- *Corresponding Author:
- Shweta Dang
Department of Biotechnology, Jaypee Institute of Information Technology, A-10, Sector 62, Noida-201 309, India
E-mail: shweta.dang@jiit.ac.in
| Date of Submission | 06 February 2017 |
| Date of Revision | 04 August 2017 |
| Date of Acceptance | 18 March 2018 |
| Indian J Pharm Sci 2018;80(3): 442-452 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present study was to develop a nanoemulsion-based delivery system containing green tea catechins called polyphenon 60 and ciprofloxacin for intravaginal delivery to treat urinary tract infection. Polyphenon 60 and ciprofloxacin were encapsulated in a single nanoemulsion system prepared using ultrasonication technique. The nanoemulsion was characterized by determining particle size, zeta potential, morphological structure and estimating in vitro release and antibacterial efficacy. To determine the in vivo pharmacokinetic parameters and intravaginal transportation of nanoemulsion in Sprague Dawley rats, gamma scintigraphy and biodistribution study was conducted with technetium pertechnetate-labelled nanoemulsion. The preliminary antibacterial investigation showed synergy between these compounds with FICindex of 0.42. The developed formulation showed zeta potential of +55.3 mV and globule size of 151.7 nm, with polydispersity index of 0.196. The percent in vitro release for polyphenon 60 at the end of 7 h was 94.8±0.9, whereas for ciprofloxacin it was 75.1±0.15 in simulated vaginal media. Antibacterial activity evaluation against extended spectrum beta lactamase and metallo beta lactamase strains revealed that nanoemulsions containing 4 and 10 mg/ml each of polyphenon 60 and ciprofloxacin effectively inhibited the growth of bacterial strains. In biodistribution study, the percent radiolabelled drug per gram was found to be 3.50±0.26 and 3.81±0.30 in kidney and urinary bladder, respectively at 3 h. From these findings it could be concluded that the developed polyphenon 60+ciprofloxacin nanoemulsion showed antibacterial activity against Escherichia coli and was transported efficiently to the target organs through vaginal mucosa.
Keywords
Ciprofloxacin, gamma scintigraphy, intravaginal drug delivery, nanoemulsion, polyphenon 60
Ciprofloxacin (CF) is a broad spectrum antibiotic of fluoroquinolone class. It has been widely used for treating urinary tract infection (UTI) caused by Escherichia coli, however, development of resistance has been reported [1]. In a recent study, CF resistance was reported to be 49.9 % against E. coli [2]. Jaktaji and Pasand [3] in a recent paper have reported that CF efficiency has decreased due to development of fluoroquinolone resistant strains. The problem of bacterial resistance to antimicrobial drugs has been attempted to be resolved by combining antibiotics with natural plant products. Jazani and Babazadeh [4] reported possible synergy between CF and aqueous green tea extracts when used for antibacterial action. Polyphenon 60 (P60) is a natural compound present in green tea (Camellia sinensis). Polyphenols exhibit strong antioxidative and free radical scavenging activities [5]. P60 irreversibly damages the cytoplasmic membrane of bacteria and acts as a potential antiadhesive agent. However, it has been reported that Gramnegative bacteria are less susceptible to catechins as lipopolysaccharides act as a barrier [6]. In a previous study, it was also reported that catechins inhibited the activity of dihydrofolate reductase, an enzyme required by pathogens for the synthesis of purines and pyrimidines and increased the epidermal thickness [7]. Roccaro et al. [8] have also reported synergistic antibacterial action of green tea with various antibiotics including tetracycline, β-lactams and fluoroquinolone. Lee et al. [9] evaluated the synergistic effect of catechins with CF on the treatment of chronic bacterial prostatitis in a rat model. Catechins combined with CF showed synergistic action and a significant inhibition of bacterial growth in chronic bacterial prostatitisinduced rats.
In the present study, CF was combined with green tea catechins and formulated as a nanoemulsion for testing against extended spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing bacterial strains. The strains selected for the study were antibiotic-resistant clinical isolates comprising of E. coli, Klibsiella pneumonia, Proteus mirabilis and Citrobacter sp.
The oral bioavailability of P60 and CF is 26 % and 69 %, respectively, which is reported to be low because of high first pass effect [10,11]. To provide for a spatial and temporal control, vaginal route of administration was explored. Prolonged residence time, biocompatibility, biodegradability were some of the key features for an ideal vaginal formulation. Oil in water (O/W) nanoemulsions are easy to prepare and scale up, have tendency to solubilize both hydrophilic and lipophilic compounds owing to oil and water phases and have their own antimicrobial effect. All these features in addition to small particle size make nanoemulsion an attractive carrier system to solubilize and transport actives across various mucosal barriers. Besides, nanoemulsions are known to provide stability to the encapsulated actives, this feature is in particular importance when encapsulating natural compounds as these are prone to oxidation and degrade/destabilize as soon as they come in contact with biological fluids. It was hypothesized that a nanoemulsion-based formulation for intravaginal (IVAG) administration would provide stability to the encapsulated CF and green tea and the nanometric size of the droplets would facilitate transport of the active across the vaginal mucosa into systemic circulation. Tuğcu-Demiröz et al. [12] compared vaginal hydroxypropylmethylcellulose-based bioadhesive gels of oxybutynin with commercial oral tablets and found higher area under curve (AUC) and bioavailability with vaginal administration of bioadhesive gels.
To study the transport of formulation across vaginal mucosa; P60 was radiolabelled with technetium pertechnetate (99mTc) and its path was studied in a comparative oral vs. IVAG gamma scintigraphy and biodistribution study. The 99mTc-P60+CF nanoemulsion was administered (IVAG and oral) to female Sprague Dawley rats and scintigrams were taken. Pharmacokinetic parameters were investigated in the blood and target organs like kidney, urinary bladder and spleen in Sprague Dawley rats after oral and IVAG administration.
