- *Corresponding Author:
- Leiming Xia
Department of Emergency Medicine, The second affiliated hospital of Anhui Medical University, Hefei, Anhui 23060, China
E-mail: 278461175@qq.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “255-261” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Autologous hematopoietic stem cell transplantation is recommended and significantly improved the prognosis of lymphoma patients. However, the preferred conditioning regimen prior to autologous hematopoietic stem cell transplantation is still a controversial topic. Current experiment was aim to explore the efficacy and safety of BEAC (carmustine, etoposide, cytarabine and cyclophosphamide), BEAM (carmustine, etoposide, cytarabine and melphalan) and Be-EAM (bendamustine, cytarabine, etoposide and melphalan) preparative regimens in lymphoma patients who underwent autologous hematopoietic stem cell transplantation. 19 lymphoma cases were enrolled and followed-up for 13 mo, 6 cases were treated with BEAC, 5 cases were treated with BEAM and 8 cases were treated with Be-EAM. We found the median progression free survival was 23 mo in BEAC group, but the progression free survival and the overall survival in the other 2 groups had not been reached due to the short follow-up time. The median of patients achieved neutrophil and platelet engraftment were 12.500 and 15.167 d in BEAC group, 11.200 and 14.000 d in BEAM group and Be-EAM cohort were 11.250 and 11.750 d. Statistically differences on platelet engraftment were observed in Be-EAM comparing with BEAC and BEAM regimens. Additionally, the volume of platelet transfusion in the Be-EAM group was the least in three groups. The main adverse events included bone marrow suppression, fever, mucositis, nausea and vomiting and all of them were acceptable. The efficacy and safety were favorable in lymphoma patients who received conditioning treatment with Be-EAM comparing with BEAC and BEAM regimens. The Be-EAM regimen has a shorter hospital stay, faster hematopoietic reconstitution, fewer platelet infusions and the superiority of storage conditions and drug cost of bendamustine, the Be-EAM regimen could be a potential optimistic option for lymphoma conditioning treatment.
Keywords
Lymphoma, BEAC, BEAM and Be-EAM conditioning regimens, autologous hematopoietic stem cell transplantation
Autologous Stem Cell Transplantation (ASCT) is the first-line consolidation treatment for patients with high- risk and the salvage treatment for relapsed, refractory lymphoma[1]. However, the optimized conditioning regimens of ASCT that chosen for lymphoma patients still inconclusive. Over last decades, carmustine based BEAM (carmustine, etoposide, cytarabine and melphalan) and BEAC (carmustine, etoposide, cytarabine and cyclophosphamide) are the most used conditioning regimens[2-4] and recent regimen that using bendamustine replacing carmustine were raised due to bendamustine is an attractive option for the management of both de novo and relapsed lymphomas[5].
Several studies have compared the efficacy and safety of the conditioning regimens, BEAM and BEAC, which are widely used in clinic. Shi et al.[2] analyzed 129 non-Hodgkin lymphoma patients who underwent autologous homologous skin construct for a median follow-up of 42.5 mo, the 5 y Overall Survival (OS) for the BEAM and BEAC groups was 77.8 % and 81.8 % and the 5 y Progression-Free Survival (PFS) in the BEAM and BEAC groups was 66.7 % and 67.5 %. Patients in BEAM group were observed significant higher ≥Grade 2 of mucositis, diarrhea and fever and no differences between BEAM and BEAC groups were observed in the time to hematopoietic recovery and the duration of hospitalization. Recent study suggested that Be-EAM (bendamustine, cytarabine, etoposide and melphalan) regimen have a better PFS[6].
Nevertheless, it is lack of evidence to comparing the efficacy and safety of Be-EAM and BEAM, BEAC conditioning regimens. Thus, we enrolled a retrospective clinical study to compare the outcomes of patients with lymphoma receiving Be-EAM and BEAM, BEAC with ASCT.
