- *Corresponding Author:
- R. B. Rupakula
Department of Chemistry, College of Science, GITAM University, Visakhapatnam - 530 045
E-mail: rrcbabu7@yahoo.in
| Date of Submission | 11 May 2010 |
| Date of Revision | 6 December 2010 |
| Date of Acceptance | 2 February 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 107-110 |
Abstract
High sensitive rapid gas chromatography-mass spectrometry method for the determination of four carcinogenic alkyl methanesulfonates viz. methyl methanesulfonate, ethyl methanesulfonate, isopropyl methanesulfonate and n-butyl methanesulfonate in doxazosin mesylate has been presented by using selective ion monitoring mode. The optimum separation was achieved between methyl methanesulfonate, ethyl methanesulfonate, isopropyl methanesulfonate and n-butyl methanesulfonate on a DB-5 (30 m×0.32 mm×1.0 μm) capillary column under programming temperature. Acetonitrile, water and ammonia (90:9:1 v/v/v) mixture was used as diluent. Various factors involved in the gas chromatography-mass spectrometry method development are also presented. This method was validated as per International Conference on Harmonization guidelines. The limit of quantitation of methyl methanesulfonate, ethyl methanesulfonate, isopropyl methanesulfonate and n-butyl methanesulfonate is 6 ppm with respect to 30 mg/ml of doxazosin mesylate.
Keywords
Alkyl methanesulfonate, doxazosin mesylate, genotoxic, method development, validation
Alkyl mesylate esters of short chain alcohols are reactive, genotoxic and possibly carcinogenic alkylating agents. Regulatory authorities made it mandatory to study and submit the impurity profile for the active pharmaceutical ingredients (APIs). Doxazosin mesylate chemically described as piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)- 4-[(2,3-dihydro-1,4-benzodioxin-2-yl)carbonyl]-, mono methanesulfonate is an antihypertensive agent [1-4] used in the treatment of benign prostatic hypertrophy..
Since, methanesulfonicacid (MSA) or mesylate is the counter ion of doxazosin mesylate (DZM), the usage of methyl, ethyl, isopropyl or n-butyl alcohol either at any stage of synthesis or crystallization, leads to the formation of genotoxic methyl, ethyl, isopropyl and n-butyl methanesulfonates, respectively. As on today, a threshold of toxicological concern (TTC) value of 1.5 μg/day intake of a genotoxic impurity is permitted. The permitted quantity in ppm is the ratio of TTC in μg/day and dose in g/ day [5]. Since, 8 mg of DZM is administered per day [6]. Permissible intake of genotoxic impurities is 188 ppm/day. Even though some analytical methods were reported [7-13] for the determination of DZM, no method was reported so far for the identification and determination of alkylmethanesulfonates in DZM, except the determination of MMS and EMS in imatinib mesylate [14]. Hence, we have developed a rapid GC-MS method that can quantitate alkyl methanesulfonates in DZM at the permissible levels. This method is validated as per ICH guidelines [15].
Methyl methanesulfonate, ethyl methanesulfonate, isopropyl methanesulfonate and n-butyl methanesulfonate (MMS, EMS, IPMS, and NBMS, respectively) were purchased from Acros Organics, Geel, Belgium. Acetonitrile and ammonia were procured from Merck, Mumbai, India. Pure sample of DZM was obtained from the synthetic division of Ogene Systems (I) Pvt Ltd (R and D), Hyderabad, India.
MMS, EMS, IPMS, and NBMS stock solutions were prepared by dissolving 10 mg individually in 100 ml of diluent. A mixture of acetonitrile, water and ammonia in the ratio of 90:9:1 v/v/v was used as diluent. MMS, EMS, IPMS, and NBMS mixture solution 1000 ppm was prepared by diluting the appropriate volume of above stock solution with diluent.
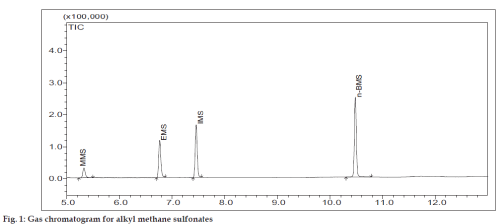
GC-MS analysis was carried out on GCMS-QP2010 system (Shimadzu Corporation, Kyoto, Japan) having GCMS solution software. AMSs were separated on DB-5 capillary column (Agilent Technologies, USA, 30 m×0.32 mm i.d.×1.0 μm film). One micro litre of 1000 ppm mixture solution with 1:5 split inlets was selected for injection. The GC oven temperature program utilized an initial temperature of 80º and an initial holding time of 3 min, and then increased at 10º/min to 220º. The final temperature was held for 8 min. The injection temperature, GC-MS interface and ion source temperature were 200, 240 and 240º, respectively. Helium was used as the carrier gas with a flow rate of 1.46 ml/min. The ionizing energy was 70 eV. The mass detector gain is 1.5 kV. The elution order was observed from the total ion chromatogram (fig. 1) using 94 ppm of alkyl methanesulfonates mixture. All the four alkyl methanesulfonates were identified using the National Institute of Standard Technology mass spectral library. Validation was done in Selective Ion Monitoring (SIM) mode.