Materials and Methods
CF hydrochloride was generously gifted by Torge Ltd. Labrasol (caprylcaproyl macrogol glyceride) was kindly gifted by Gatefosse (India). Trolox was purchased from Calbiochem (unit of Merck Millipore, India). Nutrient dehydrated agar and nutrient dehydrated broth were obtained from Qualigens, India. P60, fetal bovine serum and Dulbecco's modified Eagle medium were purchased from Sigma-Aldrich. All the other chemicals used in the study were of analytical grade or HPLC grade.
E. coli (MTCC 739) for preliminary studies was obtained from the Microbial Type Culture Collection and Gene Bank, Chandigarh, India. The bacterial culture was maintained in nutrient broth. These isolates were maintained on Luria-Bertani agar slants and stored under refrigerated conditions.
Approval to carry out animal studies was obtained from the Institute of Nuclear Medicine and Allied Sciences (INMAS), Institutional Animal Ethics Committee (IAEC), New Delhi, India, IAEC vide number INM/ IAEC/2012/05 and their guidelines were followed throughout the study. Female Sprague Dawley rats (aged 3-4 mo) weighing 180-200 g were obtained from the Central Animal House Facility of INMAS, Delhi, India. Rats were kept at normal room temperature of 25 ± 5°.
Preliminary screening of antibacterial potential of P60 and CF
Stock solutions of aqueous P60 (6.6 mg/ml), CF (20 μg/ml) and P60+CF (6.6+20 μg/ml) were prepared and diluted in the nutrient broth. To account for the effect of P60 and CF on bacterial viability, nitroblue tetrazolium (NBT) assay was performed. The wells containing 100 μl of each dilution and E. coli bacterial inoculum (5×105 cfu/ml) was incubated for 16 h at 37°. After incubation for 16 h, 25 μl of NBT (5 mg/ml) was added to the wells and plates were further incubated for 3-4 h. About 100 μl dimethyl sulfoxide was added to each well to stop the reaction and dissolve the blue crystals. To account for the colour effect of P60 and CF, absorbance (Abs) was measured at 595 nm using enzyme-linked immunosorbent assay reader at the beginning of the assay and after completion of incubation [13]. The mean percent inhibition was calculated according to the Eqn. 1 [13]: % inhibition = (1–((Abs of sample at t16 −Abs of sample at t0 )/(Abs of growth control at t16–Abs of growth control at t0 ))×100). The value which exhibited more than 90 % inhibition was used as the minimum inhibitory concentration (MIC).
Fractionated inhibitory concentration (FIC) for P60+CF in aqueous form was calculated using a checkerboard method. FIC for P60+CF in aqueous form was calculated using Eqn. 2: FIC = MICA+B/ MICA; FICB = MICB+A/MICB; FICI = FICA+FICB. Here, MICA+B was the MIC of drug A in combination with B and MICB+A was vice versa. If the FICindex was ≤0.5, the combination was defined as synergistic and additive if FICindex value was between 0.5-1 [14].
Preparation of P60+CF nanoemulsion
To select specific excipients for preparing final emulsion, solubility of P60 and CF was checked in different oils, surfactants and co-surfactants. P60+CF nanoemulsion was prepared by mixing 20 mg/ml P60 and 20 mg/ml CF in 10 % v/v labrasol as oil, 80 % v/v of 1 % cetyperidinium chloride in water as surfactant, 10 % v/v glycerol as co-surfactant to form a pre-emulsion. This pre-emulsion was subjected to homogenization at 10 000 rpm for 25 min using high speed homogenizer (Tissue Master 125 homogenizer, Omni International, Georgia) and subsequently to high energy ultra-sonication via Bench Top Ultrasonicator (Model UP400S, 24 KHz 400 W, Hielcher, Ultrasound Technology, Germany) for the time period of 150 s, at an amplitude of 40 % and pulse of 30 % (i.e. 0.3 s ON and 0.7 s OFF) to prepare the final P60+CF nanoemusion.
Determination of globule size
Globule size distribution was determined by using photon correlation spectroscopy with inbuilt Zetasizer (model: 100 HS, Malvern Instruments. UK). Before measuring size and zeta potential, the developed nanoemulsion was diluted to 1:50 v/v in HPLC water [15].
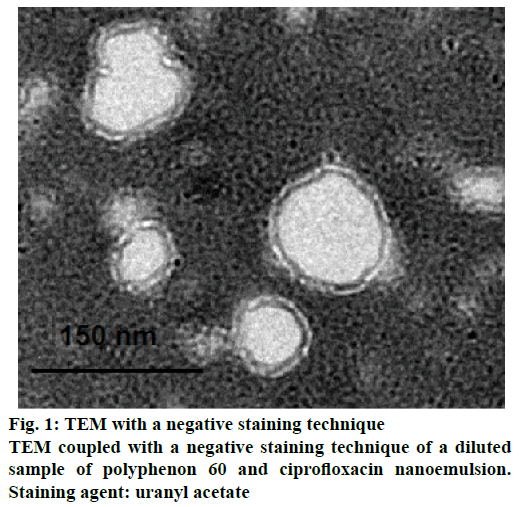
Transmission electron microscopy (TEM) of nanoemulsion
Morphological structure of developed P60+CF nanoemulsion was determined by using TEM. A drop of diluted (1:50 v/v) sample of nanoemulsion was placed on a carbon-coated copper grid (Quantifoil, Germany) and left to dry. A drop of negative stain uranyl acetate (2 % w/v) was used to stain the sample. The grid was again air dried and examined under TEM. The sample was viewed under Jeol JEM-1230 TEM operating at 80 kV.