Materials and Methods
Patients:
From December 2018 to October 2021, the 19 cases that underwent auto haematopoietic stem cell transplantation of lymphoma patients in our hospital were retrospectively analyzed; all patients were confirmed to the malignant lymphoma diagnosis criteria of the fourth criteria for diagnosis and efficacy of hematological diseases confirmed pathologically. The enrolled patients, including 10 males and 9 females, the ages range from 18 to 58, the median age was 51y. Regard to the types of diseases, it includes 5 cases Mantle Cell Lymphomas (MCL), 9 cases Diffuse Large B-Cell Lymphoma (DLBCL) (3 cases primary Central Nervous System (CNS) DLBCL), 2 cases relapsed Hodgkin Lymphomas (HL), 2 cases Peripheral T-Cell Lymphomas (PTCL), 1 case Angioimmunoblastic T-Cell Lymphoma (AITL), 1 case Burkitt Lymphoma (BL). The patient’s disease status before transplantation were recorded, 8 patients were in Complete Remission (CR), 1 patient was in near CR (nCR), 1 patient was in Very Good Partial Remission (VGPR), 8 patients were in Partial Remission (PR) and 1 patient were in Progression of Disease (PD) (the PD patient was case No. 3, the autologous peripheral stem cells were collected when patient achieved PR and disease progressed after stem cells collection). The information of patients was as shown in Table 1.
| S. no | Gender | Age | Diagnosis | Ann Arbor Stage | ECOG Score | Best response before ASCT | Pre-treatment regimen |
|---|---|---|---|---|---|---|---|
| 1 | Male | 49 | MCL | IV | 2 | CR | BEAC |
| 2 | female | 55 | DLBCL | IV | 1 | CR | BEAM |
| 3 | female | 55 | MCL | IV | 2 | PD | BEAC |
| 4 | Female | 18 | BL | II | 1 | CR | BEAC |
| 5 | Male | 39 | DLBCL | IV | 1 | PR | BEAC |
| 6 | Female | 24 | DLBCL | III | 2 | CR | BEAC |
| 7 | Male | 57 | MCL | IV | 1 | CR | BEAM |
| 8 | Male | 51 | DLBCL | III | 1 | PR | BEAC |
| 9 | Male | 30 | Relapsed HL | IV | 0 | PR | Be-EAM |
| 10 | Male | 51 | Relapsed DLBCL | IV | 1 | CR | Be-EAM |
| 11 | Male | 57 | Relapsed DLBCL | IV | 0 | PR | BEAM |
| 12 | Female | 43 | MCL | IV | 1 | PR | Be-EAM |
| 13 | Male | 52 | CNS DLBCL | IV | 2 | PR | Be-EAM |
| 14 | Female | 52 | CNS DLBCL | IV | 1 | CR | Be-EAM |
| 15 | Male | 46 | PTCL | IV | 1 | PR | Be-EAM |
| 16 | Female | 48 | AITL | III | 1 | PR | Be-EAM |
| 17 | Female | 53 | CNS DLBCL | IE | 1 | VGPR | Be-EAM |
| 18 | Female | 19 | Relapsed HL | II | 1 | nCR | BEAM |
| 19 | Male | 58 | MCL | IV | 1 | CR | BEAM |
Table 1: The Characteristics of 19 Enrolled Lymphoma Patients
Mobilization scheme:
All patients were treated with first-line or second-line therapy before ASCT and autologous hematopoietic stem cell mobilization, collection and cryopreservation were performed after diseases evaluation of PR or better. Among the 19 patients, 17 patients were directly mobilized by topside (1.6 g/m2) and 1 patient was re- mobilized by topside after a failed direct mobilization of Granulocyte Colony-Stimulating Factor (G-CSF), the other DLBCL patient (case 5) was mobilized twice, the primary stem cells collection did not reach enough Cluster of Differentiation (CD34) positive cell which was mobilized with topside 1.6 g/m2+G-CSF, so the patient was mobilized with Cyclophosphamide (CTX) 1.8 g/m2+G-CSF once again. All of the patients were successfully collected appropriate amount of stem cells for ASCT (the number of CD34 positive cells ≥2.0×106/ kg).
