- *Corresponding Author:
- J. Wang
Department of Otorhinolaryngology, South China Hospital of Shenzhen University, Shenzhen, Guangdong Province 518060, China
E-mail: jiaoba291209@163.com
| This article was originally published in a special issue,“Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “279-285” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to compare the efficacy of combined treatment with kenacort and montelukast vs. surgical treatment using low temperature plasma radiofrequency ablation in children with snoring. 70 children with snoring were selected as the study subjects at our hospital and all children met the inclusion criteria. According to the different treatments received by the children, the control group (n=35) received combined treatment with kenacort and montelukast, while the observation group (n=35) underwent surgery (low temperature plasma radiofrequency ablation). The clinical treatment effects, degree of adenoid hypertrophy, symptom improvement, ventilator function, sleep quality and quality of life were compared between the two groups of children. The total effective rate of treatment in the control group was 71.43 %, while that in the observation group was 94.29 %, which was significantly higher than that in the control group (p<0.05). Before treatment, there was no significant difference in the adenoid-nasopharyngeal ratio between the two groups (p>0.05). After treatment, the adenoidnasopharyngeal ratio in the observation group was significantly lower than that in the control group (p<0.05). Before treatment, there was no significant difference in the symptom score between the two groups (p>0.05). After treatment, the symptom score in the observation group was significantly lower than that in the control group (p<0.05). Before treatment, there was no significant difference in the LAT, oxygen desaturation index 4 % and apnea-hypopnea index levels between the two groups (p>0.05). After treatment, the LAT, oxygen desaturation index 4 % and apnea-hypopnea index levels in the observation group were significantly lower than those in the control group (p<0.05). Before treatment, there was no significant difference in the Pittsburgh sleep quality index score and obstructive sleep apnea-1 score between the two groups (p>0.05). After treatment, the Pittsburgh sleep quality index score and obstructive sleep apnea-1 score in the observation group were significantly lower than those in the control group (p<0.05). Compared with combined treatment with kenacort and montelukast, low-temperature plasma radiofrequency ablation is more effective for children with snoring and surgical treatment can better improve the clinical symptoms and ventilator function of children, thereby further improving their sleep quality and quality of life.
Keywords
Kenacort, montelukast, monotherapy, surgical treatment, pediatric snoring, comparative analysis, efficacy
Pediatric snoring refers to the sound or breathing caused by obstructed airways during a child's sleep. It is characterized by narrow airways, which causes an increase in airflow velocity, leading to vibration of the soft tissues around the throat and the production of harsh snoring sounds[1]. Pediatric snoring is a common respiratory sleep disorder in children, which can affect their sleep quality, daytime attention, learning ability and may lead to serious consequences such as breathing pauses[2]. Its etiology is complex and may be related to various factors such as genetics, upper respiratory infections, obesity and others[3]. In recent years, with the continuous development of modern medical technology, the treatment of pediatric snoring has also been continuously updated and improved. Currently, clinical treatment options for pediatric snoring mainly include conservative treatment and surgical treatment[4]. Conservative treatment mainly uses methods such as oral appliances, neck traction and drug therapy, while surgical treatment mainly includes procedures such as tonsillectomy, uvulopalatopharyngoplasty, tongue base resection and low temperature plasma radiofrequency ablation[5,6]. However, different treatment methods have different effects on the treatment of pediatric snoring, and specific treatment methods should be determined based on the patient's individual circumstances.
Mometasone furoate and montelukast are currently commonly used drugs for treating pediatric snoring. Mometasone furoate mainly improves airway ventilation by narrowing mucosal blood vessels and reducing mucosal edema[7], while montelukast can prevent mucosal edema and mucus secretion caused by allergic reactions, thereby reducing the degree of airway narrowing and obstruction[8]. Based on this, this study aims to explore the differences in the efficacy of the combined use of mometasone furoate and montelukast and surgical treatment in the treatment of pediatric snoring, in order to provide more scientific and reasonable treatment options for clinical doctors.
Materials and Methods
Study population:
A total of 70 children with snoring were selected as the study population in our hospital. Basic information such as age, gender, duration of illness and degree of tonsillar hypertrophy was collected for all patients. All patients met the inclusion criteria and were divided into two groups based on the different treatments they received; control group (n=35) received treatment with a combination of fluticasone propionate and montelukast alone, while the observation group (n=35) underwent surgery (low-temperature plasma radiofrequency ablation).
