- *Corresponding Author:
- Feng Yuan
Department of Orthopaedics, Shanghai Kaiyuan Orthopedic Hospital, Pudong, Shanghai 200120, China
E-mail: zhoulin5288@hotmail.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “1-7” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Chondrocyte dysfunction and apoptosis are the two critical features during the progression of osteoarthritis. Corilagin, a gallotannin, is isolated from Caesalpinia coriaria (Jacq.). It was reported to possess anti-inflammatory, anti-tumor and hepatoprotective activities. However, this study aims to investigate the role of corilagin in osteoarthritis and the potential mechanism of action. Primary rat chondrocytes were treated with interleukin-1 beta and various concentrations of corilagin. Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling staining, reverse transcription quantitative polymerase chain reaction, Western blot and immunofluorescence staining evaluated apoptotic levels, catabolic metabolism and relative signaling pathways. Corilagin suppressed chondrocyte cell viability reduction, lactate dehydrogenase, and inflammatory cytokine release and apoptosis triggered by interleukin-1 beta. Corilagin inhibited the interleukin-1 beta induced reduction in extracellular matrix protein collagen II and collagen type II alpha-1 and aggrecan messenger ribonucleic acid expression. Corilagin also enhanced protein expression of collagen II, ADAM metallopeptidase with thrombospondin type 1 motif 5, and sirtuin 1 and attenuated the nuclear expression of nuclear factor kappa B p65 in interleukin-1 beta treated chondrocytes. Corilagin attenuates interleukin-1 beta induce inflammatory response, apoptosis and catabolism in rat chondrocytes, and the mechanism might be associated with the activation of the sirtuin 1 nuclear factor kappa B pathway. Our results speculate that corilagin could be a potential therapeutic agent in treating osteoarthritis.
Keywords
Osteoarthritis, chondrocytes, corilagin, interleukin-1 beta, nuclear factor kappa B
Osteoarthritis (OA) is one of the main chronic joint diseases that impact and disable 100 million people globally, especially the elderly population[1]. OA is characterized by immutable cartilage degradation, tidemark disrupting, associated with angiogenesis and cartilage calcification, remodeling of the subchondral bone, osteophyte formation, and the synovial lining mild-to-moderate inflammation[2-4]. The major risk factors of OA including age, early joint injury, muscle atrophy, obesity, mechanical stress and metabolic disorders[5,6]. The disease progression is typically slow and takes a long time to symptomized resulting in stiffness, joint pain, and movement difficulties, consequently a low quality of life. Despite the massive impact of OA’s personal and social burden, there are no such potential medications available, and some conventional therapies relieve the symptoms for a relatively short term[7,8].
The pathological process of OA characterizes the provoked articular cartilage degradation and other joint constituents such as ligaments, muscles, subchondral bone and synovium. In the normal cartilage, a very low turnover observe in the chondrocyte, which secures the metabolic balance of the Extracellular Matrix (ECM)[9]. The chondrocytes molecular events including, autophagy cell death, apoptosis and necrosis, are relatively associated with the pathological process of OA[10,11]. The previous studies reported that the chondrocytes death process is initiated by the degradation of ECM, mechanical stress, oxidative stress or inflammation[12,13]. Several pro-inflammatory cytokines levels such as Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL)-6, and IL-1 Beta (β) are potentially upregulated of OA cartilage and intercede cartilage degeneration[14]. Recently reported that IL-1β is widely using to study the OA's pathophysiology[15]. Therefore, regulation of the levels of pro-inflammatory cytokine IL-1β could be a potential target of OA treatment.