In vitro release studies
About 2 ml of developed P60+CF nanoemulsion was packed in the dialysis bag (cellulose membrane mw. cut-off 12400, Sigma) immersed in 500 ml simulated vaginal medium (acetate buffer with pH 3: was prepared by dissolving sodium acetate 13.6 g and 6 ml until pH 3 of acetic acid in 1000 ml of distilled water) [12] and subjected to stirring at 100 rpm at 37° in USP II dissolution apparatus. The samples were withdrawn from the vessel and the drug release was determined spectrophotometrically for CF at 316 nm and for P60 at 274 nm.
Antibacterial studies against bio-safety level-2 strains
The effect of nanoemulsions on the test pathogens (E. coli, K. pneumoniae, P. mirabilis, C. amalonatus, C. diversus) was assayed by agar well diffusion method. The agar diffusion method was used to determine the minimal bactericidal concentration (MBC) of nanoemulsions. The bacterial culture was diluted to a final concentration of 1×106 cfu/ml and was plated on pre-prepared Muller-Hinton agar. Different concentrations of nanoemulsion (2, 4 and 10 mg/ml) were made and 10 μl was added to each well (0.5 mm) bored on agar plates. Further, the plate was incubated at 37° for 24 h and zones of inhibition were measured [16]. MBC was taken as the lowest concentration of nanoemulsion that completely inhibited the growth of pathogens [16].
Radiolabeling of P60+CF nanoemulsion with 99mTc
Radiolabeling of P60 with 99mTc pertechnetate was done using direct labelling method [17,18]. About 500 μl of P60-Sol (3 mg P60 in acetone) was taken and mixed with 200 μl of SnCl2 dihydrate solution (2 mg/ml in ethanol). To the resultant mixture (filtered through 0.22 μ nylon 66 membrane filter), required volume of sterile 99mTc-pertechnetate (5 mCi) was added with continuous mixing such that it had a radioactivity of 5 mCi/ml. The resultant formulations obtained had 100 μCi/20 μl activities. The radiolabelled P60 was then mixed with the CF nanoemulsion and was incubated at 30 ± 5° for 30 min. The radiochemical purity of 99mTc- P60+CF nanoemulsion was determined using ascending instant thin layer chromatography (Gelman Sciences, Inc., Ann Arbor, MI, USA) using acetone as mobile phase. The effect of concentration of SnCl2 and incubation time on radiolabeling efficiency was studied to achieve optimum reaction conditions by using the Eqn. 4. In vitro stability of radiolabeled formulation was evaluated and optimized in normal saline as well as in blood plasma [19,20]. Stable radiolabeled formulation was then subjected to gamma scintigraphy and biodistribution studies. Eqn. 4: % radiolabelling = radioactive counts retained in lower half of strip/total radioactive counts retained in the strip×100.
The Sprague Dawley female rats were selected for the gamma scintigraphy study. The rats were divided in three groups (3 rats for each formulation, n= 9). Group-I: rats administered P60+CF nanoemulsion (IVAG); group-II: rats administered P60+CF nanoemulsion (orally); group-III: rats administered aqueous P60+CF (IVAG).
Rats were anesthetized using 0.4 ml ketamine hydrochloride intraperitonial injection (50 mg/ml). Radiolabeled formulation 99mTc-P60+CF nanoemulsion and aqueous form (0.6 μl) with the concentration of (100 μCi/20 μl) equivalent to (20 mg/kg and 7 mg/kg body weight of P60 and CF, respectively) was administered orally and IVAG with the help of catheter made up of low density polyethylene tubing of internal diameter 0.1 mm at the delivery site respectively to the rats. Anesthetized rats were then placed on the imaging platform and imaging was performed at pre-determined time point of 0.5, 3, 6 and 24 h using a single-photon emission computerized tomography gamma camera, SPECT, LC 75-005, Diacam, Siemens AG, Erlanger, Germany.
Further biodistribution study was conducted on female rats. Similar groups were selected as in gamma scintigraphy studies and 3 rats were selected per time point in each group (n= 36). Before the administration of formulations, the rats were anesthetized using 0.4 ml ketamine hydrochloride intraperitoneal injection (50 mg/ml). Blood samples were collected through retro-orbital vein puncture and rats were sacrificed by cervical dislocation at predetermined time points (0.5, 3, 6 and 24 h) post-administration of formulations. Subsequently, urinary tract organs (kidney, spleen and urinary bladder along with ureters) were dissected, washed twice using normal saline, made free from adhering tissue/fluid, and weighed. Radioactivity present in each tissue/organ was measured using shielded well-type gamma scintillation counter. Radio pharmaceutical uptake per gram in each tissue/organ was calculated as a fraction of administered dose using Eqn. 5: radioactivity (%/g of tissue) = counts in sample×100/weight of sample×total counts injected.
Pharmacokinetic parameters for P60+CF nanoemulsion were calculated [21]. Organ targeting efficiency for kidney and urinary bladder was calculated using two Eqns. 6 and 7 mentioned below. Drug targeting efficiency (DTE %) that represents time average partitioning ratio was calculated using Eqn. 6: DTE (%) = [(AUC target organ/AUC blood))IVAG/(AUC target organ/AUC blood)oral]×100.
Direct transport percent (DTP %) of target organ was calculated using Eqn. 7: DTP (%) = [BIVAG− Bx/BIVAG]×100, where Bx = (Boral/Poral)×PIVAG, Bx is the target organ AUC fraction contributed by systemic circulation following oral administration, Boral is the AUC0–24h (target organ) following oral administration, Poral is the AUC0–24h (blood) following oral administration, BIVAG is the AUC0-24h (target organ) following IVAG administration, PIVAG is the AUC0-24h (blood) following IVAG administration, AUC is the area under the curve.
Data analysis
Results of in vitro drug release and biodistribution data were reported as mean ± SD (n= 3), and the difference between the groups were tested using two-way ANOVA using GraphPad Prism 5.0 and data analysis tool in Microsoft Excel.
