- *Corresponding Author:
- K. J. S. Khoshnaw
Department of Basic Science,
College of Medicine,
Hawler Medical University,
Erbil,
Iraq
E-mail: karim.saleh@epu.edu.iq
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “246-254” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Echinococcus granulosus sensu lato larvae cause cystic echinococcosis, a highly contagious zoonotic illness that poses a worldwide threat to human health and development. The purpose of this study was to determine the therapeutic effectiveness of albendazole and praziquantel, each alone and in combination, in vivo and to estimate the serum levels of interleukin-2, interleukin-9 and interleukin-10 as biomarkers in evaluating the responsiveness to albendazole and praziquantel treatment in experimentally infected mice. To assess treatment efficacy in vivo, mice were intraperitoneally inoculated with viable protoscolices and then gavaged daily for 1 mo with albendazole (15 mg/kg/d) and praziquantel (25 mg/kg/d), separately and in combination (albendazole 15 mg/kg/d and praziquantel 25 mg/kg/d. The mice were euthanized on d 120 of the experiment and the number, size and location of the generated secondary hydatid cysts were investigated and serum levels of interleukin-2, interleukin-9 and interleukin-10 were estimated. The size and number of hydatid cysts were decreased upon treatment with each drug, but the decrease was significant (p<0.05) in isolates 2, 3, 4, 6, 8, 9 and 10, and 2 and 3, respectively. The most often infected organ was the liver (44.91 %), followed by a peritoneal cavity (30.3 %), subcutaneous (21.1 %), spleen (3.29 %) and lungs (0.38 %). Interleukin-2 level was significantly elevated in mice inoculating with most parasite isolates, whereas interleukin-9 and interleukin-10 levels were not altered considerably experimentally in infected and treated mice compared with untreated control. Albendazole and praziquantel markedly reduced the number and size of secondary hydatid cysts compared to the untreated control group in Echinococcus granulosus-infected mice and stimulated the production of interleukin-2, which is helpful for parasite elimination. These results indicate that albendazole and praziquantel have protoscolicidal effects and the combination of albendazole and praziquantel has significant additive effects.
Keywords
Cystic echinococcosis, protoscoleces, interleukin-2, interleukin-9, interleukin-10, albendazole, praziquantel
Cystic Echinococcosis (CE) is a chronic parasitic disease caused by the tapeworm Echinococcus granulosus sensu lato (E. granulosus s. l.) metacestode stage, which is endangering human health and social development all around the world[1,2]. Dogs and other canids serve as definitive hosts for this cestode, whereas sheep and other herbivores serve as intermediate hosts[3]. Humans can become hosts by inadvertently ingesting eggs[4]. Cysts develop in the human liver and lungs but may potentially affect other organs and tissues[5,6]. Because the disease has a long incubation time, most patients experience no symptoms and eventually develop chronic infection[7]. A hydatid cyst in the liver can be treated non-operatively or operatively. Methods of operation might be conservative or radical. Percutaneous and chemotherapeutic treatments are non-operative procedures[8]. Chemotherapy may decrease the size and tension of cysts and may result in the sterilization of cyst components in rare situations[9]. Albendazole (ABZ) and Praziquantel (PZQ) are often used to treat hydatid cysts[10]. PZQ was protoscolicidal and may be combined with ABZ to enhance effectiveness[11,12]. The World Health Organization (WHO) divided hydatid cysts in the liver into five types (CE1 to CE5) and three biological stages (active, transitional and inactive CE). However, the processes underlying self-healing (inactivation) or chronic infection maintenance are still unknown[1,2,8]. Within the E. granulosus s. l. complex, currently, ten genotypes exist (G1-G10) corresponding to different species. The host specificity, morphological and genetic traits, and pathogenicity patterns of each species vary. Sheep, Tasmanian and Buffalo strains are part of the E. granulosus (sensu stricto) complex (G1- G3). The horse strain is E. equinus (G4), while the cow strain is E. ortleppi (G5), the camel and pig strains is E. intermedius (G6-G7). The American cervid strain and the Fennoscandian cervid strain are both classified as E. canadensis (G8-G10)[3,13]. Most human isolates fall under the G1-G3 genotype. Nevertheless, all genotypes except G4 (E. equinus) have been shown to infect humans. E. granulosus, E. multilocularis, E. vogeli and E. oligarthrus are the four species of the genus Echinococcus that are now recognized and considered taxonomically valid[4]. Recent research has found two additional species, E. felidis and E. shiquicus, despite the absence of evidence on their human pathogenicity[14]. Cytokines seem to have a complicated impact on the host immune system. They vary according to the genera and species of helminths, their size and location inside the host, their metabolic products and the host species[13,15]. Based on several studies conducted in CE patients and experimental models, it is widely believed that T helper (Th) 2-type cytokines were associated with illness chronicity in active CE cysts. In contrast, Th1- type cytokines were associated with disease resistance in inactive CE cysts[1,16,17].
Th-9 cell subsets are a new form of effector Cluster of Differentiation 4 (CD4+) T cells that primarily release Interleukin (IL)-9. IL-9 is a cytokine of the Th2 type that acts on a variety of inflammatory and tissue cells and is required for parasite infections and allergy diseases[7]. The current study attempts to evaluate serum levels of IL2, IL-9 and IL-10 in response to treatment with ABZ and PZQ in experimentally induced infection in Bagg Albino (BALB)/c mice and to investigate whether it can be used as biomarkers for responsiveness to treatment.
Materials and Methods
The current study took place in the animal house laboratory at the college of pharmacy, Hawler Medical University from October 1st, 2020 to July 28th, 2021.