Nowadays, the use of plant-derived natural agents is increased due to their therapeutic value for bone health, which is distinguishable in chondroprotective and osteoprotective activities[16,17]. Several of these natural agents have been reported to possess anti-proinflammatory and antioxidant activities, inhibit catabolic effects on chondrocytes, and inhibiting effects on osteoclast degeneration[18-20]. Corilagin (C27H22O18) was first derived from divi-divi (Caesalpinia coriaria (Jacq.) Willd.) in 1951[21]. In the early decades, corilagin has been reported to have different pharmacological properties such as antioxidant, hepatoprotective, anti-inflammatory and anti-tumor[22-24]. Recently, corilagin has been drawn much attention to its anti-inflammatory activities. However, we speculated that corilagin might have chondroprotective and osteoprotective activities on OA disease. To investigate this hypothesis, we conducted the present study to test the potential osteoprotective properties of corilagin.
Materials and Methods
Animal study:
Adult primary rats Sprague Dawley (SD) (n=48; 180-200 g, grade Specific-Pathogen Free (SPF)) were purchased from SLRC laboratory animal center (Shanghai, China). The procedure on animal experiments was examined and approved by the ethics committee of animal care and use of our hospital. Chondrocytes were separated from the knee cartilage of SD rats (3 d-5 d). Cartilage was rinsed with Phosphate-Buffered Saline (PBS) solution, incubated with 0.2 % collagenase (Sigma, C6885) for 1 h. Cartilage was finely cut into pieces of about 1 mm[3], filtered through a 70 mm cell strainer. Cells were cultured in high glucose Dulbecco's Modified Eagle Medium (DMEM) with 10 % Fetal Bovine Serum (FBS) (Gibco) at 37° and 5 % Carbon dioxide (CO2) atmosphere. The medium was replaced at 48 h intervals.
Cell viability:
Cell viability was evaluated by 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, primary rat articular chondrocytes (2×105 cells/well) were seeded into the 96 wells plate and growing for 24 h, treating various concentrations of corilagin (0, 10, 20, 50 100, 200 µM). Or pretreated with corilagin (0, 20, 50 µM) for 2 h, then incubated with recombinant rat IL-1β (10 ng/ml, 501-RL, R & D systems) for further 24 h. Then MTT solution was added to the cells and incubated for 4 h, and a microplate reader measured absorbance values of each well at 570 nm.
Lactate Dehydrogenase (LDH) release assay:
LDH release assay was used to evaluate the cytotoxicity. Chondrocytes were pretreated with corilagin (0, 20, 50 µM) for 2 h, then incubated with IL-1β (10 ng/ml) for further 24 h. Culture supernatant (100 μl) were collected from each well and incubated with an LDH assay kit (Jiancheng, Nanjing, China) for 20 min at 37°. The LDH activity was determined by measuring the absorbance values of each well at 570 nm using a microplate reader.
Measurement of inflammatory cytokines:
Corilagin treated supernatant of chondrocytes were collected, and TNF-α (RTA00, R & D systems) and IL-6 (R6000B, R & D systems) concentrations were determined by Enzyme-Linked Immunosorbent Assays (ELISA). A microplate reader was used to measure the absorbance values of each well at 450 nm. Standard curves for TNF-α and IL-6 were plotted to determine cytokine concentrations, expressed as pg/ml.
Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) staining:
Chondrocytes were incubated with corilagin (0, 20, 50 µM) and IL-1β for 24 h. Then cells were fixed in 4 % paraformaldehyde and permeabilized using 0.3 % Triton X-100. Then chondrocytes were stained with a TUNEL reaction mixture and treated with 4′,6-Diamidino-2-Phenylindole (DAPI). Cells were evaluated and photographed under a fluorescence microscope (IX71, Olympus, Japan) (200×magnification). The number of TUNEL-stained cells and DAPI+cells were counted, and the apoptosis rate was calculated as TUNEL-stained cells normalized to DAPI+cells.
Caspase-3 activity:
Cells were pretreated with corilagin for 2 h and then incubated with IL-1β for 24 h. Chondrocytes were collected and lysed by cell lysis buffer, incubated with 10 μl of caspase-3 substrate (Ac-DEVD-pNA, Cat. No: C1115; Beyotime, Shanghai, China) for 1 h at 37°. Then the absorbance at 405 nm (excitation at 380 nm) was measured using a microplate reader. The caspase-3 activity was normalized with that of controls.