All the four alkyl methanesulfonates are liquids, hence it was planned to separate them by gas chromatography, identify and confirm them by mass spectrometry. DZM is insoluble in dimethyl formamide, dimethyl sulfoxide. Hence acetonitrile, water and ammonia (90:9:1) v/v/v mixture was used as diluent. Ammonia was used to reduce peak tailing. AMSs mixture solution (1000 ppm) was prepared as stock solution. Initially the experiments were carried out by using DB-1 column (100%-dimethylpolysiloxane) for the separation of AMSs, but the resolutions were found to be very poor. Then, this column was replaced by DB-Wax column and same result was found. Hence, DB-5 column (5%-phenyl-95%-dimethylpolysiloxane) was used and good resolutions were observed. An optimum injection volume of 1 μl was chosen. The split ratio was fixed as 1:5 depending on the detector response. An initial column temperature of 800 was found to be optimum.
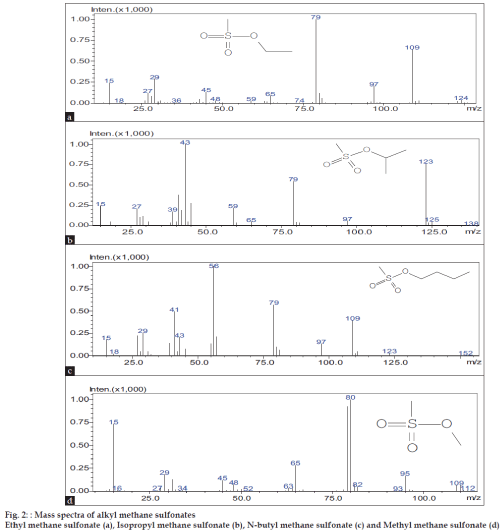
The present method is validated as per ICH guidelines [10]. MMS, EMS, IPMS and NBMS mixture solution (1000 ppm) was injected and the limit of detection (LOD) and the limit of quantification (LOQ) values were determined at the lowest concentrations at which signal-to-noise ratio is 3 and 10, respectively. LOD and LOQ values for all the AMSs were found to be 2 and 6 ppm, respectively. Linearity of the method was checked by plotting calibration curves between the peak areas versus the concentration of AMSs over the range 6-225 ppm. The slope, intercept and correlation coefficient values were derived from liner least-square regression treatment. The correlation coefficient values reported in (Table 1) indicate the best linearity of the method. The precision of the method was evaluated in terms of repeatability and intermediate precision. The repeatability is determined by calculating the relative standard deviation (% RSD) of six replicate determinations by injecting freshly prepared 6 ppm mixture solution separately on the same day. For intermediate precision, 6 ppm mixture solution was injected on six different days. The low % RSD values via peak areas confirm the good precision of the developed method (Table 1). AMSs were not detected when three pure R and D samples of DZM (30 mg/ ml) were analyzed in the present method. Hence, theaccuracy of the method was determined by spiking AMSs mixture at three concentration levels (6, 188 and 225 ppm) to 300 mg of DZM and making the volume to 10 ml with diluent. Each determination was carried out for three times. The recovery data presented in (Table 1) indicates the accuracy of the method. The method is specific because mass detector was used in the analysis. The mass spectra of EMS, IPMS, NBMS and MMS, shows Parent peaks in fig. 2 at m/z 124 (C3H8O3S (a)), 138 (C4H10O3S(b)) 152 (C5H12O3S (c)), and 110 (C2H6O3S (d)), respectively corresponding to their molecular weights. In the varied gas chromatographic conditions of ±5 % on the carrier gas flow, ±50 on the initial oven temperature, ±10/ min on the ramp rate, the retention times and peak areas of AMSs were found to be same indicating the robustness of the method.