Protoscolices isolation:
Infected sheep’s livers with multiple hydatid cysts were obtained from a slaughterhouse in Erbil (one sample per animal) during post mortem examination. A cold box and ice were employed to minimize contamination and then brought to the animal house laboratory at the college of pharmacy within an hour. The cysts surfaces were then sterilized with 70 % methanol and cyst fluid (25 ml) was drained using a sterilized syringe and put into glassware cylinders for 30 min. The protoscolices dropped to the bottom of the cylinders and stayed there. The supernatant was removed and the protoscolices were repeatedly rinsed with Phosphate Buffered Saline (PBS), including antibiotic antimycotic solution. The viability of the protoscolices (fig. 1) was determined using 0.1 % eosin and microscopic observation. Protoscolices samples with more than 90 % viability were used[17,18].
Materials:
ABZ and PZQ were obtained from European Pharmacopoeia (EP) Ltd. Co., China, with the following catalog numbers: P2670000 and A0325100, respectively. Dimethyl Sulfoxide (DMSO) extra pure, EP, United States Pharmacopeia (USP) and antibiotic antimycotic solution purchased from sigma Aldrich (St. Louis, Missouri, United States). Mouse IL-2, IL-9 and IL-10 Enzyme-Linked Immunosorbent Assay (ELISA) kits were purchased from Sunlong Biotech Co. Ltd, China. ABZ and PZQ were diluted with 0.1 % DMSO to get the required concentration levels[19].
Animal studies:
Albino BALB/c mice (n=21; age, 6 w; weight, 20 to 25 g) were obtained from the College of Medicine- University of Sulaimani. The mice were reared and reproduced at the college of pharmacology’s animal home in a temperature-controlled (21°), light-cycled (12 h light/dark cycle) environment with 45 % to 55 % humidity. During the experiment, mice were given ad libitum with regular pellet meal and tap water in plastic cages[20]. A total of 288 male and female inbred mice were separated into 12 groups, with each group divided into four sub-groups of six mice. For each mouse, the first, second and third groupings were injected with 2000 protoscolices in 200 μl of PBS, while the fourth group was left as a negative control, injected with 0.9 % normal saline[3]. 30 d post-inoculation, all mice were treated orally (0.25 ml) by gavage with ABZ (15 mg/ kg/d), PZQ (25 mg/kg/d) and a combination of ABZ (15 mg/kg/d) and PZQ (25 mg/kg/d). The fourth mice group (positive control) was administrated with the same volume of Distilled Water (DW) containing 0.1 % DMSO. For a month, all administrations were done simultaneously every day. The mice were euthanized on d 120 of the experiment and the number, size, and location of the generated secondary hydatid cysts were investigated[19].
Blood collection:
Before scarification, blood samples were obtained from the mice through heart puncture on d 120 of the study. Before centrifugation (3000 rpm for 5 min), the blood was left to clot at room temperature for 10 min; roughly 200 μl of serum was taken from each animal and kept at -20° until used to estimate cytokines[20].
Cytokine assays:
According to the manufacturer’s recommendations, ELISA commercial kits were used to determine IL-2, IL-9 and IL-10 in mouse serum. All tests were carried out twice. IL-2 and IL-9 had 1.2 pg/ml-100 pg/ml assay range and IL-10 had 1 pg/ml-100 pg/ml assay range.
Statistical analysis:
Means and Standard Deviation (SD)[21] were used to represent the data. Student’s t-test and one-way Analysis of Variance (ANOVA) were used to examine differences based on data attributes, with the Least Significant Difference (LSD) utilized in comparative analysis. The International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) 25.0 version was used to analyze the data, p-values less than 0.05 were deemed statistically significant.
Results and Discussion
The mice were euthanized after a 1 mo treatment period, the peritoneal cavity was opened and the secondary hydatid cysts were carefully removed. The size, quantity and location of hydatid cysts were calculated in each treatment group and compared to the untreated control group. According to Table 1, compared to the positive control groups, the number of cysts emerging in mice after ABZ, PZQ and ABZ+PZQ treatment was significantly reduced (p<0.05) in isolates 2, 3, 4, 6, 8, 9 and 10. In contrast, in isolates 11 and 12, the number of secondary hydatid cysts was reduced but not significantly (p>0.05). On the other hand, only ABZ treatment was significant in isolate 1 and in isolate 5, both PZQ and ABZ+PZQ were significant. While the size decreased considerably in isolate 7 only when treated with PZQ.