Immunofluorescence:
Chondrocytes were seeded on coverslips in 6-well culture plates for various treatments. After washing with PBS 3 times, chondrocytes were fixed with 4 % paraformaldehyde (pH 7.4) and blocked with 0.1 % Triton X-100. The chondrocytes were treated with a primary antibody against collagen II (sc-52658, Santa Cruz) overnight at 4°, with a specific secondary antibody for 1 h. Then chondrocytes were incubated with DAPI (10 mg/ml) for 5 min to stain nuclei. Cultures were mounted on glass slides, evaluated and photographed under a fluorescence microscope (IX71, Olympus, Japan) (400× magnification).
Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR) analysis:
Total Ribonucleic Acid (RNA) was extracted from cultured cells using TRIzol reagent (Invitrogen), and 1 μg RNA was reversely transcribed into complementary Deoxyribonucleic Acid (cDNA) by a reverse-transcription kit (TaKaRa, Kusatsu, Japan). The synthesized cDNA was amplified using SYBR Green PCR Master Mix (TaKaRa Company, Kusatsu, Japan). The following thermal cycling conditions were pre-denaturation at 95° for 30 s, followed by 40 cycles at 95° for 10 s and annealing at 58° for 20 s. The primers were as follows; Collagen Type II Alpha 1 Chain (COL2A1): forward 5′-CCC CTG CAG TAC ATG CGG-3′; reverse 5′-CTC GAC GTC ATG CTG TCT CAA G-3′; aggrecan, forward 5′-TAA ACC CGG TGT GAG AAC CG-3′; reverse 5′-CCT GGG TGA CAA TCC AGT CC-3′; Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), forward 5′-GGA ATC CAC TGG CGT CTT CA-3′; reverse 5′-GGT TCA CGC CCA TCA CAA AC-3′. GAPDH was used as the internal control. Relative expression was evaluated using the 2-ΔΔCt method.
Western blot:
Total protein was extracted by the Radioimmunoprecipitation Assay (RIPA) lysis buffer (Beyotime Biotechnology, Shanghai, China), and Nuclear Factor Kappa B (NF-κB p65) protein was extracted using nuclear extraction reagents (Pierce Biotechnology, Inc., Rockford, Illinois United States of America (USA)). After quantification, protein samples (50 μg) were separated on Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transfected onto Polyvinylidene Difluoride (PVDF) membrane. After blocking with 5 % Bovine Serum Albumin (BSA), the membrane was incubated with the primary antibodies of Bcl-2-associated X Protein (BAX) (1:500, ab182734, Abcam), B-cell lymphoma 2 (Bcl-2) (1:500, ab194583, Abcam), collagen II (1:200, sc-52658, Santa Cruz), ADAM Metallopeptidase with Thrombospondin Type 1 Motif 5 (ADAMTS5) (1:500, ab41037, Abcam), Sirtuin 1 (SIRT1) (1:500, ab189494, Abcam), NF-κB p65 (1:400, sc-8008, Santa Cruz), β-actin (1:2000, ab8226, Abcam), and PNCA (1:1000, ab18197, Abcam) overnight at 4°. The cell membrane was then incubated with a Horseradish Peroxidase (HRP) conjugated secondary antibody (1:5000). Finally, the blots were visualized by an Enhanced Chemiluminescence (ECL) detection system (Millipore), and the intensity of the bands was quantified using densitometry scans and analyzed by ImageJ software.
Statistical analysis:
All data were expressed as means±Standard Deviation (SD) from three independent experiments and analyzed by Statistical Package for the Social Sciences (SPSS) 20.0 statistical software (SPSS, Inc., Chicago, Illinois, USA). One-way Analysis of Variance (ANOVA) was conducted to compare the differences between three or more groups, followed by Dunnett’s post-hoc multiple comparison test. p<0.05 was considered as the statistical significance.