Results and Discussion
The antimicrobial activity of P60 and CF was quantified by calculating the MIC, the lowest concentration at which the agent inhibits the growth of the pathogen for E. coli [14]. The results (Table 1) indicated that CF is highly effective against E. coli at lower concentrations (20 ng/ml) as compared to P60 (3 mg/ml). Hoshino et al. [22] also demonstrated that catechin-copper (II) complexes did damage to the cytoplasmic membrane of E. coli, which was an important mechanism in killing E. coli. Ikigai et al. [23] demonstrated that catechins induced leakage of molecules from the intra-liposomal space by disrupting the membrane, suggesting that this action is responsible for bactericidal activity of catechin against E. coli. However, on combining CF and P60, the MIC was reduced up to three folds as compared to individual MIC. FICindex value against E. coli was found to be 0.424 and the combination can said to be synergistic (FICindex<0.5). The results were in agreement with Lee et al. [9] where it was reported that combination of catechins and CF was more effective than CF alone in treating chronic bacterial prostatitis. Isogai et al. [24] evaluated the synergistic effect of catechins and levofloxacin in mouse intestine with E. coli infection. It was suggested that combination of P60 with CF was effective for eliminating the bacteria and able to reduce the rate of reoccurrence of infection.
| Preliminary screening of P60+CF | |||
| MIC (P60) | 3.3 mg | MIC (ciprofloxacin) | 20 ng |
| MIC (P60 in combination) | 0.412 mg | MIC (ciprofloxacin in combination) | 6 ng |
| FIC (aq.P60) | 0.412/3.30= 0.124 | FIC (aqueous ciprofloxacin) | 6/20= 0.3 |
Table 1: Preliminary Screening of the Polyphenon 60+Ciprofloxacin Nanoemulsion
In the present work, the nanoemulsion was prepared and loaded with 20 mg/ml of CF and 20 mg/ml of P60. The loaded nanoemulsion was characterized using particle size analysis and zeta potential. The results (Table 2) showed average particle size of both placebo and drug-loaded nanoemulsion was 142.7 and 151.7 nm, respectively. This indicated that the nanoemulsions approached a monodisperse stable system. Based on previous study reported by Neves et al., [25] this size range of 100 to 200 nm was selected as potentially optimal in terms of vaginal drug delivery, particularly when trying to target the epithelial layer. The zeta potential of placebo and drug-loaded nanoemulsion was +39.7 and +55.3 mV, respectively indicating a high stability of droplets against coalescence of dispersed phase.
| Drug concentration (mg/ml) | MDS (nm) | PDI | Zeta potential (mV) | |
|---|---|---|---|---|
| Placebo | - | 142.7 | 0.433 | +39.7 |
| NE (ciprofloxacin+P60) | 20+20 | 151.7 | 0.196 | +55.3 |
Table 2: Characterization of the Nanoemulsion Formulation
The negative stained TEM picture of this o/w nanoemulsion was shown in Figure 1. The image represented the particle size (around 150 nm), which appeared in agreement with the measured particle size by zetasizer. In addition, these electron microscopy pictures showed good monodispersity of the particles, thus indicating the relatively good quality of formulation.
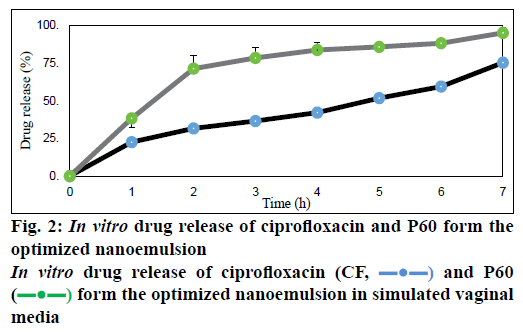
Dissolution studies done in simulated vaginal medium at pH 3 showed percent release of P60 to be 94.8 ± 0.9 % and of CF to be 75.1 ± 0.15 % at 7 h (Figure 2). Owing to high aqueous solubility, the release of both P60 and CF was high [26]. The release was found to be sustained for a period of 7 h. Both the compounds showed prolonged release in vaginal media, which proves that both P60 and CF were soluble at vaginal acidic pH and the nanoemulsion could be able to control the release in a sustained manner [25].
Gram-negative resistant isolates were collected from different health care centers of Mumbai and were identified using conventional cultural, biochemical and morphological tests, and comprised of E. coli, K. pneumoniae, Proteus mirabilis, C. amalonatus, C. diversus [16]. MBC was identified and the findings demonstrated that in both ESBL and MBL-producing isolates, the P60+CF nanoemulsion showed no inhibition of growth of all the isolates at 1:10 dilution containing 2 mg/ml of each P60 and CF whereas growth was inhibited at 1:5 and 1:2 dilutions containing 4 and 10 mg/ml of each P60 and CF, respectively. The placebo showed no growth inhibition of all strains at 1:10 and 1:5 dilutions whereas effective inhibition of growth was observed at 1:2 dilution. Therefore, the 1:5 and 1:2 dilutions containing 4 and 10 mg/ml of each drug were effective for the growth inhibition (Table 3). The results indicated that both P60 and CF are potent against ESBL and MBL-producing uropathogens. Higher antimicrobial effect of nanoemulsions can be attributed to the formation of nanodrops that increase the surface tension and thereby force themselves to merge with the lipids present in the bacterial cell membrane [27]. On a mass scale, this effectively disintegrates the membrane and kills the bacteria. Moreover, water present in nanoemulsion system is tightly bound to the internal oil phase and therefore not available to bacteria for its growth [28]. Aruna et al. [16] also observed in the study that uropathogens showed higher degree of resistant towards antibiotics. It also revealed that (72.05 %) ESBL produces isolates were resistant to CF, one of the most commonly used fluoroquinolone drugs for UTI. But our results demonstrated that CF when combined with natural green tea catechins in a single nano carrier system showed inhibition of resistant strains.