Conditioning regimens:
6 of the 19 patients received the BEAC regimen (carmustine 300 mg/m2/d 6 d+ cyclophosphamide 1 g/ m2/d-5 d-2+topodside 100 mg/m2/d-5 d -2+cytarabine 100 mg/m2/q 12 h 5 d-2 d), 8 of the 19 patients received the Be-EAM regimen (carmustine 160 mg/m2/d 8 d-7 d+topodside 100 mg/m2/d 6 d-3+cytarabine 100 mg/ m2/q 12 h 6 d 3+melphalan 140 mg/m2/d 2 d), 5 of the 19 patients BEAM regimen (carmustine 300 mg/m2/d 7 d+etopodside 100 mg/m2/d 6 d-3+cytarabine 100 mg/ m2/q 12 h 6 d-3+melphalan 140 mg/m2/d d-2). After the pretreatment, the transfusion of autologous stem cells was performed on 1 d.
Outcome information and endpoints:
The primary outcome information was hematopoietic reconstitution and supporting care of blood infusion, the hematopoietic reconstitution including platelet and neutrophil engraftment and the supporting care of blood infusion including platelet, erythrocyte and plasma infusion. The endpoints were PFS which defined the time of patients from receive treatment to the time they observe disease progression and the OS was defined the time from treatment to death from any cause and adverse reactions during transplantation.
Statistical analysis:
GraphPad 9.0 software was used for data statistical analysis. Log-rank test was used for survival comparison between groups, Statistical Package for Social Sciences (SPSS) 16.0 was used for measurement data between groups, mean±standard deviation was used for description, and unpaired t test was used for difference comparison. p<0.05 was considered statistically significant.
Results and Discussion
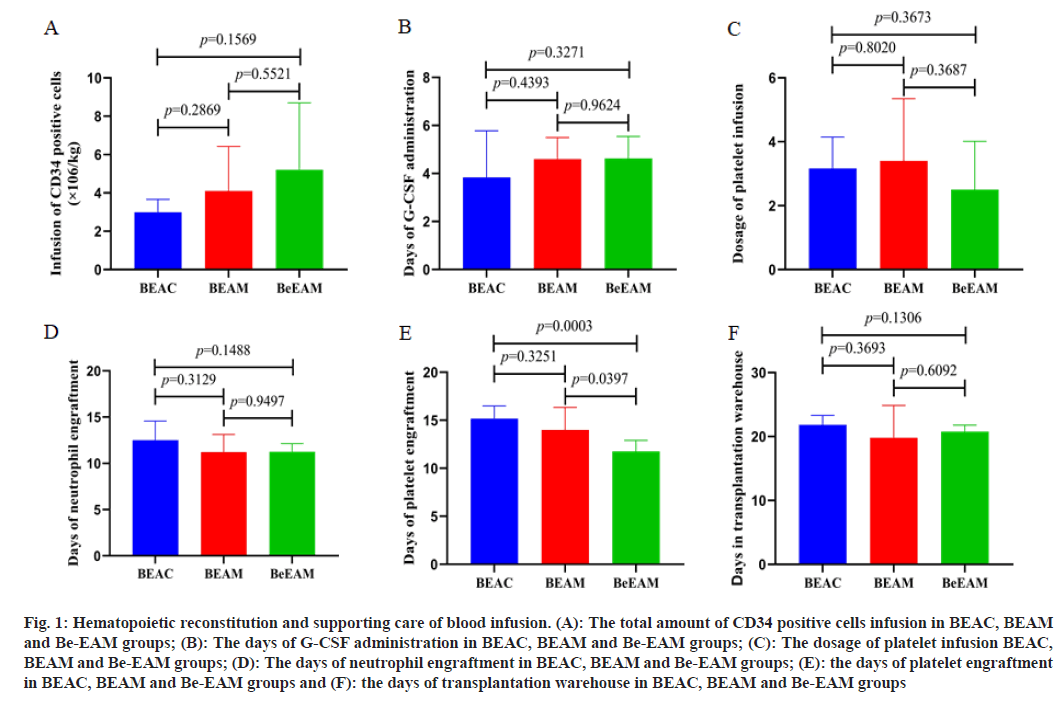
In 19 lymphoma patients, the median transfusion amount of the CD34 positive cells were 3.33×106/ kg (range from 2.2 to 13×106/kg), G-CSF was administrated to promote neutrophil homing. The mean time of neutrophil and platelet engraftment were 12 d (range from 9th d to 16th d) and 13 d (range from 10th d to 17th d), respectively. The baseline of CD34 positive cells infusion and G-CSF administration in BEAC, BEAM, Be-EAM groups did not show any differences. All patients were supported with platelet infusion, the median amount of platelet infusion were 2 therapeutic doses. 4 of the 19 cases were infused erythrocyte, 4 of the 19 cases were infused plasma. The amount of platelet transfusion showed no differences in BEAC, BEAM, Be-EAM groups. Statistical analysis was performed to comparing the differences of supporting administration of blood infusion and hemolytic engraftment in BEAC, BEAM, Be-EAM groups. We founded that the platelet engraftment was shorten in Be- EAM group (11.750±1.165 d), which was significantly different from BEAM (14.000±2.345 d) and BEAC (15.167±1.329) group. Meanwhile, the differences of neutrophil engraftment in BEAC, BEAM, Be-EAM groups were showed no significant differences. The days in transplantation warehouse in BEAC, BEAM, Be-EAM groups were 21.833±1.472, 19.800±5.070 and 20.750±1.035, which showed no differences in these groups as shown in fig. 1 and Table 2.