Results and Discussion
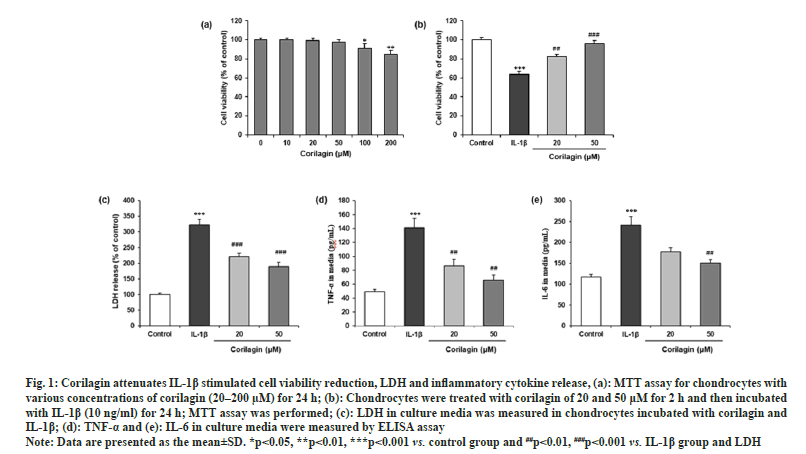
The possible cytotoxic effects of corilagin on the chondrocyte cells were evaluated with incubation of different concentrations of corilagin for 24 h. The MTT assay showed no cytotoxic effects on chondrocytes at 10, 20 and 50 μM. Thus, corilagin had no negative effects on chondrocytes viability within this range (fig. 1a). Moreover, the MTT assay revealed that IL-1β (10 ng/ml) significantly reduced cell viability, which was significantly attenuated by corilagin at 20 µM and 50 µM (fig. 1b). However, corilagin also suppressed the IL-1β stimulated LDH release (p<0.05) as shown in fig. 1c.
Fig. 1: Corilagin attenuates IL-1β stimulated cell viability reduction, LDH and inflammatory cytokine release, (a): MTT assay for chondrocytes with
various concentrations of corilagin (20–200 μM) for 24 h; (b): Chondrocytes were treated with corilagin of 20 and 50 μM for 2 h and then incubated
with IL-1β (10 ng/ml) for 24 h; MTT assay was performed; (c): LDH in culture media was measured in chondrocytes incubated with corilagin and
IL-1β; (d): TNF-α and (e): IL-6 in culture media were measured by ELISA assay.
Note: Data are presented as the mean±SD. *p<0.05, **p<0.01, ***p<0.001 vs. control group and ##p<0.01, ###p<0.001 vs. IL-1β group and LDH.
The IL-1β stimulation significantly increased the expression of TNF-α and IL-6 in culture media of chondrocytes compared to the control. The effect of corilagin on the secretion levels of TNF-α and IL-6 were assessed by ELISA. Chondrocytes were co-treated with corilagin showed statistically significant (p<0.05) decreases compared treated with IL-1β alone as shown in fig. 1d and fig. 1e.
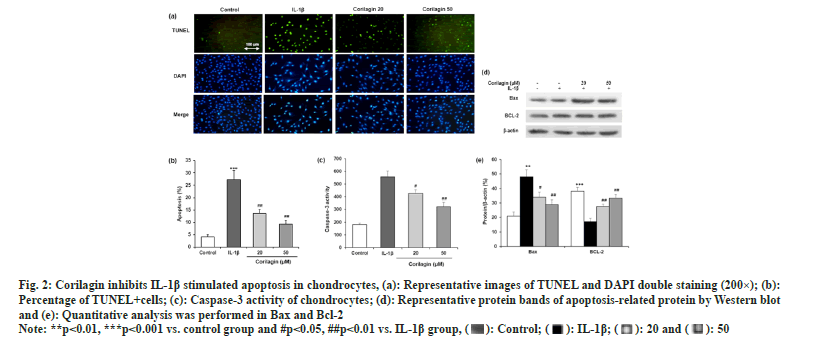
In TUNEL and DAPI double staining, apoptotic cells showed the green-stained nuclei (fig. 2a). The number of cells with green TUNEL staining was reduced in corilagin groups compared to the IL-1β group (fig. 2b). The colorimetric method was carried out to detect the activity of caspase-3, a biomarker of apoptosis. Corilagin significantly reversed the IL-1β induced increase in caspase-3 activity (fig. 2c). In addition, Western blotting was used to determine two apoptosis-related proteins, BAX and Bcl-2 (fig. 2d). Corilagin suppressed apoptosis of chondrocytes by reducing BAX expression and increasing Bcl-2 expression in cells with IL-1β incubation as shown in fig. 2e.