| Antibacterial activity of nanoemulsions on pathogens | ||||||||
|---|---|---|---|---|---|---|---|---|
| Activity of various dilutions of nanoemulsions against ESBL cultures | ||||||||
| P60+ciprofloxacin NE (dilutions) | Placebo (dilutions) | |||||||
| 1:10 | 1:5 | 1:2 | 1:10 | 1:5 | 1:2 | |||
| 250 | E. coli | + | - | - | + | + | - | |
| 253 | E. coli | + | - | - | + | + | - | |
| 254 | E. coli | + | - | - | + | + | - | |
| 255 | E. coli | + | - | - | + | + | - | |
| 258 | E. coli | + | - | - | + | + | - | |
| 260 | E. coli | + | - | - | + | + | - | |
| 261 | E. coli | + | - | - | + | + | - | |
| 262 | E. coli | + | - | - | + | + | - | |
| 263 | E. coli | + | - | - | + | + | - | |
| 264 | E. coli | + | - | - | + | + | - | |
| 210 | K. pneumoniae | + | - | - | + | + | - | |
| 275 | K. pneumoniae | + | - | - | + | + | - | |
| 296 | K. pneumoniae | + | - | - | + | + | - | |
| 466 | K. pneumoniae | + | - | - | + | + | - | |
| 507 | K. pneumoniae | + | - | - | + | + | - | |
| 610 | K. pneumoniae | + | - | - | + | + | - | |
| 645 | K. pneumoniae | + | - | - | + | + | - | |
| 674 | K. pneumonia | + | - | - | + | + | - | |
| 9b | P. mirabilis | + | - | - | + | + | - | |
| 110 | P. mirabilis | + | - | - | + | + | - | |
| 150 | P. mirabilis | + | - | - | + | + | - | |
| 191 | P. mirabilis | + | - | - | + | + | - | |
| 15 | C. diversus | + | - | - | + | + | - | |
| 30 | C. diversus | + | - | - | + | + | - | |
| 59 | C. amalonatus | + | - | - | + | + | - | |
| 123 | C .diversus | + | - | - | + | + | - | |
| Activity of various dilutions of nanoemulsions against MBL cultures | ||||||||
| 95 | K. pneumoniae | + | - | - | + | + | - | |
| 101 | K. pneumoniae | + | - | - | + | + | - | |
| 457 | K. pneumoniae | + | - | - | + | + | - | |
| 202 | K. pneumoniae | + | - | - | + | + | - | |
| 462 | K. pneumoniae | + | - | - | + | + | - | |
| 614 | K. pneumoniae | + | - | - | + | + | - | |
| 618 | K. pneumoniae | + | - | - | + | + | - | |
| 85 | P. aeruginosa | + | - | - | + | + | - | |
| 214 | P. aeruginosa | + | - | - | + | + | - | |
| 216 | P. aeruginosa | + | - | - | + | + | - | |
| 463 | E. coli | + | - | - | + | + | - | |
| 467 | E. coli | + | - | - | + | + | - | |
| 469 | E. coli | + | - | - | + | + | - | |
| 471 | E. coli | + | - | - | + | + | - | |
| 473 | E. coli | + | - | - | + | + | - | |
| 479 | E. coli | + | - | - | + | + | - | |
| 493 | E. coli | + | - | - | + | + | - | |
| 519 | E. coli | + | - | - | + | + | - | |
| 520 | E. coli | + | - | - | + | + | - | |
| 522 | E. coli | + | - | - | + | + | - | |
| 525 | E. coli | + | - | - | + | + | - | |
| 526 | E. coli | + | - | - | + | + | - | |
| 621 | E. coli | + | - | - | + | + | - | |
| 623 | E. coli | + | - | - | + | + | - | |
Table 3: Antibacterial Potential of P60+Ciprofloxacin Nanoemulsion Against BSL-2 Strains (ESBL and MBL Producing Uropathogens)
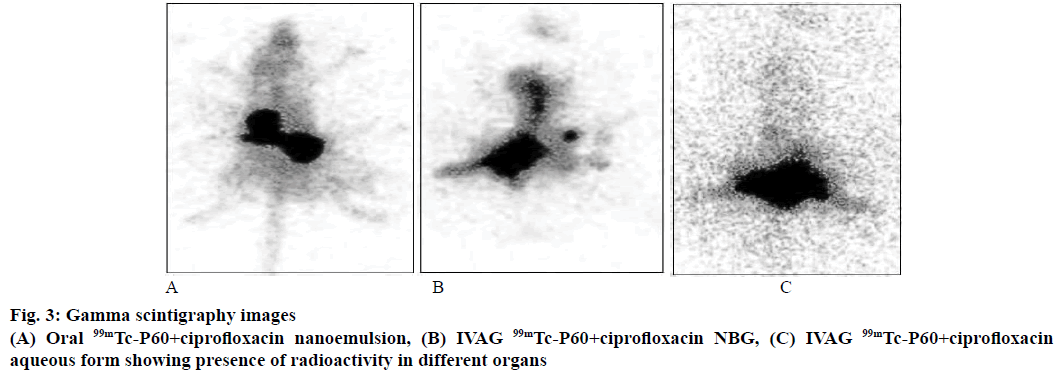
P60+CF nanoemulsion was used to label with 99mTc because of its suitable chemical properties for labelling process. P60+CF nanoemulsion was successfully labelled with 99mTc and labelling efficiency of 99mTc- P60+CF nanoemulsion and 99mTc-P60+CF aqueous form was 99.7 ± 1.3 and 94.4 ± 0.9 %, respectively. The distribution and retention time of radiolabelled P60+CF nanoemulsion was studied by gamma scintigraphic images of Sprague Dawley rats after IVAG and oral administration at predetermined time points of 0.5, 3, 6 and 24 h. Scintigrams of urinary tract of rat administered with P60+CF nanoemulsion by IVAG route showed the presence of drug in kidney, urinary bladder and vaginal tract for 6 h and trace amounts at 24 h and maximum distribution at 3 h (Figure 3). Whereas images of rat administered with P60+CF nanoemulsion orally showed distribution of drug in gastric tract and images of female rats, which received IVAG P60+CF aqueous form showed less distribution of drug in urinary tract (Figure 3). The literature [29] suggested that it was due to the permeation of nano droplets across the vaginal mucosa. Our results were in agreement to previous study of Neves et al., [25] where fluorescent images of the deprivin-loaded nanoparticles administered (IVAG) in female mice were taken and higher uptake of drug was detected at vaginal level and in uterine tissue. Woolfson in 2003 [30] also observed in a study that radiolabelled microspheres given intravaginally in sheep model showed higher retention time up to 12 h in the urinary tract.