| Isolates | Number of mice | Number of cysts (Mean±SD) Controls | Number of mice | Number of cysts (Mean±SD) ABZ | Number of mice | Number of cysts (Mean±SD) PZQ | Number of mice | Number of cysts (Mean±SD) combination |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 105 (17.5±9.02) | 4 | 12 (3±2.16)* | 6 | 50 (8.33±7.84) | 5 | 23 (4.6±2.607)* |
| 2 | 5 | 60(12.0±7.54) | 4 | 0 (0.03±0.05)* | 5 | 0 (0.02±0.04) * | 3 | 0 (0.03±0.05)* |
| 3 | 5 | 76 (15.2±10.94) | 4 | 0 (0.03±0.06)* | 6 | 0 (0.02±0.05)* | 6 | 0 (0.02±0.05)* |
| 4 | 5 | 100 (20±12.84) | 4 | 2 (0.5±0.01)* | 3 | 5 (1.66±0.57)* | 4 | 1 (0.25±0.01)* |
| 5 | 4 | 103 (25.75±8.95) | 4 | 60 (15±4.082) | 3 | 78 (1.67±0.58)* | 3 | 5 (0.25±0.01)* |
| 6 | 6 | 93 (15.5±10.55) | 5 | 11 (0.02±0.04)* | 4 | 19 (0.03±0.05)* | 6 | 7 (0.83±0.26)* |
| 7 | 6 | 154 (25.66±23.93) | 6 | 43 (12.67±9.16) | 6 | 21 (3.50±1.76)* | 6 | 32 (5.33±5.125) |
| 8 | 4 | 115 (28.75±12.36) | 4 | 10 (2.5±1.73)* | 5 | 24 (4.8±4.919)* | 4 | 6 (0.03±0.05)* |
| 9 | 5 | 128 (25.6±15.58) | 5 | 8 (1.6±0.894)* | 5 | 20 (4.0±2.345)* | 5 | 24 (4.8±3.701)* |
| 10 | 4 | 115 (28.75±14) | 6 | 6 (1.00±0.0)* | 5 | 8 (1.60±0.8)* | 6 | 33 (5.50±4.85)* |
| 11 | 4 | 134 (33.5±30.59) | 6 | 101 (16.83±17.6) | 6 | 30 (5.0±2.1) | 6 | 272 (45.33±17.53) |
| 12 | 4 | 238 (59.5±67.71) | 6 | 125 (20.83±18.33) | 0 | 0 (0.02±0.04) | 4 | 2 (0.50±0.58) |
Note: *Significant difference
Table 1: Treatme nt Of Mice Infected With 12 Isolates Of E. Granulosus Protoscoleces Regarding The Number Of Secondary Hydatid Cysts That Developed Over 1 MO Of Infection
As seen in Table 2, the results obtained in this study revealed statistical differences (p<0.05) between control groups and treated groups when the size of hydatid cysts was measured (diameter) in isolates 2 and 3. In contrast to the control groups, the size of secondary hydatid cysts was decreased no significantly (p>0.05) in isolates 1, 4, 5, 7, 9, 10 and 11. In isolates 8 and 12, the size of cysts was decreased significantly only in the groups treated with combination and PZQ alone, respectively. While in isolate 6, the size of cysts was reduced considerably in both ABZ and PZQ treated groups.
| Isolates | Number of cysts | Mean of cyst size (mm)±SD Controls | Number of cysts | Mean of cyst size (mm±SD ABZ | Number of cysts | Mean of cyst size (mm)±SD PZQ | Number of cysts | Mean of cyst size (mm)±SD combination |
|---|---|---|---|---|---|---|---|---|
| 1 | 105 | 4.21±19.04 | 12 | 1.33±2.01 | 50 | 1.12±3.88 | 23 | 2.10±5.52 |
| 2 | 60 | 3.78±14.49 | 0 | 0.05±0.00* | 0 | 0.05±0.00* | 0 | 0.05±0.00* |
| 3 | 76 | 3.25±15.46 | 0 | 0.05±0.00* | 0 | 0.05±0.00* | 0 | 0.05±0.00* |
| 4 | 100 | 4.24±20.83 | 2 | 0.5±0.00 | 5 | 0.5±0.00 | 1 | 4±0.02 |
| 5 | 103 | 3.43±15.43 | 60 | 1±4.21 | 78 | 2.89±21.46 | 5 | 1±1.024 |
| 6 | 93 | 2.59±22.95 | 0 | 0.05±0.00* | 0 | 0.05±0.00* | 5 | 0.5±0.12 |
| 7 | 154 | 4.13±26.33 | 76 | 1.06±5.31 | 21 | 1.47±2.731 | 32 | 1.06±2.97 |
| 8 | 115 | 4.22±23.78 | 10 | 2.4±4.76 | 24 | 1.104±2.911 | 0 | 0.05±0.00* |
| 9 | 128 | 4.35±24.43 | 8 | 2.37±3.42 | 20 | 4.15±10.02 | 24 | 3.31±8.92 |
| 10 | 115 | 4.54±25.75 | 6 | 1.83±0.75 | 8 | 0.5±0.462 | 33 | 1.16±3.09 |
| 11 | 134 | 4.08±29.25 | 101 | 1.60±7.80 | 30 | 2.45±5.65 | 272 | 1.59±11.24 |
| 12 | 238 | 2.78±26.40 | 125 | 1.25±6.97 | 0 | 0.05±0.00* | 2 | 1.05±0.04 |
Note: *Significant difference
Table 2: Treatment of Mice Infected with 12 isolates of E. Granulosus Protoscoleces Regarding the Size of Secondary Hydatid Cysts that Developed over 1 MO of Infection
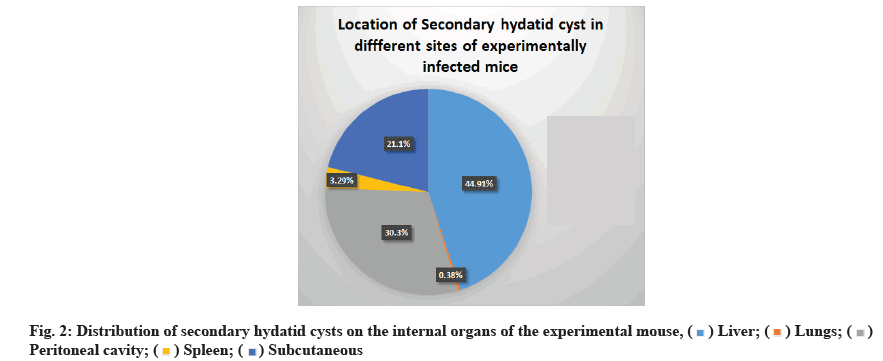
As shown in fig. 2, the location of developing hydatid cysts on the internal organs of mice treated with ABZ (15 mg/kg), PZQ (25 mg/kg) and ABZ+PZQ (15 mg/ kg+25 mg/kg) was variable. However, the liver was the most common site of infection (44.91 %), followed by a peritoneal cavity (30.3 %), subcutaneous (21.1 %), spleen (3.29 %) and lungs (0.38 %).