Fig. 2: Corilagin inhibits IL-1β stimulated apoptosis in chondrocytes, (a): Representative images of TUNEL and DAPI double staining (200×); (b):
Percentage of TUNEL+cells; (c): Caspase-3 activity of chondrocytes; (d): Representative protein bands of apoptosis-related protein by Western blot
and (e): Quantitative analysis was performed in Bax and Bcl-2.
Note: **p<0.01, ***p<0.001 vs. control group and #p<0.05, ##p<0.01 vs. IL-1β group,  .
.
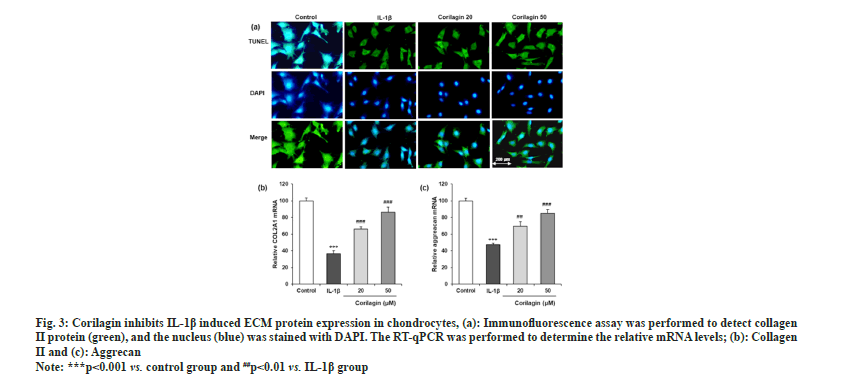
An immunofluorescence assay revealed IL-1β decreased collagen II-positive staining in chondrocytes, and this effect was markedly attenuated by corilagin (fig. 3a). RT-qPCR also showed that IL-1β upregulated the mRNA levels of COL2A1 (collagen II) and aggrecan, and these changes were reversed by corilagin as shown in fig. 3b and fig. 3c.
Fig. 3: Corilagin inhibits IL-1β induced ECM protein expression in chondrocytes, (a): Immunofluorescence assay was performed to detect collagen
II protein (green), and the nucleus (blue) was stained with DAPI. The RT-qPCR was performed to determine the relative mRNA levels; (b): Collagen
II and (c): Aggrecan.
Note: ***p<0.001 vs. control group and ##p<0.01 vs. IL-1β group.
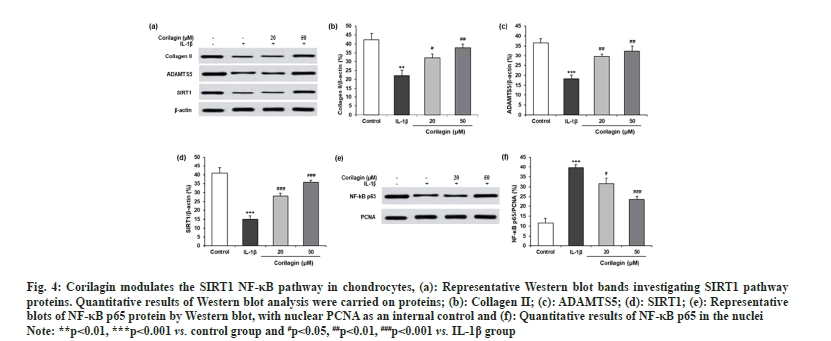
To evaluate the detailed mechanisms involved in the protective effects of corilagin on chondrocyte injury, we next measured protein levels of ECM-related proteins and SIRT1 (fig. 4a). IL-1β increased the protein expression of collagen II (fig. 4b), ADAMTS5 (fig. 4c), and SIRT1 (fig. 4d), where reversed effect was observed for corilagin. In chondrocytes with IL-1β, corilagin decreased the nuclear expression of NF-κB p65 as shown in fig. 4e and fig. 4f.