| BEAC | BEAM | Be-EAM | p value | |||
|---|---|---|---|---|---|---|
| 6 patients | 5 patients | 8 patients | BEAC vs. BEAM | BEAC vs. Be-EAM | BEAM vs. Be-EAM | |
| Infusion of CD34 positive cells (×106/kg) | 2.992±0.675 | 4.108±2.323 | 5.200±3.499 | 0.2869 | 0.1569 | 0.5521 |
| Days of G-CSF administration | 3.833±1.941 | 4.600±0.894 | 4.625±0.916 | 0.4393 | 0.3271 | 0.9624 |
| Dosage of platelet infusion | 3.167±0.983 | 3.400±1.949 | 2.500±1.512 | 0.802 | 0.3673 | 0.3687 |
| Days of neutrophil engraftment | 12.500±2.074 | 11.200±1.924 | 11.250±0.886 | 0.3129 | 0.1488 | 0.9497 |
| Days of platelet engraftment | 15.167±1.329 | 14.000±2.345 | 11.750±1.165 | 0.3251 | 0.0003 | 0.0397 |
| Days in transplantation warehouse | 21.833±1.472 | 19.800±5.070 | 20.750±1.035 | 0.3693 | 0.1306 | 0.6092 |
Table 2: Hematopoietic Reconstitution and Supporting Care of Blood Infusion
Fig. 1: Hematopoietic reconstitution and supporting care of blood infusion. (A): The total amount of CD34 positive cells infusion in BEAC, BEAM and Be-EAM groups; (B): The days of G-CSF administration in BEAC, BEAM and Be-EAM groups; (C): The dosage of platelet infusion BEAC, BEAM and Be-EAM groups; (D): The days of neutrophil engraftment in BEAC, BEAM and Be-EAM groups; (E): the days of platelet engraftment in BEAC, BEAM and Be-EAM groups and (F): the days of transplantation warehouse in BEAC, BEAM and Be-EAM groups
We analyzed the Adverse Events (AEs) which were followed by World Health Organization guideline. The most common AEs was infection, 15 of 19 (78.95 %) patients were observed fever, including febrile neutropenia 9 cases, pulmonary infection 3 cases, abdominal infection 1 case, perianal infection 1 case, the mixed infection of pulmonary and perianal infection 1 case. 6 of 19 patients were observed grade 1-2 mucositis. The gastrointestinal symptom also monitored, nausea and vomiting were monitored in all 19 patients during conditioning treatment, including 2 patients in grade 1, 12 patients in grade 2 and 5 patients in grade 3.3 patients were noticed that suffered gastrointestinal bleeding, the symptom was cured after treatment. 11 of 19 patients were observed diarrhea, most cases were in grade 1-2, including 1 case in grade 4 with obvious bloody diarrhea. 5 of 19 patients occurred grade 1 hepatic injury, the symptoms cured after treatment. Impairment of renal function was founded, 1 MCL patient previously suffered hypertensive nephrosclerosis of chronic kidney disease grade 3, creatinine was 126 µmol/l before conditioning treatment and 132.1-140.7 µmol/l during conditioning treatment, creatinine was decreased to 106.9-126.8 µmol/l after stem cell infusion. Arrhythmia was founded in 2 patients, one was supraventricular tachycardia and the other was infection induced persistent sinus velocity. No hemorrhagic cystitis and hepatic vein occlusion were observed in 3 groups as shown in Table 3.