The serum level of IL-2 in mice was shown in Table 3. The mean concentration of IL-2 in isolates 1, 2, 3 and 4 for ABZ, PZQ and ABZ+ PZQ were considerably (p<0.05) higher in comparison to the control groups (fig. 2). Conversely, all other isolates (5, 6, 7, 8, 9, 10, 11 and 12) indicated no significant variations (p>0.05) when compared to comparable control groups.
| Isolates | Negative control (Mean±SD) | ABZ (15 mg/kg) (Mean±SD) | PZQ (25 mg/kg) (Mean±SD) | Combination (15+25 mg/kg) (Mean±SD) |
|---|---|---|---|---|
| 1 | 113.89±10.5 | (147.64±1.18)* | (149.74±1.78)* | (149.32±8.31)* |
| 2 | 113.89±10.5 | (147.85±15.15)* | (147.85±2.07)* | (73.9±2.07)* |
| 3 | 113.89±10.5 | (140.71±5.64)* | (144.7±20.79)* | (158.36±0.29)* |
| 4 | 113.89±10.5 | (156.05±10.1)* | (153.31±8.02)* | (144.7±4.75)* |
| 5 | 113.89±10.5 | (139.45±4.45) | (128.1±36.54) | (139.24±21.98) |
| 6 | 113.89±10.5 | (131.05±4.45) | (132.1±10.1) | (128.52±16.93) |
| 7 | 113.89±10.5 | (125.37±23.76) | (95.54±5.94) | (122.01±13.66) |
| 8 | 113.89±10.5 | (112.77±12.47) | (124.32±8.02) | (115.71±2.37) |
| 9 | 113.89±10.5 | (116.13±6.3) | (90.5±4.2) | (116.34±12.81) |
| 10 | 113.89±10.5 | (151±0.59) | (134.2±39.21) | (120.12±2.07) |
| 11 | 113.89±10.5 | (121.38±0.29) | (124.01±5.49) | (102.16±0.74) |
| 12 | 113.89±10.5 | (122.12±1.932) | (120.12±5.05) | (126.42±1) |
Note: *Significant difference
Table 3: Concentration of Serum IL-2 In Mice Following Treatment With ABZ, PZQ And ALZ+PZQ Among Isolates
The serum level of IL-9 in the study mice groups was shown in Table 4 for all isolates. The mean concentration of IL-9 in isolates 1 was significantly (p<0.05) decreased in mice treated with ABZ+PZQ (Table 4). Conversely, all other isolates (2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12) revealed no statistically significant (p>0.05) differences compared with relevant control groups.
| Isolates | Negative control (Mean±SD) | ABZ (15 mg/kg) (Mean±SD) | PZQ (25 mg/kg) (Mean±SD) | Combination (15+25 mg/kg) (Mean±SD) |
|---|---|---|---|---|
| 1 | 143.28±24.34 | 135.93±0.73 | 137.49±5.4 | 35.93±2.7* |
| 2 | 143.28±24.34 | 134.02±0.49 | 182.45±55.24 | 120.65±8.59 |
| 3 | 143.28±24.34 | 152.42±20.62 | 146.34±1.71 | 145.82±21.11 |
| 4 | 143.28±24.34 | 167.18±9.08 | 146.34±15.95 | 148.43±10.55 |
| 5 | 143.28±24.34 | 142.87±10.06 | 176.72±7.85 | 162.49±20.62 |
| 6 | 143.28±24.34 | 154.5±3.928 | 150.86±9.08 | 161.1±8.83 |
| 7 | 143.28±24.34 | 163.53±26.02 | 136.79±1.96 | 149.29±4.91 |
| 8 | 143.28±24.34 | 162.31±5.64 | 151.55±27.25 | 155.37±0.73 |
| 9 | 143.28±24.34 | 139.05±5.64 | 163.18±10.31 | 148.95±11.29 |
| 10 | 143.28±24.34 | 136.27±15.95 | 152.59±10.55 | 164.75±7.61 |
| 11 | 143.28±24.34 | 143.39±14.24 | 169.09±18.16 | 152.94±0.73 |
| 12 | 143.28±24.34 | 139.66±8.22 | 140.35±9.94 | 153.11±17.67 |
Note: *Significant difference
Table 4: Concentration of Serum IL-9 In Mice Following Treatment With ABZ, PZQ and ALZ+PZQ Among Isolates
Table 5 shows the serum level of IL-10 in the studied mice. The mean concentration of IL-10 in isolates 3 and 9 revealed a significant (p<0.05) difference when treated with ABZ and ABZ+PZQ, respectively. Conversely, no significant (p>0.05) difference was found between the other isolates (2, 4, 5, 6, 7, 8, 10, 11 and 12) and relevant control groups.