| Parameter | MMS | EMS | IMS | n-BMS |
|---|---|---|---|---|
| LOD (ppm) | 2 | 2 | 2 | 2 |
| LOQ (ppm) | 6 | 6 | 6 | 6 |
| Linear Range (ppm) | 2-225 | 2-225 | 2-225 | 2-225 |
| Slope | 835 | 945 | 687 | 993 |
| Intercept | 831 | 678 | 898 | 915 |
| Correlation coefficient | 0.9999 | 0.9998 | 0.9999 | 0.9997 |
| Precision (% RSD) | 0.5 | 1.08 | 1.38 | 1.5 |
| Intermediate precision (% RSD) | 1.14 | 1.65 | 1.12 | 1.84 |
| % Recovery (Mean ± % RSD) at | ||||
| LOQ | 98.3±0.15 | 99.4±0.78 | 99.6±0.23 | 99.8 ±0.11 |
| 188 ppm | 99.4±0.29 | 99.6±0.52 | 98.5±0.65 | 99.6±0.71 |
| 225 ppm | 99.3±0.21 | 99.6±0.44 | 99.5±0.22 | 99.4±0.42 |
LOD, LOQ and Linear ranges are given in ppm with respect to 30 mg/ml of Doxazosinmesylate, six determinations using 6 ppm of AMS with respect to 30 mg/ml of Doxazosinmesylate for three determinations.
Table 1: Analytical Data Of Proposed Method
The aim of this study is to develop a GCMS method that can quantify four alkyl methanesulfonates in doxazosin mesylate. The developed GC-MS method was optimized based on the resolutions of AMSs peaks and validated as per ICH guidelines. The method well suits for the intended purpose.
Acknowledgements
The authors are grateful to Dr. B. M. Choudary, Managing Director, Ogene Systems (I) Pvt. Ltd, Hyderabad for providing facilities to carry out this research work.
References
- Roger LW. United States Pharmacopoeia. 31st ed. Rockville MD: The US Pharmacopeial Convention; 2008. p. 2016.
- O′Neill M, Heckelman P, Koch C, Roman K, Kenny C, editors. The Merck Index. 14th ed. Whitehouse Station NJ: Merck and Co., Inc.; 2007. p. 581.
- European Pharmacopeia. 6th ed. France: Council of Europe; 2008. p. 1756.
- British Pharmacopeia. The Department of Health. London: The British Pharmacopoeia Commission; 2008. p. 761.
- Physicians Desk Reference. 61st ed. Montvale NJ: Thomson Publishers; 2007. p. 2492.
- Rao PN, Nagaraju D, Nivedita J, Kumaraswamy G. Development and validation of reversed-Phase HPLC method for monitoring of synthetic reaction during the manufacture of a key intermediate of an antihypertensive drug. J Sep Sci 2006;29:2303-9.
- Sane RT, Francis M, Hijli PS, Pawar S, Arvind RP. Determination of doxazosin in its pharmaceutical formulation by high-performance thin-layer chromatography. J Planar Chromatogr 2002;15:34-7.
- Rao RN, Nagaraju D, Narasaraju A. Enatiomeric resolution of doxazosinmesylate and its process-related substances on polysaccharide chiral stationary phases. J Pharm Biomed Anal 2006;41:766-73.
- Bebawy LI, Moustafa AA, Talib NF. Stability indicating methods for the determination of doxazosinmesylate and celecoxib. J Pharm Biomed Anal 2002;27:779-93.
- Altiokka G, Atkosar Z. Flow injection analysis of doxazosinmesylate using detection. J Pharm Biomed Anal 2002;27:841-4.
- Kim YJ, Lee Y, Kang MJ, Huh JS, Yoon M, Lee J, et al. High-performance liquid chromatographic determination of doxazosin in human plasma for bioequivalance study of controlled release doxazosin tablets. Biomed Chromatogr 2006;20:1172-7.
- Sripalakit P, Nermhom P, Aurasorn S. Validtion and pharmacokinetic application of a method for determination of doxazosin in human plasma by high-performence liquid chromatography, Biomed Chromatogr 2006;20:729-35.
- Ning M, Liu W, Li H, Chen B, Zhu Y, Liu X, et al. LC-MS determination and relative bioavailability of doxazosinmesylate tablets in healthy Chinese male volunteers. J Pharm Biomed Anal 2007;43:1049-56.
- Ramakrishna K, Raman NV, NarayanaRao KM, Prasad AV, Subhaschander Reddy K. Development and validation of GC-MS method for the determination of methyl methanesulfonate and ethyl methanesulfonate in imatinibmesylate. J Pharm Biomed Anal 2008;46:780-3.
- Validation of analytical procedures; text and methodology Q2 (Rl), International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. Geneva, Switzerland 2005.