| BEAC | BEAM | Be-EAM | |
|---|---|---|---|
| Fever | 5/6 | 4/5 | 6/8 |
| Mucositis | 1/6 | 1/5 | 4/8 |
| Hepatotoxicity | 3/6 | 0/5 | 2/8 |
| Diarrhea | 2/6 | 3/5 | 6/8 |
| Gastrointestinal bleeding | 1/6 | 1/5 | 1/8 |
| Arrhythmia | 1/6 | 1/5 | 0/8 |
| Severe nausea and vomiting | 2/6 | 0/5 | 3/8 |
Table 3: Adverse Reactions were Observed in BEAC, BEAM and Be-EAM Groups
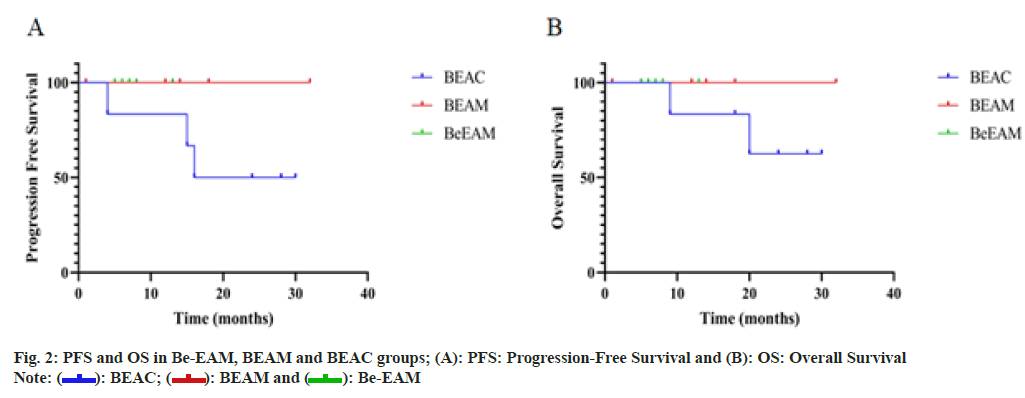
Follow-up ended on November 30, 2021. Follow-up range from, the median time of follow-up was 13 (1 to 32) mo, 17 of 19 (89.5 %) patients were surviving, 2 of 19 (10.5 %) patients were died of disease relapse or progression. The median of PFS was 23 mo in BEAC group, the median of PFS in BEAM and Be-EAM cohort and the median of OS in three cohorts has not been reached due to the short of follow-up as shown in fig. 2.
Lymphoma is one of the common malignant hematological diseases[7]. Unfortunately, treatment reaction to conventional radiotherapy and chemotherapy is not ideal, the disease control and prognosis are poor in high-risk and relapse, refractory patients[8-10]. Recurrence and drug resistance seriously limit the further improvement of efficacy[11]. ASCT can significantly improve outcome of the lymphoma patients, which was recommended as the preferred treatment option for lymphoma patients with high- risk and the salvage treatment for relapsed, refractory disease[12].