| Isolates | Negative control (Mean±SD) | ABZ (15 mg/kg) (Mean±SD) | PZQ (25 mg/kg) (Mean±SD) | Combination (15+25 mg/kg) (Mean±SD) |
|---|---|---|---|---|
| 1 | 83.43±18.75 | 125.63±12.22 | 170.93±69.38 | 109.09±1.06 |
| 2 | 83.43±18.75 | 79.96±18.34 | 99.51±9.83 | 99.32±46.25 |
| 3 | 83.43±18.75 | 105.33±26.58 | 88.04±14.35 | 126.01±77.09 |
| 4 | 83.43±18.75 | 117.18±26.84 | 91.24±13.55 | 447.81±468.92 |
| 5 | 83.43±18.75 | 126.01±36.15 | 100.82±16.48 | 78.83±0.26 |
| 6 | 83.43±18.75 | 104.39±7.7 | 123.38±33.49 | 99.13±8.24 |
| 7 | 83.43±18.75 | 91.24±30.57 | 82.03±30.3 | 99.69±10.1 |
| 8 | 83.43±18.75 | 94.81±5.31 | 119.43±55.55 | 96.5±12.49 |
| 9 | 83.43±18.75 | 83.53±27.64 | 119.06±24.72 | 94.81±10.63 |
| 10 | 83.43±18.75 | 105.9±2.39 | 99.51±0.26 | 121.69±15.68 |
| 11 | 83.43±18.75 | 104.58±1.06 | 95.18±15.41 | 99.88±0.26 |
| 12 | 83.43±18.75 | 105.71±38.81 | 106.46±5.84 | 102.89±16.74 |
Note: *Significant difference
Table 5: Concentration of Serum IL-10 In Mice After Treatment With ABZ, PZQ and ALZ+PZQ Among 12 Different Isolates
Identifying markers indicating treatment efficacy is one of the most pressing issues in CE patients after surgical or pharmacological therapy immunosurveillance. In the pharmacological therapy of CE, the frequent recurrence of illness after an initial treatment success is a significant issue[22]. This study aimed to determine the serum levels of IL2, IL-9 and IL10 in response to treatment with ABZ and PZQ in experimentally induced infection in BALB/c mice.
Generally, benzimidazole derivatives (mebendazole and ABZ) and PZQ are used to treat hydatid cysts[10]. ABZ and mebendazole may, in certain situations, reduce the size of cysts and result in cyst component sterilization[23]. ABZ is more effective when used with PZQ[24,25].
In the present study, the number of secondary hydatid cysts arising in mice after ABZ, PZQ and ABZ+PZQ treatment was significantly reduced (p<0.05) in isolates 2, 3, 4, 6, 8, 9 and 10 when compared to the untreated control group. These findings support previous research[26]. In contrast, the number of secondary hydatid cysts was reduced in isolates 11 and 12, but not significantly (p>0.05). These results are inconsistent with the previous studies[19,27-29]. In our results, the size of hydatid cysts was decreased in all isolates upon treatment with each drug. These results agree with the previous studies[27,28]. However, the decrease was significant (p<0.05) in isolates 1 and 2 compared with the untreated control group that was consistent with previously published data[26].
In previous studies, secondary hydatid cysts were identified in the livers of BALB/c and abdominal cavities of Naval Medical Research Institute NMRI mice 4 mo following protoscolices injection[30,31]. In the present study, the location of developing secondary hydatid cysts on the internal organs of mice treated with ABZ, PZQ and ABZ+PZQ was variable. However, the liver was the most often infected organ (44.91 %), followed by a peritoneal cavity (30.3 %), subcutaneous (21.1 %), spleen (3.29 %) and lung (0.38 %). This result agrees with the previously published data[28].
In the current study, response to treatment with ABZ, PZQ and both in combination was variable in mice groups harboring secondary hydatid cysts from different parasite isolates. Cyst development was not seen in mice inoculated with protoscolices from isolates 1, 2, 6 upon treatment with ABZ and PZQ separately, isolate 8 when both drugs were administered in combination (ABZ+PZQ) and isolate 12 when treated with PZQ. Such inconsistent response to treatment might be due to host and parasite factors such as the strain of E. granulosus[32]. The cyst’s age, the patient’s age, the cysts localization and morphological features have been indicated as factors influencing the therapeutic results[33].
Trying to find biochemical markers that can monitor the progression of hydatid disease, some studies explored the correlation between cytokine levels in the serum and disease outcome and infection stage. Th2 and Th1 serum cytokine levels were increased in CE patients through the aggressive phase of the disease before treatment, according to Naik et al.[22]. However, IL-4 and IL-10 levels decreased significantly 2 y after chemotherapy. An experimental study found that the Th1 cytokine profile was predominant in the early postinfection phase (3-4 w), then shifted to the Th2 cytokine profile in the 4th w[34]. Furthermore, Torcal et al.[35] found a link between IL-1, IL-2 and IL-4 levels and cysts number, characteristics and location in the liver. Touil-Boukoffa et al.[36] proposed that serum cytokine levels measurement after surgical removal of hydatid cysts could allow early relapse detection.
In the mouse model with E. granulosus infection, an initial elevation in the secretion level of Th1 cytokines was fatal for the parasite. Then, the Th1 response was progressively shifted towards the Th2 response, resulting in increased production of anti-inflammatory cytokines, beneficial for oncosphere survival[3,37]. Clinical investigations have also shown that successful therapy is linked to the Th1 profile. In contrast, treatment resistance is associated with increased Th2 cytokine production, suggesting that the Th1/Th2 imbalance is critical for controlling CE immunopathogenesis[38,39].
This study found a significant (p<0.05) rise in IL-2 cytokine in isolates 1, 2, 3 and 4. Previous studies have supported these findings[2,6,19,37,40-45]. Other isolates (5, 6, 7, 8, 9, 10, 11 and 12) showed no significant differences (p>0.05). These results were in agreement with previous studies[17,40]. Human subjects receiving treatment have a higher Th1 cytokine profile than Th2 subjects[46]. The host immune system may be exposed to various antigens during therapy, such as antigens 5 and B, and antigen E and tegumental antigen (Teg)[3,47]. This may assist in explaining why our study’s cytokine expression was so variable. Additionally, host genetic variables may be implicated[48].