Fig. 4: Corilagin modulates the SIRT1 NF-κB pathway in chondrocytes, (a): Representative Western blot bands investigating SIRT1 pathway
proteins. Quantitative results of Western blot analysis were carried on proteins; (b): Collagen II; (c): ADAMTS5; (d): SIRT1; (e): Representative
blots of NF-κB p65 protein by Western blot, with nuclear PCNA as an internal control and (f): Quantitative results of NF-κB p65 in the nuclei.
Note: **p<0.01, ***p<0.001 vs. control group and #p<0.05, ##p<0.01, ###p<0.001 vs. IL-1β group.
In this study, we used primary rat chondrocytes treated with IL-1β and various concentrations of corilagin to investigate the potential effect of Corilagin on OA. Our data suggest that corilagin significantly reduced the IL-1β induce inflammatory response, apoptosis and catabolism in rat chondrocytes by the activation of the SIRT1-NF-κB pathway under OA.
Corilagin is a natural tannin family member discovered in a wide range of medicinal plants, including Phyllanthus species[24]. Several study results showed that the potential efficacy of corilagin against cancer and neurodegenerative diseases[25,26]. Recently, corilagin has drawn much attention to its anti-inflammatory activities. The early study reported the effectiveness of different corilagin doses on chronic liver disease in rats, and up to 1000 µM did not show any adverse effects on Huh7 hepatoma cells during cell viability assay. In between 10 µM and 200 µM, corilagin doses potentially reduced cell death in the infectious cell[27]. Our corilagin 20 µM and 50 µM doses showed a potential effect to suppress the IL-1β stimulated LDH release and did not observe any toxicity on chondrocytes during an in vitro cell viability assay, suggesting corilagin could significantly affect OA.
For the development of OA, the pro-inflammatory cytokines IL-6, IL-8, IL-1β and TNF-α plays an important role and their attenuation is the main treatment strategy in OA[28]. Three major pro-inflammatory cytokines, IL-6, IL-1β, and TNF-α, were implicated, and the results showed to stimulate chondrocytes. Thus, the resulted in apoptosis and enhanced the expression of inflammatory factors (Cyclooxygenase-2 (COX-2) and Metalloproteinase (MMP)), which are important to OA's starting and progression[29,30]. The recruitment of IL-1β and TNF-α death-related compounds by the combinations with specific ligands resulting the apoptotic pathway activation and consequently internucleosomal DNA fragmentation[15]. The inflammatory response pretends potential impacts on metabolic processes in the ECM, such as the inflammation-associated upregulation of MMPs leading to the matrix catabolism progression[14]. Corilagin treatment efficiently attenuated the IL-1ꞵ induced the pro-inflammatory cytokines expression.
OA is a degenerative joint disease that is characterized by dysfunction and excessive chondrocytes apoptosis. Environment and genetic factors, aging play critical roles in its pathogenesis[2,31]. The reduction of chondrocytes results in lacking some ECM proteins, a consequence of the cartilage matrix degradation[13]. Normal and OA cartilage showed increased apoptosis in human chondrocytes, associated with the cell’s inflammatory response[32,33]. The destruction of cartilage could exacerbate different intracellular signaling pathways by activation of catabolic enzymes secretions and other catabolic factors, which increase the apoptotic levels in OA chondrocytes, consequently the cartilage degradation in ECM[34,35]. We reported in our study that corilagin significantly reduced the apoptosis of chondrocytes and promoted chondrocyte function to inhibit IL-1β mediated ECM proteins in chondrocytes are therefore vital.