Inclusion and exclusion criteria:
Inclusion criteria: Children aged between 2 y and 13 y old with good physical condition; children diagnosed with simple snoring after preliminary examination, excluding other sleep-disordered breathing; snoring seriously affects the sleep quality and quality of life of the child; both the child and the parent voluntarily participate in the study, and sign the informed consent form.
Exclusion criteria: Children with severe systemic diseases such as cardiovascular, pulmonary, nervous and endocrine diseases; children with severe respiratory infections or other respiratory diseases; children with oral or throat inflammation, such as tonsillitis, pharyngitis, etc.; children with severe sleep disorders such as night terrors, sleepwalking, etc.; children who have received relevant surgical treatment or medication for snoring and parents who do not agree to participate in the study.
Methods:
Control group: The control group of patients received simple medication treatment with budesonide mometasone furoate nasal spray (Zhejiang Xianju Pharmaceutical Co., Ltd., National Medical Products Administration Approval No. H20113481) at a dose of 1 spray per nostril per day and montelukast sodium chewable tablets (Qilu Pharmaceutical (Hainan) Co., Ltd., National Medical Products Administration Approval No. H20203124) at a dose of 4 mg/d for children under 6 y old and 5 mg/d for children aged 6 y and older, once daily before bedtime, for a continuous treatment period of 3 mo.
Observation group: The observation group of patients received treatment with low-temperature plasma radiofrequency ablation after surgery. Specifically, after general anesthesia, the tonsils were removed using an Evac70° scalpel. The tonsil membrane was fully exposed and located, and then cut from top to bottom and from outside to inside, with complete removal of the tonsils outside the tonsil membrane. If there were any bleeding points, electro cautery was used to stop the bleeding. After the tonsils were removed, low-temperature plasma radiofrequency ablation was performed under nasal endoscopy. A soft guide wire was inserted from the nasal cavity, pulled out from the mouth, and grasped at both ends for traction to expose the nasopharynx. A hemostatic forceps was used to bend the front end of the plasma knife to facilitate endoscopic ablation through the mouth. During the operation, normal tissues were protected, bleeding was stopped in a timely manner, and the knife was removed after ensuring a clean removal and the operation was completed.
Observational indicators:
Clinical treatment efficacy: The clinical treatment efficacy of the patients was classified into cured, markedly effective, effective and ineffective. The criteria for judgment are as follows; cured clinical symptoms of the patients disappeared completely after treatment; markedly effective clinical symptoms of the patients improved significantly and approached disappearance after treatment; effective clinical symptoms of the patients improved to some extent after treatment and ineffective clinical symptoms of the patients did not improve or even worsened after treatment.
Degree of adenoid hypertrophy: The degree of adenoid hypertrophy in the patients was evaluated using the Adenoid-Nasopharyngeal ratio (A/N ratio) [9] before and after treatment. The A/N ratio of ≥0.71 was used as the pathological hypertrophy standard and surgical indication. The higher the A/N ratio of the patients, the more severe their degree of adenoid hypertrophy.
Improvement of symptoms: Before and after treatment, the "Pediatric Snoring Symptom Evaluation Scale" developed by our hospital was used to evaluate the improvement of symptoms in the patients. This scale includes four aspects; snoring volume, duration of breathing cessation, mouth breathing and daytime sleepiness. Each aspect adopts a 0-5 point system, and the total score of the scale is 20 points. The lower the score of the patients, the more significant their symptom improvement.
Ventilation function: Before and after treatment, the multi-channel sleep test was used to measure the ventilation function of the patients. The ventilation function indicators included in this study were; Longest Apnea Time (LAT), the frequency of oxygen saturation levels below 4 % of the baseline per hour (Oxygen Desaturation Index (ODI)-4 %), and the number of apnea and hypopnea events per hour of sleep (Apnea-Hypopnea Index (AHI)).