According to Li et al.[41], patients with active cysts had higher Th1 cytokines before treatment than normal controls; despite this, no noticeable change from Th1 to Th2 response was detected in patients with inactive cysts after therapy.
According to our findings, the serum cytokine IL-9 is significantly (p<0.05) reduced in mice treated with ABZ+PZQ in isolate 1. On the other hand, none of the other isolates (2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12) demonstrated a statistically significant difference (p>0.05) when compared to the untreated control group. These findings agree with a previous study[40].
Other ex vivo experiments that found cytokines in CE patient’s serum supported the link between the production of cytokines and the prognosis of the illness. Rigano et al.[49] found higher serum levels of IL-4 and IL-10 in patients who did not respond to therapy than those who did; Bayraktar et al.[15] discovered higher serum levels of IL-2, IL-4 and IL-10 in CE patients before treatment compared to those who were treated and healthy controls.
The patient’s age, chemotherapy schedule, prior surgery, cyst location, metacestode integrity, cyst form and age, and parasite strain may impact the immune response and account for the widely different clinical presentation of illness[46].
In the present study, the mean concentration of IL-10 in isolates 1 and 10 revealed a significant (p<0.05) difference when treated with PZQ and ABZ+PZQ, respectively. These findings are consistent with previous researches[17,37,46]. In contrast, no significant (p>0.05) difference was found between all other isolates (2, 4, 5, 6, 7, 8, 9, 11 and 12) and relevant control groups, and this might be due to cytokines are not specific for a particular disease, as they are produced in every Th1 or Th2-mediated inflammatory. These results agree with previously published data[40,49,50]. It’s known that the immune response to hydatid cysts in intermediate hosts is complicated and contradictory. The Th1/Th2 cytokine imbalance plays a significant role in promoting the disease’s immunopathogenesis change through Th1 and Th2 cytokines[38].
The cytokine response may be contradictory due to different T cell activation. It may also be related to the study design, time frame and classification of cysts. Recent research discovered no significant variations in serum concentrations of Th1 and Th2 cytokines between individuals with liver cysts at various stages (CE1-CE5), revealing several limitations in using cytokines as biomarkers of hydatid cyst biological activity[27]. According to our results, we believe that longitudinal monitoring in a time frame that allows for observation of changes in cyst development and the host’s immune response after the intervention may be the most valuable use of cytokine as a marker.
ABZ and PZQ reduced the number and size of secondary hydatid cysts markedly compared to the untreated control group in infected mice. They stimulated the release of IL-2, which is beneficial to parasite clearance and decreases IL-9 and IL-10 cytokines. Overall, high IL-2 production with low IL-9 and IL-10 production in complete responders and enhanced IL-9 and IL-10 production with low IL-2 output in non-responders suggest Th-l cell activation in protective immunity and Th2/Th9 cell activation in susceptibility to hydatid disease. Such variable response to treatment and varying cytokine expression levels observed in mice experimentally infected with different local isolates of E. granulosus protoscoleces necessitate further investigations to identify the local strain of E. granulosus impact on the immunological and chemotherapeutical response in both human and animal hydatidosis.
Ethical consideration:
The operations and management of the animals were done following the World Medical Association statement on animal use in biomedical research (https://www.wma.net/policies-post/wma-statementon- animal-use-in-biomedical-research). The protocols authorized by the research ethics committee in the College of Medicine, Hawler Medical University (meeting code: 6, paper code: 11, Date: 23-11-2020).
Acknowledgements:
We would like to thank Mr. Halmat Hamza and the meat inspection team at the Erbil slaughterhouse for assisting us in obtaining hydatid cyst samples from infected killed sheep.
Conflict of interests:
The authors declared no conflict of interest.
References
- Li Z, Zhang C, Li L, Bi X, Yang S, Zhang N, et al. The local immune response during Echinococcus granulosus growth in a quantitative hepatic experimental model. Sci Rep 2019 3;9(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang J, Yang Q, Li Z, Xu X, Chen C, et al. Synergism therapeutic and immunoregulatory effects of albendazole+rAd-mIL-28B against echinococcosis in experiment-infected mice with protoscoleces. PLoS Negl Trop Dis 2021;15(11):e0009927.
[Crossref] [Google Scholar] [PubMed]
- Tamarozzi F, Mariconti M, Neumayr A, Brunetti E. The intermediate host immune response in cystic echinococcosis. Parasite Immunol 2016;38(3):170-81.
[Crossref] [Google Scholar] [PubMed]
- Regassa B. Review on hydatidosis in small ruminant and its economic and public health significance. J Dairy Vet Sci 2019;11(2):1-8.
- Nazligul Y, Kucukazman M, Akbulut S. Role of chemotherapeutic agents in the management of cystic echinococcosis. Int Surg 2015;100(1):112-4.
[Crossref] [Google Scholar] [PubMed]
- Yasen A, Li W, Aini A, Ran B, Jiang T, Shao Y, et al. Th1/Th2/Th17 cytokine profile in hepatic cystic echinococcosis patients with different cyst stages. Parasite Immunol 2021;43(7):e12839.
[Crossref] [Google Scholar] [PubMed]
- Pang N, Zhang F, Ma X, Zhang Z, Zhao H, Xin Y, et al. Th9/IL-9 profile in human echinococcosis: Their involvement in immune response during infection by Echinococcus granulosus. Mediators Inflamm 2014.