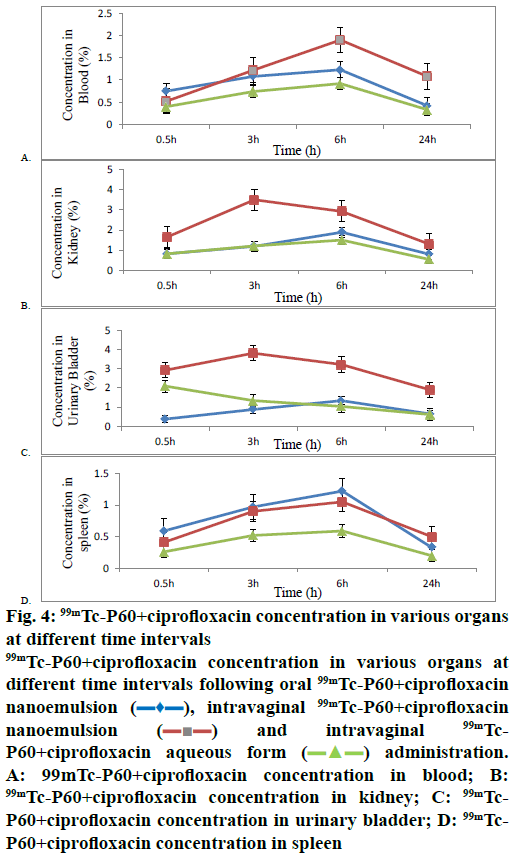
Further, biodistribution studies of 99mTc-P60 CF nanoemulsion and 99mTc-P60+CF aqueous form following IVAG and oral administration in Sprague- Dawley rats were performed, and the radioactivity in percent per gram of organ of administered dose was estimated at predetermined time intervals up to 24 h (Table 4). The concentration of radiolabelled drug in blood at all sampling time points for different formulations was also assessed and recorded (Figure 4A). The analysis indicated that the percent per gram concentration of 99mTc-P60+CF nanoemulsion in kidney and urinary bladder following the IVAG administration (figs. 4B and C) were found to be 3.50 ± 0.26 and 3.81 ± 0.30 at 3 h, which was significantly higher as compared to both 99mTc-P60+CF nanoemulsion administered orally (1.19 ± 0.12 and 0.88 ± 0.16) and 99mTc-P60+CF aqueous form administered intravaginally (1.21 ± 0.11 and 1.34 ± 0.12). While the spleen concentration (Figure 4D) of 99mTc-P60+CF nanoemulsion after IVAG administration was comparable to that of oral administration of 99mTc-P60+ CF nanoemulsion and IVAG administration of 99mTc-P60+CF aqueous form at all the time points. Tedajo et al. [31] also demonstrated the applicability of emulsions as a new drug carrier system for vaginal delivery with a good retention of the emulsion in the vagina. The pharmacokinetic parameters for the 99mTc- P60+CF nanoemulsion and 99mTc-P60+CF aqueous form were calculated (Table 5). The lower Tmax values (3 h) for target organs after IVAG administration when compared to oral administration (6 h) may also be attributed to preferential target drug delivery following IVAG administration of 99mTc-P60+CF nanoemulsion. This confirmed that 99mTc-P60+CF nanoemulsion crossed epithelium rapidly and reached the target organs when given intravaginally as compared to oral administration. When the Cmax and AUC of kidney and urinary bladder concentration of 99mTc-P60+CF nanoemulsion (IVAG), 99mTc-P60+CF nanoemulsion (orally) and 99mTc-P60+CF aqueous form (IVAG) were compared, the Cmax kidney (3.50 %/g) and Cmax urinary bladder (3.81 %/g) and AUC kidney (54.28 h %/g) and AUC urinary bladder (65.85 h %/g) of 99mTc-P60+CF nanoemulsion (IVAG) were found to be significantly higher. This could be due to small size of particles and large surface area of nanoemulsion. Vaginal absorption and bioavailability of multiple emulsions were studied and it was observed that the application of multiple emulsions may facilitate application, and reduce irritation and burning in the vulvovaginal region as compared to tablets formulations [32-34]. The % DTP and DTE represent the percent of drug directly transported to the target organs via the IVAG pathway. DTP and DTE % were calculated using tissue/organ distribution data following IVAG and oral administration and are recorded in Table 6. 99mTc-P60+CF nanoemulsion (IVAG) showed the highest DTE % kidney (106.29), DTE % urinary bladder (175.40) and DTP % kidney (54.5), DTP % urinary bladder (43.416) for kidney and urinary bladder amongst 99mTc-P60+CF nanoemulsion (oral) and 99mTc-P60+CF aqueous form (IVAG). The higher DTP % and DTE % suggest that 99mTc-P60+CF nanoemulsion (IVAG) has better target organ efficiency and the observations were in sync with the reports of Cu et al. [35] who observed that nanoemulsion particles were found at significantly higher concentration in the reproductive tissues, beyond the mucus layer, at 2 and 6 h after IVAG delivery. Vaginal drug delivery bypasses the first pass effect; avoiding the gastrointestinal degradation and hepatic metabolism. Cicinelli et al. [36] also reported that radiotracer was absorbed through vaginal mucosa and raised its concentration in the paravaginal spaces, lymph, and vaginal venous vessels and reached ultimately in the systemic circulation. Similarly, it was observed that 99mTc-P60+CF nanoemulsion travelled efficiently throughout the target organs and reached systemic circulation and remained active up to 24 h.