Currently, sufficient stem cells mobilization and appropriate protocol of conditioning treatment were significantly highlighted in the success of ASCT[13]. In our published data, we analyzed the risky factors of autologous stem cells mobilization and collection in patients with hematological malignancy and found that combination of VP16 and G-CSF could significantly enhance the achieved amount of stem cells than CTX plus G-CSF or G-CSF alone[14]. Thus, we further analyzed the efficacy and safety of BEAC, BEAM and Be-EAM conditioning regimens prior to ASCT in lymphoma patients based on the conditioning treatment of VP16 plus G-CSF. The information of treatment- related adverse reactions, neutrophil and platelet engraftment and days in transplantation warehouse and supporting administration of blood infusion were recorded and compared, in order to propose a relatively reasonable and safe conditioning regimen.
Three different conditioning schemes of BEAC, BEAM and Be-EAM were included in current study. There was no significant difference in the number of CD34 positive cells reverted in patients receiving different conditioning regimens. Previous publications indicated that PFS and OS could not be further prolonged by ASCT using carmustine based conditioning treatments. Robinson et al.[15] reviewed lymphoma patients who received ASCT and registered in European cooperative group for bone marrow transplantation and found no statistical differences in PFS and OS between the BEAC and BEAM conditioning regimens (PFS 63 % vs. 64 %, OS 78 % vs.77 %, respectively). However, bendamustine based regimen was reported could prolong the survival of lymphoma patients. Hueso et al.[16] recruited 60 and 108 patients in the Be-EAM and the BEAM groups, respectively, and indicated a 3 y PFS was significantly higher in the Be-EAM group than that in the BEAM group (84 % vs. 63 %, p=0.03). However, the OS was not statistically different between the two groups (p=0.2)[16]. In current study, the rate of OS and PFS in BEAC group were lower than BEAM and Be-EAM group, but no significant difference was observed, which might largely due to the limited patient size and the short follow-up, further conclusion should enlarge the sample size and extended follow-up time. Regarding to the hematopoietic reconstruction, we found that the median of neutrophil engraftment in BEAC regimen was within 12.5 d, 11 d for the BEAM regimen and 11.5 d for the Be-EAM. There was no significant difference among the three groups. But in terms of platelet engraftment, the median period was 15 d in the BEAC group, 13 d in the BEAM group and 12 d in the Be-EAM group, the time of platelet engraftment was significantly shorter in patients with Be-EAM conditioning treatment. The study published in 2021 found no statistical difference in OS, PFS and neutrophil and platelet engraftment between the Be-EAM and BEAM groups, but the total cost of hospitalization and the infusion requirements of blood in Be-EAM group was lower than that of the BEAM group[11]. Our data indicated the hospitalization in Be- EAM group was 20.750±1.035, which slightly shorter than BEAC and BEAM group with no difference, and the data was similar to Chantepie et al.[17].
Severe grade 3 and 4 AEs in the transplantation were not observed. Patients in the 3 groups experienced varying degrees of fever during transplantation; the incidence of fever is 83.3 % in the BEAC group, 80 % in the BEAM group and 75 % in the Be-EAM group. This is similar to the French multicenter retrospective analysis published by Chantepie et al.[17], which reported incidence of infection was 78.2 % in 474 lymphoma patients pretreated with Be-EAM. In addition, our data reported the occurrence of mucositis and diarrhea was higher in Be-EAM regimen group than that in BEAC and BEAM group. In the part of organ toxicity, Robinson et al. [15] reported the incidence of non-relapse mortality events including cardio toxicity and multiple organ failure and carmustine-related pulmonary toxicity (idiopathic pneumonia syndrome) and secondary malignancy in BEAC group was similar to that of BEAM group. We found cardiac arrhythmias occurred in both the BEAC and BEAM groups, but no case was observed in Be-EAM group and the statistical analysis cannot be performed due to the small size of cases. The incidence of renal impairment was reported as common AEs in Be-EAM regime, Saleh et al.[18] reported 12 % and 6 % in the Be-EAM vs. BEAM groups, and Hahn et al.[19] reported a incidence of nephrotoxicity high to 32 % in the Be- EAM group[19]. Most of the nephrotoxicity occurred several days after bendamustine administration, most of which were grades 1-2, and a few are grade 3 or above. Therefore, the dose of bendamustine above 200 mg/m2 is not recommended to reduce the occurrence of nephrotoxicity. In the Be-EAM group of our center, the dosage of bendamustine was 160 mg/m2, the liver and renal function was carefully monitored, 2 patients (25 %) were found a nephrotoxicity, the creatinine increased more than 1.5 times in the first 2-3 d after bendamustine administration comparing with baseline, similar events were reported by Hahn et al.[19].