There are several molecular signaling pathways were reported which are involved during the OA's pathophysiology. The investigations of the mechanisms of action of OA are a hotspot of the research. The NF-kB family members are extensively expressed transcription factors that regulate immunity, cell proliferation, inflammation, apoptosis and other cellular processes[36]. Abnormal association by harmful factors could induce nuclear translocation of the NF-kB, followed by binding with specific genes and consequently triggering their transcription[37]. During the progression of OA in chondrocytes, these genes are involved with those to encode with different main catabolic enzymes such as MMP-1, MMP-2, and MMP-3, and catabolic factors, including COX-2 and NO[34]. Therefore, the NF-κB signaling pathway is essential for OA progression. Moreover, therapeutic molecules could target through the NF-κB pathway to induce the chondrocytes inflammatory response in OA. In this study, corilagin dose-dependently induced the p65 level and the degradation of SIRT1, suggesting that corilagin attenuated the NF-κB pathway.
In conclusion, corilagin inhibits the IL-1β induced inflammatory response and the levels of apoptosis in rat chondrocytes by attenuating the NF-κB pathway. Our results also suggest that corilagin mediated inhibition of ECM catabolism in OA. Thus, corilagin could be a potential therapeutic agent for the treatment of OA.
Acknowledgements:
Zhenghai Shao wrote the manuscript, performed experiments, analyzed the data, and Feng Yuan designed and supervised the study, revised the manuscript. All authors have read and approved the manuscript.
Ethical approval:
This study is based on the cell line. We have obtained a statement from the ethics committee of Shanghai Sixth People's Hospital, ruled that no formal ethics approval was required in this particular in vitro study.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wight L, Owen D, Goldbloom D, Knupp M. Pure ankle dislocation: A systematic review of the literature and estimation of incidence. Injury 2017;48(10):2027-34.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum 2012;64(6):1697.
[Crossref] [Google Scholar] [PubMed]
- Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat Rev Rheumatol 2016;12(11):632-44.
[Crossref] [Google Scholar] [PubMed]
- Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol 2012;8(11):665-73.
[Crossref] [Google Scholar] [PubMed]
- Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2018;14(11):674-81.
[Crossref] [Google Scholar] [PubMed]
- Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 2016;59(3):134-8.
[Crossref] [Google Scholar] [PubMed]
- da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017;390(10090):e21-33.
[Crossref] [Google Scholar] [PubMed]
- Spetea M. Opioid receptors and their ligands in the musculoskeletal system and relevance for pain control. Curr Pharm Des 2013;19(42):7382-90.
[Crossref] [Google Scholar] [PubMed]
- Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: Use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys 1998;350(1):61-71.
[Crossref] [Google Scholar] [PubMed]
- Kuhn K, Dlima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthr Cartil 2004;12(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Zhai X, Meng R, Li H, Li J, Jing L, Qin L, et al. miR-181a modulates chondrocyte apoptosis by targeting glycerol-3-phosphate dehydrogenase 1-like protein (GPD1L) in osteoarthritis. Med Sci Monit 2017;23:1224-31.
- Del Carlo Jr M, Loeser RF. Cell death in osteoarthritis. Curr Rheumatol Rep 2008;10(1):37-42.
[Crossref] [Google Scholar] [PubMed]
- Gao G, Ding H, Zhuang C, Fan W. Effects of hesperidin on H2O2-treated chondrocytes and cartilage in a rat osteoarthritis model. Med Sci Monit 2018;24:9177-86.
[Crossref] [Google Scholar] [PubMed]
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7(1):33-42.
[Crossref] [Google Scholar] [PubMed]
- Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res Ther 2009;11(6):165.
[Crossref] [Google Scholar] [PubMed]
- Henrotin Y, Clutterbuck AL, Allaway D, Lodwig EM, Harris P, Mathy-Hartert M, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthr Cartil 2010;18(2):141-9.
