, but there are still the following problems; the sample size of this study is small, and only a single-center trial was conducted, which might lead to bias; during the research process, the test results could not be evaluated blindly and there might be measurement bias and the observation period of this study was short and there was no longterm follow-up. Although the short-term clinical efficacy was observed, the long-term efficacy could not be evaluated.
Roxadustat, a pioneering inhibitor of HIF-α proline residue by prolyl hydroxylase, has been approved for the treatment of renal anemia, including promoting EPO production and improving iron metabolism. In addition to the treatment of renal anemia, roxadustat can also be used to treat diseases such as renal disease, CKD-related renal anemia, ischemic diseases, etc. However, most studies are preclinical studies at the cell or animal level, requiring further research to determine whether these preclinical findings and applications can be transplanted into humans. It is hoped that in the future, the clinical studies with larger samples can be conducted to provide more clinical evidence for application of this method.
Author’s contributions:
Wenwen Li and Fei Zha have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Quiroga B. Effectiveness of biosimilar drugs in the treatment of renal anemia: A case series. Medwave 2021;21(9):e8474.
[Crossref] [Google Scholar] [PubMed]
- Mima A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: Advantages and disadvantages. Eur J Pharmacol 2021;912:174583.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Deng Y, Gong R. Efficacy and safety of intravenous iron with different frequencies for renal anaemia: A systematic review and meta-analysis. J Clin Pharm Ther 2022;47(6):713-21.
[Crossref] [Google Scholar] [PubMed]
- Akizawa T, Yamada T, Nobori K, Matsuda Y, Hayashi Y, Hayasaki T, et al. Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int Rep 2021;6(10):2604-16.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Ren Q, Hu R, Zheng K, Qin Y, Li X. Does HIF-PHI increased risk of gastrointestinal hemorrhage in patients with renal anemia: A review of cases reported to the US Food and drug administration adverse event reporting system. Ren Fail 2021;43(1):1170-1.
[Crossref] [Google Scholar] [PubMed]
- Laurentius A, Wiyono L, Subali AD, Rossimarina V. Cardiorenal anaemia syndrome vs. left ventricular ejection fraction: Which is a better mortality prognostic factor for heart failure? A systematic review and meta-analysis. Eur Heart J Suppl 2021;23:123.
- Gao M, Zhang Z, Zhang Y, Li M, Che X, Cui X, et al. Steamed Panax notoginseng attenuates renal anemia in an adenine-induced mouse model of chronic kidney disease. J Ethnopharmacol 2022;288:114941.
[Crossref] [Google Scholar] [PubMed]
- Yu Y, Yang F, Yu Q, Liu S, Wu C, Su K, et al. Discovery of a potent and orally bioavailable hypoxia-inducible factor 2? (HIF-2?) agonist and its synergistic therapy with prolyl hydroxylase inhibitors for the treatment of renal anemia. J Med Chem 202;64(23):17384-402.
[Crossref] [Google Scholar] [PubMed]
- Fei M, Wen XQ, Yu ZL, Kang T, Wu WH, Ou ST. Roxadustat as treatment for a blood transfusion-dependent maintenance hemodialysis patient: A case report and review of literature. World J Clin Cases 2021;9(15):3680.
[Crossref] [Google Scholar] [PubMed]
- Nakai T, Saigusa D, Iwamura Y, Matsumoto Y, Umeda K, Kato K, et al. Esterification promotes the intracellular accumulation of roxadustat, an activator of hypoxia-inducible factors, to extend its effective duration. Biochem Pharmacol 2022;197:114939.
[Crossref] [Google Scholar] [PubMed]
- Hara R, Goto N, Furuya D, Kitahara T, Numata H, Watanabe S, et al. The effect of roxadustat on transfusion-dependent myelodysplastic syndrome complicated by chronic kidney disease. Case Rep Oncol 2021;14:1574-9.
[Crossref] [Google Scholar] [PubMed]
- Shutov E, Su?owicz W, Esposito C, Tataradze A, Andric B, Reusch M, et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: A phase 3, randomized, double-blind, placebo-controlled study (ALPS). Nephrol Dial Transpl 2021;36(9):1629-39.
[Crossref] [Google Scholar] [PubMed]
- Zheng L, Tian J, Liu D, Zhao Y, Fang X, Zhang Y, et al. Efficacy and safety of roxadustat for anaemia in dialysis-dependent and non-dialysis-dependent chronic kidney disease patients: A systematic review and meta-analysis. Br J Clin Pharmacol 2022;88(3):919-32.
[Crossref] [Google Scholar] [PubMed]
- Cai KD, Zhu BX, Lin HX, Luo Q. Successful application of roxadustat in the treatment of patients with anti-erythropoietin antibody-mediated renal anaemia: A case report and literature review. J Int Med Res 2021;49(4):03000605211005984.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Xu Y, Xie J, Liu J, Zhang R, Yan X. Roxadustat does not affect platelet production, activation and thrombosis formation. Arterioscler Thromb Vasc Biol 2021;41(10):2523-37.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Zhao B, Wang J, Wang L, Tao M, Lu J, et al. Red blood cell lifespan in long-term hemodialysis patients treated with roxadustat or recombinant human erythropoietin. Ren Fail 2021;43(1):1428-36.
[Crossref] [Google Scholar] [PubMed]
- Ichii M, Mori K, Miyaoka D, Sonoda M, Tsujimoto Y, Nakatani S, et al. Suppression of thyrotropin secretion during roxadustat treatment for renal anemia in a patient undergoing hemodialysis. BMC Nephrol 2021;22:1-5.