Sleep quality: Before and after treatment, the Pittsburgh Sleep Quality Index (PSQI) was used to evaluate the sleep quality of the children[10,11]. The scale includes 7 sleep quality assessment indicators; sleep quality, sleep duration, sleep efficiency, sleep disorders, use of sedatives or sleeping pills, daytime dysfunction and subjective evaluation of sleep quality. The score range for each indicator is 0-3 points and the total score is 21 points. The higher the score, the worse the child's sleep quality.
Quality of life: Before and after treatment, the Obstructive Sleep Apnea (OSA-1) scale was used to evaluate the quality of life of the children[12]. The scale includes 8 items; degree of sleepiness, restlessness, headaches, coughing, nasal congestion, breathing difficulties, dry mouth and nocturia. The score range for each item is 0-3 points and the total score is 24 points. The higher the total score, the worse the child's quality of life.
Statistical analysis:
GraphPad Prism 8 was used for graphing and Statistical Package for the Social Sciences (SPSS) 22.0 was used to analyze the data. For continuous data, the mean and standard deviation were used to describe the distribution and t-test or Analysis of Variance (ANOVA) was used to compare differences between the two groups. For categorical data, frequency and percentage were used to describe the distribution, and Chi-square (χ2) or Fisher's exact tests were used to compare differences between the two groups. p<0.05 were considered statistically significant.
Results and Discussion
Among the 35 patients in the control group, there were 20 males and 15 females, aged 2 y-12 y with a mean age of (6.2±1.7) y, and a disease course of 4 mo to 3 y with an average of (1.3±0.8) y. The degree of tonsillar hypertrophy was as follows; grade I in 8 cases, grade II in 9 cases and grade III in 18 cases. Among the 35 patients in the observation group, there were 22 males and 13 females, aged 2 y-13 y with a mean age of (6.4±1.6) y, and a disease course of 5 mo to 3 y with an average of (1.4±0.8) y. The degree of tonsillar hypertrophy was as follows; grade I in 9 cases, grade II in 9 cases and grade III in 17 cases. The baseline information of the two groups of patients was similar and there was no statistically significant difference (p>0.05) as shown in Table 1.
| Basic data information | Control group (n=35) | Observation group (n=35) | t/χ2 | p |
|---|---|---|---|---|
| Gender | 0.238 | 0.625 | ||
| Male | 20 | 22 | ||
| Female | 15 | 13 | ||
| Average age (years) | 6.2±1.7 | 6.4±1.6 | 0.506 | 0.613 |
| Average duration of disease (years) | 1.3±0.8 | 1.4±0.8 | 0.522 | 0.602 |
| Degree of tonsil hypertrophy | 0.077 | 0.780 | ||
| Class I | 8 | 9 | ||
| Class II | 9 | 9 | ||
| Class III | 18 | 17 |
Table 1: Comparison of Baseline Information Between the Two Groups of Patients
The total effective rate of treatment in the control group was 71.43 %, while the total effective rate of treatment in the observation group was 94.29 %. The total effective rate of treatment in the observation group was significantly higher than that in the control group (p<0.05) as shown in Table 2.
| Group (n) | Cure | Markedly effective | Efficient | Invalid | Total effective rate (%) |
|---|---|---|---|---|---|
| Control group (n=35) | 2 | 11 | 12 | 10 | 0.7143 |
| Observation group (n=35) | 10 | 14 | 9 | 2 | 0.9429 |
| χ2 | - | - | - | - | 6.436 |
| p | - | - | - | - | 0.011 |
Table 2: Comparison of Clinical Treatment Efficacy Between the Two Groups of Patients
Before treatment, there was no significant difference in the A/N ratio between the two groups of patients (p>0.05). After treatment, the A/N ratio in the observation group was significantly lower than that in the control group (p<0.05) as shown in Table 3.
| Group | Degree of A/N ratio | |
|---|---|---|
| Before treatment | After treatment | |
| Control (n=35) | 0.82±0.33 | 0.69±0.23 |
| Observation (n=35) | 0.83±0.36 | 0.60±0.12 |
| t | 0. 121 | 2.052 |
| p | 0. 903 | 0.044 |
Table 3: Comparison of Adenoid Hypertrophy Between the Two Groups of Patients
Before treatment, there was no significant difference in the symptom scores between the two groups of patients (p>0.05). After treatment, the symptom scores of the observation group were significantly lower than those of the control group (p<0.05), as shown in Table 4.