| Formulation and route of administration | Distribution of radiolabelled P60+cipro in counts/g of tissues in different organs at different sampling time points | ||||
|---|---|---|---|---|---|
| Organ | 0.5 h | 3 h | 6 h | 24 h | |
| Oral 99mTc-P60+ciprofloxacin nanoemulsion | Blood | 0.75 ± 0.25 | 1.08 ± 0.12 | 1.23 ± 0.22 | 0.42 ± 0.11 |
| Kidney | 0.82 ± 0.20 | 1.19 ± 0.12 | 1.89 ± 0.14 | 0.80 ± 0.13 | |
| Urinary bladder | 0.38 ± 0.08 | 0.88 ± 0.16 | 1.34 ± 0.11 | 0.65 ± 0.10 | |
| Spleen | 0.59 ± 0.15 | 0.97 ± 0.23 | 1.62 ± 0.10 | 0.33 ± 0.09 | |
| IVAG 99mTc-P60+ciprofloxacin nanoemulsion | Blood | 0.52 ± 0.10 | 1.22 ± 0.21 | 1.9 ± 0.16 | 1.08 ± 0.22 |
| Kidney | 1.65 ± 0.15* | 3.50 ± 0.26* | 2.92 ± 0.24* | 1.30 ± 0.18* | |
| Urinary bladder | 2.92 ± 0.32* | 3.81 ± 0.30* | 3.22 ± 0.25* | 1.91 ± 0.14* | |
| Spleen | 0.41 ± 0.18 | 0.90 ± 0.30 | 1.05 ± 0.29 | 0.50 ± 0.15 | |
| IVAG 99mTc P60+ciprofloxacin aqueous form | Blood | 0.4 ± 0.31 | 0.74 ± 0.10 | 0.92 ± 0.08 | 0.33 ± 0.09 |
| Kidney | 0.82 ± 0.09 | 1.21 ± 0.11 | 1.50 ± 0.12 | 0.54 ± 0.09 | |
| Urinary bladder | 2.10 ± 0.20 | 1.34 ± 0.12 | 1.04 ± 0.09 | 0.62 ± 0.13 | |
| Spleen | 0.26 ± 0.07 | 0.52 ± 0.20 | 0.59 ± 0.21 | 0.20 ± 0.09 | |
Table 4: Distribution of 99mtc-P60+Ciprofloxacin Nanoemulsion (Orally and IVAG), 99mtc-P60+Ciprofloxacin Aqueous form (IVAG) at Different Time Intervals in Sprague Dawley Female Rats
Figure 4: 99mTc-P60+ciprofloxacin concentration in various organs at different time intervals
99mTc-P60+ciprofloxacin concentration in various organs at different time intervals following oral 99mTc-P60+ciprofloxacin nanoemulsion ( ), intravaginal 99mTc-P60+ciprofloxacin nanoemulsion (
), intravaginal 99mTc-P60+ciprofloxacin nanoemulsion ( ) an intravaginal 99mTc- P60+ciprofloxacin aqueous form (
) an intravaginal 99mTc- P60+ciprofloxacin aqueous form ( ) administration. A: 99mTc-P60+ciprofloxacin concentration in blood; B: 99mTc-P60+ciprofloxacin concentration in kidney; C: 99mTc-P60+ciprofloxacin concentration in urinary bladder; D: 99mTc-P60+ciprofloxacin concentration in spleen
) administration. A: 99mTc-P60+ciprofloxacin concentration in blood; B: 99mTc-P60+ciprofloxacin concentration in kidney; C: 99mTc-P60+ciprofloxacin concentration in urinary bladder; D: 99mTc-P60+ciprofloxacin concentration in spleen
| Formulation and route of administration | Organ | Cmax (%/g) | Tmax (h) | AUC |
|---|---|---|---|---|
| Oral 99mTc-P60+ciprofloxacin nanoemulsion | Blood | 1.23 | 6 | 20.77 |
| Kidney | 1.89 | 6 | 31.54 | |
| Urinary bladder | 1.34 | 6 | 22.9 | |
| Spleen | 1.62 | 6 | 19.32 | |
| IVAG 99mTc-P60+ciprofloxacin nanoemulsion | Blood | 1.9 | 6 | 33.8 |
| Kidney | 3.50* | 3 | 54.28* | |
| Urinary bladder | 3.81* | 3 | 65.85* | |
| Spleen | 1.05 | 6 | 18.6 | |
| IVAG 99mTc P60+ciprofloxacin aqueous form | Blood | 0.92 | 6 | 8.78 |
| Kidney | 1.50 | 6 | 25.15 | |
| Urinary bladder | 2.10 | 0.5 | 23.33 | |
| Spleen | 0.59 | 6 | 9.805 |
Table 5: Pharmacokinetics of 99mtc-P60+Ciprofloxacin Nanoemulsion (Orally and IVAG) and 99mtc-Ciprofloxacin Aqueous Form (IVAG) at Different time Intervals in Sprague Dawley Rats
| Formulation and route of administration | Target organ | Drug targeting efficiency (DTE %) | Direct target organ transport (DTP %) |
|---|---|---|---|
| P60+ciprofloxacin nanoemulsion (IVAG) | Kidney | 106.29 | 54.5 |
| P60+ciprofloxacin nanoemulsion (IVAG) | Urinary bladder | 175.40 | 43.416 |
Table 6: Drug Targeting Efficiency and Direct Target Organ Transport Following Intravaginal Administration of 99mtc-P60+Ciprofloxacin Nanoemulsion
In the present investigations, it was concluded that P60 and CF showed synergistic effect as per the FICindex against E. coli and were successfully encapsulated in an o/w nanoemulsion. The drug release from the encapsulated nanoemulsion was estimated in simulated vaginal media using in vitro dissolution apparatus and findings demonstrated sustained release for both P60 and CF. The optimized nanoemulsion was tested for its antibacterial action on resistant representative isolates of E. coli, K. pneumonia, P. aeruginosa and P. mirabilis, respectively and findings confirmed the potential of nano formulations to be used against ESBL and MBL producing uropathogens. Our findings revealed that radiolabelled P60+CF nanoemulsion penetrated vaginal mucus most rapidly and reached the target organs such as kidney and urinary bladder. The percent per gram of radiolabelled drug reaching to target organs was significantly higher via IVAG route
Acknowledgements
The authors are grateful to the Jaypee Institute of Information Technology, Noida, UP (India), for the infrastructural support. The author would also like to thank Dr Aruna, Wilson College, Mumbai for giving facilities and support to carry antibacterial studies.