[Crossref] [Google Scholar] [PubMed]
- Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, et al. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int 2008;82(6):475-88.
[Crossref] [Google Scholar] [PubMed]
- Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res 2009;58(12):899-908.
[Crossref] [Google Scholar] [PubMed]
- Umar S, Umar K, Sarwar AH, Khan A, Ahmad N, Ahmad S, et al. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014;21(6):847-56.
[Crossref] [Google Scholar] [PubMed]
- Wu X, Li Z, Yang Z, Zheng C, Jing J, Chen Y, et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways. J Bone Miner Res 2012;27(6):1298-308.
[Crossref] [Google Scholar] [PubMed]
- Schmidt OT, Lademann R. Corilagin, ein weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung über natürliche Gerbstoffe. Justus Liebigs Annalen der Chemie 1951;571(3):232-7.
- Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa island and its tannin Corilagin. Phytomedicine 2007;14(11):755-62.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Zhang SL, Tao JY, Pang R, Jin F, Guo YJ, et al. Preliminary exploration on anti-inflammatory mechanism of corilagin (beta-1-O-galloyl-3, 6-(R)-hexahydroxydiphenoyl-D-glucose) in vitro. Int Immunopharmacol 2008;8(7):1059-64.
[Crossref] [Google Scholar] [PubMed]
- Jia L, Jin H, Zhou J, Chen L, Lu Y, Ming Y, et al. A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement Altern Med 2013;13(1):33.
[Crossref] [Google Scholar] [PubMed]
- Li X, Deng Y, Zheng Z, Huang W, Chen L, Tong Q, et al. Corilagin, a promising medicinal herbal agent. Biomed Pharmacother 2018;99:43-50.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Chen C. Corilagin prevents tert-butyl hydroperoxide-induced oxidative stress injury in cultured N9 murine microglia cells. Neurochem Int 2011;59(2):290-6.
[Crossref] [Google Scholar] [PubMed]
- Reddy BU, Mullick R, Kumar A, Sharma G, Bag P, Roy CL, et al. A natural small molecule inhibitor corilagin blocks HCV replication and modulates oxidative stress to reduce liver damage. Antiviral Res 2018;150:47-59.
[Crossref] [Google Scholar] [PubMed]
- Shen S, Guo J, Luo Y, Zhang W, Cui Y, Wang Q, et al. Functional proteomics revealed IL-1β amplifies TNF downstream protein signals in human synoviocytes in a TNF-independent manner. Biochem Biophys Res Commun 2014;450(1):538-44.
[Crossref] [Google Scholar] [PubMed]
- Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol 2011;7(1):50-6.
[Crossref] [Google Scholar] [PubMed]
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6(11):625-35.
[Crossref] [Google Scholar] [PubMed]
- Brandi ML, Gennari L, Cerinic MM, Becherini L, Falchetti A, Masi L, et al. Genetic markers of osteoarticular disorders: Facts and hopes. Arthritis Res Ther 2001;3(5):270-80.
[Crossref] [Google Scholar] [PubMed]
- Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Annal Rheum Dis 2000;59(12):959-65.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Wang L, Teng X, Chen Q, Pan C. N-methyl pyrrolidone (NMP) alleviates lipopolysaccharide (LPS)-induced inflammatory injury in articular chondrocytes. Med Sci Monit 2018;24:6480.
[Crossref] [Google Scholar] [PubMed]
- Ou Y, Tan C, An H, Jiang D, Quan Z, Tang K, et al. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med Sci Monit 2012;18(6):BR247-52.
[Crossref] [Google Scholar] [PubMed]
- Rigoglou S, Papavassiliou AG. The NF-κB signaling pathway in osteoarthritis. Int J Biochem Cell Biol 2013; 45:2580-4.
- Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009;1(4):a000034.
[Crossref] [Google Scholar] [PubMed]
- Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer 2012;12(2):121-32.
[Crossref] [Google Scholar] [PubMed]