| Group | Symptom index score (points) | |
|---|---|---|
| Before treatment | After treatment | |
| Control (n=35) | 14.23±2.74 | 8.61±1.85 |
| Observation (n=35) | 14.31±2.59 | 5.54±1.13 |
| t | 0.125 | 8.378 |
| p | 0.900 | <0.001 |
Table 4: Comparison of Symptom Improvement Between the Two Groups of Patients
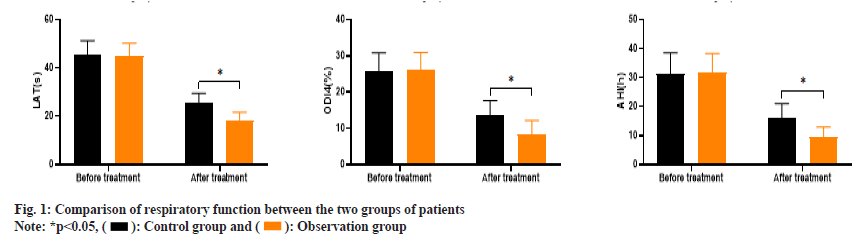
As shown in fig. 1, the LAT values of the control group before and after treatment were (45.21±6.09 and 25.34±4.12) and the ODI 4 % values were (25.63±5.19 and 13.45±4.21), and the AHI values were (31.35±7.17 and 15.91±5.13), respectively. The LAT values of the observation group before and after treatment were (44.83±5.47 and 18.45±3.23), the ODI 4 % values were (26.07±4.89 and 8.32±3.84) and the AHI values were (31.72±6.62 and 9.48±3.46).
Before treatment, there was no significant difference in LAT, ODI 4 % and AHI levels between the two groups (p>0.05). After treatment, the LAT, ODI 4 % and AHI levels of the observation group were significantly lower than those of the control group (p<0.05).
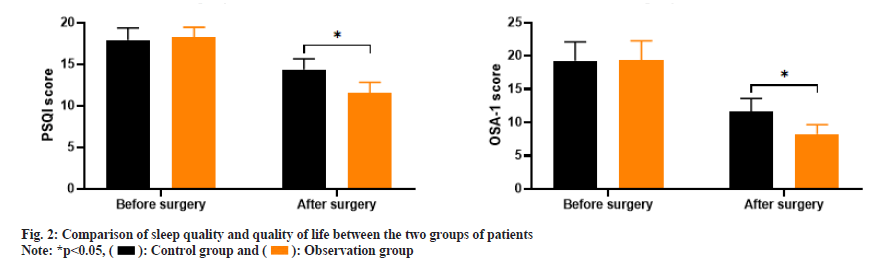
As shown in fig. 2, the PSQI scores before and after treatment in the control group were (17.92±1.43 and 14.38±1.26) and the OSA-1 scores were (19.26±2.85 and 11.64±1.98); the PSQI scores before and after treatment in the observation group were (18.21±1.23 and 11.57±1.25) and the OSA-1 scores were (19.37±2.91 and 8.26±1.39). Before treatment, there was no significant difference in PSQI scores and OSA-1 scores between the two groups (p>0.05); after treatment, the PSQI and OSA-1 scores in the observation group were significantly lower than those in the control group (p<0.05).
Pediatric snoring refers to the condition in which children produce snoring sounds during sleep, mainly manifested as loud snoring or long duration of snoring, accompanied by varying degrees of impaired sleep quality[13]. Another study found that in addition to impaired sleep quality, pediatric snoring may also lead to some other physical problems[14]. For example, prolonged snoring symptoms may lead to the occurrence of sleep apnea syndrome, which can cause hypoxemia, hypercapnia and other problems, and in the long run, it may also lead to complications such as cardiovascular and cerebrovascular diseases. In addition, the occurrence of snoring can also lead to an increase in nasal and pharyngeal secretions, which can cause persistent stimulation to the child's tonsils and/or adenoids[15]. Therefore, the course of the disease is usually longer, and the impact on the daily life and growth, and development of affected children is also more severe.