The results showed that the efficacy and side effects in lymphoma patients whom conditioning treated with BEAC, BEAM and Be-EAM regimen are acceptable and controllable. Bendamustine based Be-EAM regimen offered lymphoma patients a quick platelet engraftment, OS rate could not analyze currently, but a high OS rate was reported from the other center[6,16,19]. Considering the strict storage conditions and quick expiration of carmustine, bendamustine based regimen seems to be an economic and promising conditioning treatment. However, more evidence of outcome and AEs from enlarged patient size needs to be evaluated.
Authors contributions:
Yingying Chen and Lingling Jiang contributed equally to this work. Leiming Xia and Fengbo Jin designed the study. Leiming Xia, Lingling Jiang and Yingying Chen were the major contributors in writing the manuscript. Limei He, Yu Zhang, Jianjun Li, Lixia Liu, Min Ruan and Zhicheng Zhou collected the clinical data. All authors participated in reading and sorting the relevant literature. All authors have read and approved the final manuscript.
Funding:
This work was supported by the Research Fund of Anhui Institute of Translational Medicine (2021zhyx-C70) and the Research Fund of Anhui Medical University (2018xkj061).
Conflict of interest:
The authors declare no conflicts of interest, financial or otherwise.
References
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J 2021;134(7):783-91.
[Crossref] [Google Scholar] [PubMed]
- Shi Y, Liu P, Zhou S, Yang J, Han X, He X, et al. Comparison of CBV, BEAM and BEAC high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation in non-Hodgkin lymphoma: Efficacy and toxicity. Asia Pac J Clin Oncol 2017;13(5):e423-9.
[Crossref] [Google Scholar] [PubMed]
- Shin HJ, Lee WS, Lee HS, Kim H, Lee GW, Song MK, et al. Busulfan-containing conditioning regimens are optimal preparative regimens for autologous stem cell transplant in patients with diffuse large B-cell lymphoma. Leuk Lymphoma 2014;55(11):2490-6.
[Crossref] [Google Scholar] [PubMed]
- Kim KH, Kim WS, Kim SJ, Yoon DH, Suh C, Kang HJ, et al. Treatment with intravenous busulfan, melphalan, and etoposide followed by autologous stem cell transplantation in patients with non-Hodgkin's lymphoma: A multicenter study from the consortium for improving survival of lymphoma. Transpl Int 2020;33(10):1211-9.
[Crossref] [Google Scholar] [PubMed]
- Shabbir-Moosajee M, Jehangir S, Sawani S, Muhammed T, Ali N, Sheikh U, et al. Safety and efficacy of bendamustine in the conditioning regimen for autologous stem cell transplantation in patients with relapsed/refractory lymphoma. Blood Res 2019;54(2):108-13.
[Crossref] [Google Scholar] [PubMed]
- Costes-Tertrais D, Hueso T, Gastinne T, Thieblemont C, Oberic L, Bouabdallah K, et al. Bendamustine-EAM vs. R-BEAM after high-dose cytarabine-based induction in newly diagnosed patients with mantle cell lymphoma, a LYSA retrospective study. Bone Marrow Transpl 2022;57(4):627-32.
[Crossref] [Google Scholar] [PubMed]
- Han XL, Zhang XY, Li JD, Wang WL, Yang C. Abnormal expression and clinical significance of B lymphocyte stimulating factor in b cell-derived malignant hematologic diseases. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25(1):138-41.
[Google Scholar] [PubMed]
- Alonso-Álvarez S, Manni M, Montoto S, Sarkozy C, Morschhauser F, Wondergem MJ, et al. Primary refractory follicular lymphoma: A poor outcome entity with high risk of transformation to aggressive B cell lymphoma. Eur J Cancer 2021;157:132-9.