- Shams-Ul-Bari SH, Malik AA, Khaja AR, Dass TA, Naikoo ZA. Role of albendazole in the management of hydatid cyst liver. Saudi J Gastroenterol 2011;17(5):343.
[Crossref] [Google Scholar] [PubMed]
- Kandil A, Keles AG, Balci H, Tansel CD. The effects of nitric oxide and inhibitor, and combination of albendazole and praziquantel on liver in mice injected with Echinococcus granulosus larvae. Acta Trop 2021;219:105917.
[Crossref] [Google Scholar] [PubMed]
- Roos MH, Kwa MS, Veenstra JG, Kooyman FN, Boersema JH. Molecular aspects of drug resistance in parasitic helminths. Pharmacol Ther 1993;60(2):331-6.
[Crossref] [Google Scholar] [PubMed]
- Polat E, Aslan M, Cakan H, Saribas S, Ipek T, Kocazeybek B. The effects of albendazole and povidone iodine for hydatid cysts protoscoleces, in vitro and in vivo. Afr J Microbiol Res 2009;3(11):743-6.
- Sharafi SM, Sefiddashti RR, Sanei B, Yousefi M, Darani HY. Scolicidal agents for protoscolices of Echinococcus granulosus hydatid cyst: Review of literature. J Res Med Sci 2017;22.
[Crossref] [Google Scholar] [PubMed]
- Dell B, Newman SJ, Purple K, Miller B, Ramsay E, Donnell R, et al. Retrospective investigation of Echinococcus canadensis emergence in translocated elk (Cervus canadensis) in Tennessee, USA and examination of canid definitive hosts. Parasit Vectors 2020;13(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, et al. Hepatic echinococcosis: Clinical and therapeutic aspects. World J Gastroenterol 2012;18(13):1448.
[Crossref] [Google Scholar] [PubMed]
- Bayraktar MR, Mehmet N, Durmaz R. Th1 and Th2 inducing cytokines in cystic echinococcosis. Turkiye Parazitol Derg 2005;29(3):167-70.
[Google Scholar] [PubMed]
- Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Microbiol Immunol 2006;47(1):24-41.
[Crossref] [Google Scholar] [PubMed]
- Pan W, Hao WT, Shen YJ, Li XY, Wang YJ, Sun FF, et al. The excretory-secretory products of Echinococcus granulosus protoscoleces directly regulate the differentiation of B10, B17 and Th17 cells. Parasit Vectors 2017 10(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Norouzi R, Ataei A, Hejazy M, Noreddin A, El Zowalaty ME. Scolicidal effects of nanoparticles against hydatid cyst protoscolices in vitro. Int J Nanomedicine 2020;15:1095.
[Crossref] [Google Scholar] [PubMed]
- Ma XM, Bao G, Wan JM, Liao DJ, Yin SH, Meng XQ, et al. Therapeutic effects of Sophora moorcroftiana alkaloids in combination with albendazole in mice experimentally infected with protoscolices of Echinococcus granulosus. Braz J Med Biol Res 2007;40(10):1403-8.
- Küster T, Zumkehr B, Hermann C, Theurillat R, Thormann W, Gottstein B, et al. Voluntary ingestion of antiparasitic drugs emulsified in honey represents an alternative to gavage in mice. J Am Assoc Lab Anim Sci 2012;51(2):219-23.
[Google Scholar] [PubMed]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and together with TGF-β, generates IL-9+ IL-10+ Foxp3−effector T cells. Nat Immunol 2008;9(12):1347-55.
[Crossref] [Google Scholar] [PubMed]
- Naik MI, Tenguria RK, Haq E. Detection of serum cytokines before and after pharmacological and surgical treatment in patients with cystic echinococcosis. J Helminthol 2016;90(1):91-5.
[Crossref] [Google Scholar] [PubMed]
- Smego Jr RA, Sebanego P. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis 2005;9(2):69-76.
[Crossref] [Google Scholar] [PubMed]
- Cobo F, Yarnoz C, Sesma B, Fraile P, Aizcorbe M, Trujillo R, et al. Albendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatisosis caused by Echinococcus granulosus. Trop Med Int Health 1998;3(6):462-6.
[Crossref] [Google Scholar] [PubMed]
- Mohamed AE, Yasawy MI, Al Karawi MA. Combined albendazole and praziquantel versus albendazole alone in the treatment of hydatid disease. Hepatogastroenterology 1998;45(23):1690-4.
[Google Scholar] [PubMed]
- Jelowdar A, Rafiei A, Abbaspour MR, Rashidi I, Rahdar M. Efficacy of combined albendazol and praziquntel and their loaded solid lipid nanoparticles components in chemoprophylaxis of experimental hydatidosis. Asian Pac J Trop Biomed 2017;7(6):549-54.
- Piccoli L, Meroni V, Genco F, Tamarozzi F, Tinelli C, Filice C, et al. Serum cytokine profile by ELISA in patients with echinococcal cysts of the liver: A stage-specific approach to assess their biological activity. Clin Dev Immunol 2012.
[Crossref] [Google Scholar] [PubMed]
- Ahmadnia S, Moazeni M, Mohammadi-Samani S. Hydatid cyst formation in male Balb/c mice following the intraperitoneal injection of live protoscoleces and activated oncospheres: A comparative study. J Parasit Dis 2014;38(1):77-80.
[Crossref] [Google Scholar] [PubMed]
- Ali WR, Ibrahim AA. A trial treatment of hydatidosis in white mice by immunization and chemicals. J Phys Conf Ser 2020;1660(1):012003.