Currently, the treatment methods for pediatric snoring mainly include conservative treatment and surgical treatment. Drug therapy is one of the conservative treatment methods. Due to its non-invasive nature and lack of anesthesia, drug therapy has become the most acceptable treatment method for children and their families. The drug therapy method mainly involves the use of anti-inflammatory drugs and oral expanders to quickly relieve symptoms in children[16]. The control group in this study used kenalog, a common topical glucocorticoid, which has strong anti-itch, anti-inflammatory, anti-allergic, vasoconstrictive and cell division inhibition effects. It can reduce local inflammation in children and shrink adenoid tissue to relieve clinical symptoms such as nasal congestion and runny nose[17]. Montelukast sodium can block the combination of leukotriene’s and receptors produced by the body's cells induced by external pathogenic factors, thus blocking airway inflammation and improving mucosal edema, reducing adenoid growth, and decreasing secretions[18]. Studies have shown that the combination of these two drugs can have a synergistic effect, further improving the treatment effectiveness for children. Surgical treatment is achieved by removing enlarged lesions in children to improve clinical symptoms[19]. The traditional surgical methods for pediatric snoring are tonsillectomy and adenoidectomy, and the traditional surgical treatment effective rate can reach 85 % to 90 %[20]. However, this method can easily cause damage to normal tissues near the lesions, so the acceptance level of children and their families is relatively low. Low-temperature plasma ablation is a new surgical technique used to treat pediatric snoring[21]. It uses a radio frequency electric field to activate gas molecules and produce low-temperature plasma, which accurately ablates soft tissue in the nasopharynx without damaging surrounding tissues, thereby improving pediatric snoring symptoms. Clinical studies have shown that compared with traditional surgical methods[22], low-temperature plasma ablation can significantly reduce damage to normal tissues in children while ensuring treatment effectiveness. However, there is no clear conclusion yet about the differences in efficacy between drug therapy with kenalog and montelukast, and lowtemperature plasma ablation for the treatment of pediatric snoring.
Although this study to some extent demonstrated the difference in efficacy between drug treatment and surgical treatment, there are still some limitations in the study such as; the sample size was relatively small, including only 70 children, which may lead to bias in the results. In the future, increasing the sample size could further verify the reliability and stability of the results; this study adopted a single-center research design, which has certain geographical and institutional limitations. In the future, a multicenter, randomized controlled research design could be used to expand the sample range and improve the generalizability of the research; the study did not involve the observation of long-term efficacy and only evaluated the short-term effects. In the future, longer follow-up observations could be used to assess the long-term efficacy of lowtemperature plasma radiofrequency ablation; the study did not track and observe the psychological and behavioral development of children. In the future, a more detailed evaluation of these factors could be conducted to fully understand the effects and impact of low-temperature plasma radiofrequency ablation on the treatment of pediatric sleep apnea.
Conflict of interests:
The authors declared no conflict of interests.
References
- Gulotta G, Iannella G, Vicini C, Polimeni A, Greco A, de Vincentiis M, et al. Risk factors for obstructive sleep apnea syndrome in children: State of the art. Int J Env Res Public Health 2019;16(18):3235.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Celestin J, Lockey RF. Pediatric sleep apnea syndrome: An update. J Allergy Clin Immunol 2016;4(5):852-61.
[Crossref] [Google Scholar] [PubMed]
- Bitners AC, Arens R. Evaluation and management of children with obstructive sleep apnea syndrome. Lung 2020;198(2):257-70.
[Crossref] [Google Scholar] [PubMed]
- Koka V, de Vito A, Roisman G, Petitjean M, Filograna Pignatelli GR, Padovani D, et al. Orofacial myofunctional therapy in obstructive sleep apnea syndrome: A pathophysiological perspective. Medicina 2021;57(4):323.
[Crossref] [Google Scholar] [PubMed]
- Babakurban ST, Aydin E. Adenoidectomy: Current approaches and review of the literature. Kulak Burun Bogaz Ihtis Derg 2016;26(3):181-90.
[Crossref] [Google Scholar] [PubMed]
- Huynh NT, Desplats E, Almeida FR. Orthodontics treatments for managing obstructive sleep apnea syndrome in children: A systematic review and meta-analysis. Sleep Med Rev 2016;25:84-94.