[Crossref] [Google Scholar] [PubMed]
- Bentolila G, Pavlovsky A. Relapse or refractory Hodgkin lymphoma: Determining risk of relapse or progression after autologous stem-cell transplantation. Leuk Lymphoma 2020;61(7):1548-54.
[Crossref] [Google Scholar] [PubMed]
- Yhim HY, Eshet Y, Metser U, Lajkosz K, Cooper M, Prica A, et al. Risk stratification for relapsed/refractory classical Hodgkin lymphoma integrating pretransplant Deauville score and residual metabolic tumor volume. Am J Hematol 2022;97(5):583-91.
[Crossref] [Google Scholar] [PubMed]
- Gu J, Liu S, Cui W, Dai H, Cui Q, Yin J, et al. Identification of the predictive models for the treatment response of refractory/relapsed B-Cell ALL patients receiving CAR-T therapy. Front Immunol 2022;13:858590.
[Crossref] [Google Scholar] [PubMed]
- Kaloyannidis P, Omari R, Eldebawy E, Al Shaibani E, Apostolidis J, Hindi T, et al. Favorable outcome after adjuvant involved-field radiotherapy after autologous hematopoietic stem-cell transplantation in patients with high-risk relapsed/refractory lymphoma: A single-center experience. Clin Lymphoma Myeloma and Leuk 2021;21(2):e112-9.
- Kambhampati S, Hunter B, Varnavski A, Fakhri B, Kaplan L, Ai WZ, et al. Ofatumumab, etoposide, and cytarabine intensive mobilization regimen in patients with high-risk relapsed/refractory diffuse large B-cell lymphoma undergoing autologous stem cell transplantation. Clin Lymphoma Myelom Leuk 2021;21(4):246-56.
[Crossref] [Google Scholar] [PubMed]
- ZHAO Yi-ming, Q. W. J. L. Analysis of the influencing factors of mobilization and collection of autologous hematopathy in patient with malignant blood disease. J Bengbu Med Coll 2020;9(45):1216-9.
- Robinson SP, Boumendil A, Finel H, Dreger P, Sureda A, Hermine O, et al. High-dose therapy with BEAC conditioning compared to BEAM conditioning prior to autologous stem cell transplantation for non-Hodgkin lymphoma: No differences in toxicity or outcome. A matched-control study of the EBMT-Lymphoma Working Party. Bone Marrow Transplant 2018;53(12):1553-9.
[Crossref] [Google Scholar] [PubMed]
- Hueso T, Gastinne T, Garciaz S, Tchernonog E, Delette C, Casasnovas RO, et al. Bendamustine-EAM vs. BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: A multicenter retrospective study from Lymphoma Study Association (LYSA) centers. Bone Marrow Transplant 2020;55(6):1076-84.
[Crossref] [Google Scholar] [PubMed]
- Chantepie SP, Garciaz S, Tchernonog E, Peyrade F, Larcher MV, Diouf M, et al. Bendamustine‐based conditioning prior to autologous stem cell transplantation (ASCT): Results of a French multicenter study of 474 patients from Lymphoma Study Association (LYSA) centers. Am J Hematol 2018;93(6):729-35.
[Crossref] [Google Scholar] [PubMed]
- Saleh K, Danu A, Koscielny S, Legoupil C, Pilorge S, Castilla-Llorente C, et al. A retrospective, matched paired analysis comparing bendamustine containing Be-EAM vs. BEAM conditioning regimen: Results from a single center experience. Leuk Lymphoma. 2018;59(11):2580-7.
[Crossref] [Google Scholar] [PubMed]
- Hahn L, Lim H, Dusyk T, Sabry W, Elemary M, Stakiw J, Danyluk P, Bosch M. Be-EAM conditioning regimen is a safe, efficacious and economical alternative to BEAM chemotherapy. Sci Rep 2021;11(1):1-9.
 Be-EAM
Be-EAM