[Crossref] [Google Scholar] [PubMed]
- Takada A, Shibata T, Shiga T, Groenendaal-van de Meent D, Komatsu K. Population pharmacokinetics of roxadustat in Japanese dialysis-dependent chronic kidney disease patients with anaemia. Br J Clin Pharmacol 2022;88(2):787-97.
[Crossref] [Google Scholar] [PubMed]
- Miao AF, Liang JX, Yao L, Han JL, Zhou LJ. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against renal ischemia/reperfusion injury by inhibiting inflammation. Ren Fail 2021;43(1):803-10.
[Crossref] [Google Scholar] [PubMed]
- Liu F, Wang J, Ye Q, Fu H, Mao J. Roxadustat for renal anemia in ESRD from PKD patients: Is it safe enough? J Am Soc Nephrol 2021;32(4):1005.
[Crossref] [Google Scholar] [PubMed]
- Wu T, Qi Y, Ma S, Zhang L, Pu X, Chen K, et al. Efficacy of roxadustat on anemia and residual renal function in patients new to peritoneal dialysis. Ren Fail 2022;44(1):529-40.
[Crossref] [Google Scholar] [PubMed]
- Zhou QQ, Li J, Liu B, Wang CL. Roxadustat for treatment of anemia in a cancer patient with end-stage renal disease: A case report. World J Clin Case 2022;10(19):6587.
[Crossref] [Google Scholar] [PubMed]
- Tang M, Zhu C, Yan T, Zhou Y, Lv Q, Chuan J. Safe and effective treatment for anemic patients with chronic kidney disease: An updated systematic review and meta-analysis on roxadustat. Front Pharmacol 2021;12:658079.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Li Z, Zhang H, Hu J, Wang J, Zhou H, et al. A prospective, self-controlled pilot study of the efficacy of roxadustat for erythropoietin hyporesponsiveness in patients requiring chronic ambulatory peritoneal dialysis. J Ren Nutr 2022;32(5):595-604.
[Crossref] [Google Scholar] [PubMed]
- Kurata Y, Tanaka T, Nangaku M. An evaluation of roxadustat for the treatment of anemia associated with chronic kidney disease. Exp Opin Pharmacother 2022;23(1):19-28.
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Wang X. A case report of rhabdomyolysis caused by the use of roxadustat in the treatment caused by renal anaemia. Int J Clin Prac 2021;75(6):e14011.
[Crossref] [Google Scholar] [PubMed]
- Yang Z, Ma T, Xu X, Fu G, Zhao J, Xu Y, et al. Randomized study on the efficacy of standard vs. low roxadustat dose for anemia in patients on peritoneal dialysis. Kidney Int Rep 2022;7(3):455-64.
[Crossref] [Google Scholar] [PubMed]
- Ugawa T, Ashizaki M, Murata A, Majikawa Y. Roxadustat (Evrenzo® tablet), a therapeutic drug for renal anemia: Pharmacological characteristics and clinical evidence in Japan. Nihon Yakurigaku zasshi. Folia Pharmacol Japonica 2021;156(3):187-97.
- Yu WH, Li XJ, Yuan F. Roxadustat for treatment of erythropoietin-hyporesponsive anemia in a hemodialysis patient: A case report. World J Clin Cases 2020;8(23):6048.
[Crossref] [Google Scholar] [PubMed]
- Zhu XW, Zhang CX, Xu TH, Jiang GN, Yao L. Efficacy of roxadustat in treatment of peritoneal dialysis patients with renal anaemia. World J Clin Cases 2021;9(26):7682.
[Crossref] [Google Scholar] [PubMed]
- Eleftheriadis T, Pissas G, Mavropoulos A, Nikolaou E, Filippidis G, Liakopoulos V, et al. In mixed lymphocyte reaction, the hypoxia-inducible factor prolyl-hydroxylase inhibitor roxadustat suppresses cellular and humoral alloimmunity. Arch Immunol Ther Exp 2020;68:1-2.
[Crossref] [Google Scholar] [PubMed]
- Tanaka M, Shinohara K, Ono A, Ikuma M. Role of roxadustat for ESA-resistant renal anemia??Read with caution. J Am Soc Nephrol 2020;31(11):2737.
[Crossref] [Google Scholar] [PubMed]
- Li ZL, Tu Y, Liu BC. Treatment of renal anemia with roxadustat: Advantages and achievement. Kidney Dis 2020;6(2):65-73.
[Crossref] [Google Scholar] [PubMed]
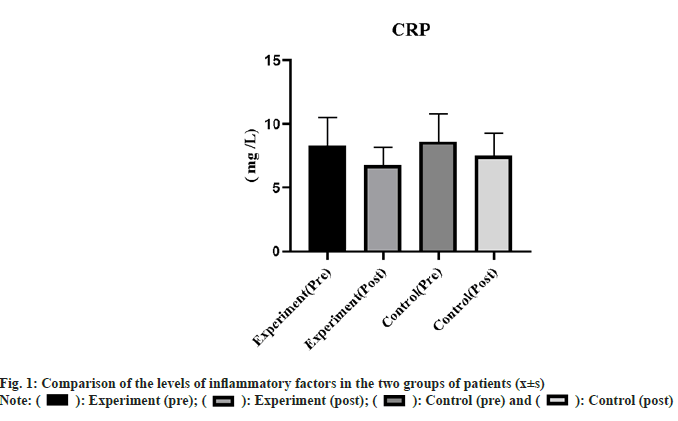
 ): Experiment (pre); (
): Experiment (pre); ( ): Experiment (post); (
): Experiment (post); ( ): Control (pre) and (
): Control (pre) and ( ): Control (post)
): Control (post)