- Mondragón-De-La-Pena C, Ramos-Solis S, Barbosa-Cisneros O, Rodriguez-Padilla C, Tavizon-Garcia P, Herrera-Esparza R. Echinococcus granulosus down regulates the hepatic expression of inflammatory cytokines IL-6 and TNFα in BALB/c mice. Parasite 2002;9(4):351-6.
[Crossref] [Google Scholar] [PubMed]
- Urrea-Paris MA, Moreno MJ, Casado N, Rodriguez-Caabeiro F. In vitro effect of praziquantel and albendazole combination therapy on the larval stage of Echinococcusgranulosus. Parasitol Res 2000;86(12):957-64.
[Crossref] [Google Scholar] [PubMed]
- Eckert J, Deplazes P. Biological, epidemiological and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004;17(1):107-35.
[Crossref] [Google Scholar] [PubMed]
- Teggi A, Lastilla MG, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother 1993;37(8):1679-84.
[Crossref] [Google Scholar] [PubMed]
- Rostami-Rad S, Jafari R, Darani HY. Th1/Th2-type cytokine profile in C57 black mice inoculated with live Echinococcus granulosus protoscolices. J Infect Public Health 2018;11(6):834-9.
[Crossref] [Google Scholar] [PubMed]
- Torcal J, Navarro-Zorraquino M, Lozano R, Larrad L, Salinas JC, Ferrer J, et al. Immune response and in vivo production of cytokines in patients with liver hydatidosis. Clin Exp Immunol 1996;106(2):317-22.
[Crossref] [Google Scholar] [PubMed]
- Touil-Boukoffa Ch, Sancëau J, Tayebi B, Wietzerbin J. Relationship among circulating interferon, tumor necrosis factor-α and interleukin-6 and serologic reaction against parasitic antigen in human hydatidosis. J Interferon Cytokine Res 1997;17(4):211-7.
- Mourglia-Ettlin G, Marqués JM, Chabalgoity JA, Dematteis S. Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl Trop Dis 2011;5(8):e1293.
[Crossref] [Google Scholar] [PubMed]
- Siracusano A, Delunardo F, Teggi A, Ortona E. Cystic echinococcosis: Aspects of immune response, immunopathogenesis and immune evasion from the human host. Endocr Metab Immune Disord Drug Targets 2012;12(1):16-23.
[Crossref] [Google Scholar] [PubMed]
- Gottstein B, Soboslay P, Ortona E, Wang J, Siracusano A, Vuitton DΑ. Immunology of alveolar and cystic echinococcosis (AE and CE). Adv Parasitol 2017;96:1-54.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Ma HZ, Apaer S, Anweier N, Zeng Q, Fulati X, et al. Impact of albendazole on cytokine and chemokine response profiles in Echinococcus multilocularis-inoculated mice. Biomed Res Int 2021.
- Li ZD, Mo XJ, Yan S, Wang D, Xu B, Guo J, et al. Multiplex cytokine and antibody profile in cystic echinococcosis patients during a three-year follow-up in reference to the cyst stages. Parasit Vectors 2020;13(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Ali WR, Sadek AA, Ghazi HF. Evaluation of IL-2, IL-1 evaluation of IL-2, IL-10 and IFN-gamma immune expression in liver and spleen after treatment of experimental cystic echinococcosis. TOFIQ J Med Sci 2016;3(1):26-38.
- Li ZJ, Wang YN, Qi W, Wei Z. Echinococcus granulosus 14-3-3 protein: A potential vaccine candidate against challenge with Echinococcus granulosus in mice. Biomed Environ Sci 2012;25(3):352-8.
[Crossref] [Google Scholar] [PubMed]
- Abo-Aziza FA, Hendawy SH, Oda SS, Aboelsoued D, El Shanawany EE. Cell-mediated and humoral immune profile to hydatidosis among naturally infected farm animals. Vet World 2020;13(1):214.
[Crossref] [Google Scholar] [PubMed]
- Yang S, Du X, Wang C, Zhang T, Xu S, Zhu Y, et al. Coding and noncoding RNA expression profiles of spleen CD4+ T lymphocytes in mice with echinococcosis. Research Square 2021.
- Rigano R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, et al. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol 1995;102(2):281-5.
[Crossref] [Google Scholar] [PubMed]
- Rigano R, Buttari B, Profumo E, Ortona E, Delunardo F, Margutti P, et al. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect Immun 2007;75(4):1667-78.
[Crossref] [Google Scholar] [PubMed]
- Rigano R, Profumo E, Buttari B, Teggi A, Siracusano A. Cytokine gene expression in peripheral blood mononuclear cells (PBMC) from patients with pharmacologically treated cystic echinococcosis. Clin Exp Immunol 1999;118(1):95-101.
[Crossref] [Google Scholar] [PubMed]
- Rigano R, Profumo E, Ioppolo S, Notargiacomo S, Teggi A, Siracusano A. Serum cytokine detection in the clinical follow up of patients with cystic echinococcosis. Clin Exp Immunol 1999;115(3):503-7.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Yang SH, Niu N, Tao J, Du XC, Yang JH, et al. lncRNA028466 regulates Th1/Th2 cytokine expression and associates with Echinococcus granulosus antigen P29 immunity. Parasit Vectors 2021;14(1):1-11.
[Crossref] [Google Scholar] [PubMed]
 ) Liver; (
) Liver; ( ) Lungs; (
) Lungs; ( )
Peritoneal cavity; (
)
Peritoneal cavity; ( ) Spleen; (
) Spleen; ( ) Subcutaneous
) Subcutaneous 