[Crossref] [Google Scholar] [PubMed]
- Spada F, Barnes TM, Greive KA. Comparative safety and efficacy of topical mometasone furoate with other topical corticosteroids. Australas J Dermatol 2018;59(3):e168-74.
[Crossref] [Google Scholar] [PubMed]
- Naqi SA, Ashfaq AH, Umar MA, Karmani JK, Arshad N. Clinical outcome of montelukast sodium in children with adenoid hypertrophy. Pak J Med Sci 2021;37(2):362.
- Li P, Li T, Yu L, Chen A, Wu Y, Wan Y, et al. Predictive value of adenoid-nasopharyngeal ratio in the diagnosis of secretory otitis media. Ear Nose Throat J 2022:01455613221144496.
[Crossref] [Google Scholar] [PubMed]
- Leong KW, Griffiths A, Adams AM, Massie J. How to interpret polysomnography. Arch Dis Child Edu Prac 2020;105(3):130-5.
[Crossref] [Google Scholar] [PubMed]
- Sancho-Domingo C, Carballo JL, Coloma-Carmona A, Buysse DJ. Brief version of the Pittsburgh Sleep Quality Index (B-PSQI) and measurement invariance across gender and age in a population-based sample. Psychol Assessment 2021;33(2):111-21.
[Crossref] [Google Scholar] [PubMed]
- Hyung PG, Kyung KT, Kweon KS, Woo YB, Hoon LS, Lok JC, et al. The relationship between sleep quality, daytime sleepiness and rapid eye movement obstructive sleep apnea (REM-OSA). Sleep Breath 2022:1-7.
[Crossref] [Google Scholar] [PubMed]
- Luzzi V, Ierardo G, di Carlo G, Saccucci M, Polimeni A. Obstructive sleep apnea syndrome in the pediatric age: The role of the dentist. Eur Rev Med Pharmacol Sci 2019;23:9-14.
[Crossref] [Google Scholar] [PubMed]
- Lai CC, Lin PW, Lin HC, Friedman M, Chang HW, Salapatas AM, et al. Clinical predictors of pediatric obstructive sleep apnea syndrome. Ann Otol Rhinol Laryngol 2018;127(9):608-13.
[Crossref] [Google Scholar] [PubMed]
- Urakov A, Urakova N, Fisher E, Yagudin I, Darya S, Svetova M, et al. Inhalation of an aerosol solution of hydrogen peroxide and sodium bicarbonate for the urgent recanalization of the respiratory tract after blockage by mucus and pus. J Mod Biol Drug Discov 2022;1(2):2.
- Xu Z, Wu Y, Tai J, Feng G, Ge W, Zheng L, et al. Risk factors of obstructive sleep apnea syndrome in children. J Otolaryngol Head Neck Surg 2020;49(1):1-7.
- Song Y, Pan K, Chen Y, Wang X, Tian J. The efficacy of mometasone furoate for children with asthma: A meta-analysis of randomized controlled trials. Postępy Dermatol Alergol 2020;38(5):740-5.
[Crossref] [Google Scholar] [PubMed]
- Ji T, Lu T, Qiu Y, Li X, Liu Y, Tai J, et al. The efficacy and safety of montelukast in children with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med 2021;78:193-201.
[Crossref] [Google Scholar] [PubMed]
- Jin W, Zhao Z, Zhou D. Effect of montelukast sodium combined with budesonide aerosol on airway function and T lymphocytes in asthmatic children. Pak J Med Sci 2022;38(5):1265.
[Crossref] [Google Scholar] [PubMed]
- Grosse L, Bahr K. Obstructive sleep apnea syndrome in children. HNO 2021;69(4):325-34.
- Wang C, Zhao Y, Li C, Song Q, Wang F. Meta-analysis of low temperature plasma radiofrequency ablation and CO2 laser surgery on early glottic laryngeal carcinoma. Comput Math Methods Med 2022;2022:3417005.
[Crossref] [Google Scholar] [PubMed]
- Long X, Li Z, Liu Y, Zhen H. Clinical application of low-temperature plasma radiofrequency in the treatment of hemangioma in nasal cavity, pharynx and larynx. Ear Nose Throat J 2021:01455613211062443.
[Crossref] [Google Scholar] [PubMed]
 ): Control group and (
): Control group and ( ): Observation group
): Observation group