Financial support and sponsorship
This work was supported financially by the Department of Biotechnology, Government of India to conduct the research work (DBT project No.BT/PR7215/ NNT/28/654/2013).
Conflict of interest
The authors declare that this paper content has no conflict of interests.
References
- Arslan H, Azap ÖK, Ergönül Ö, Timurkaynak F. Urinary tract infection study group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother 2005;56:914-18.
- Yılmaz N, Ağuş N, Bayram A, Şamlıoğlu P, Şirin MC, Derici YK, et al. Antimicrobial susceptibilities of Escherichia coli isolates as agents of community-acquired urinary tract infection (2008–2014). Turk J Urol 2016;42:32.
- Jaktaji RP, Pasand S. Overexpression of SOS genes in ciprofloxacin resistant Escherichia coli mutants. Gene 2016;576:115-18.
- Jazani NH, Babazadeh H. Evaluation of the sensitivity of Pseudomonas aeruginosa clinical isolates to ciprofloxacin. J Biol Sci 2008;8:486-89.
- Sosa MV, Rodríguez-Rojo S, Mattea F, Cismondi M, Cocero MJ. Green tea encapsulation by means of high pressure antisolvent co-precipitation. J Supercrit Fluids 2011;56:304-11.
- Sharma A, Gupta S, Sarethy IP, Dang S, Gabrani R. Green tea extract: possible mechanism and antibacterial activity on skin pathogens. Food Chem 2012;135:672-75.
- Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, et al. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J 2003;17:1913-15.
- Roccaro AS, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother 2004;48:1968-73.
- Lee YS, Han CH, Kang SH, Lee SJ, Kim SW, Shin OR, et al. Synergistic effect between catechin and ciprofloxacin on chronic bacterial prostatitis rat model. Int J Uro 2005;12:383-9.
- Chow HS, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res 2005;11:4627-33.
- Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J. Absolute oral bioavailability of ciprofloxacin. Antimicrob Agents Chemother 1986;30:444-46.
- Tuğcu-Demiröz F, Acartürk F, Erdoğan D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int J Pharma 2013;457:25-39.
- Kahlmeter G, Brown DF, Goldstein FW, Dannaoui E, Lass‐Florl C, Sandven P, et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infec 2006;12:501-3.
- Sharma G, Raturi K, Dang S, Gabrani R. Combinatorial antimicrobial effect of curcumin with selected phytochemicals on Staphylococcus epidermidis. J Asian Nat Prod Res 2014;16:535-41.
- Attwood D, Mallon C, Ktistis G, Taylor CJ. A study on factors influencing the droplet size in nonionic oil-in-water microemulsions. Int J Pharm 1992;88:417-22.
- Aruna K, Mobashshera T. Prevalence of extended spectrum beta-lactamase production among uropathogens in south Mumbai and its anti-biogram pattern. EXCLI J 2012;11:363-72.
- Saha GB. Methods of radiolabeling. Physics and radiobiology of nuclear medicine. New York: Springer; 1993. p. 100-6.
- Babbar AK, Singh AK, Goel HC, Chauhan UP, Sharma RK. Evaluation of 99m Tc-labeled photosan-3, a hematoporphyrin derivative, as a potential radiopharmaceutical for tumor scintigraphy. Nucl Med Biol 2000;27:419-26.
- Sharma D, Sharma RK, Sharma N, Gabrani R, Sharma SK, Ali J, et al. Nose-to-brain delivery of PLGA-diazepam nanoparticles. AAPS PharmSciTech 2015;16:1108-21.
- Rastogi R, Sultana Y, Aqil M, Ali A, Kumar S, Chuttani K, et al. Alginate microspheres of isoniazid for oral sustained drug delivery. Int J pharm 2007;334:71-77.
- Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J pharm 2008;358:285-91.
- Hoshino N, Kimura T, Yamaji A, Ando T. Damage to the cytoplasmic membrane of Escherichia coli by catechin-copper (II) complexes. Free Radical Biol Med 1999;27:1245-50.
- Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta 1993;1147:132-36.
- Isogai E, Isogai H, Hirose K, Hayashi S, Oguma K. In vivo synergy between green tea extract and levofloxacin against enterohemorrhagic Escherichia coli O157 infection. Curr Microbial 2007;42:248-51.
- das Neves J, Nunes R, Machado A, Sarmento B. Polymer-based nanocarriers for vaginal drug delivery. Adv Drug Deliv Rev 2015;92:53-70.
- Reisner DE. Bionanotechnology II: Global Prospects. Vol. 2. Boca Raton, Florida: CRC Press; 2011.
- Hamouda T, Baker JR. Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram‐negative bacilli. J Appl Microbial 2000;89:397-403.
- AL-Janabi AA. Potential activity of the purine compounds caffeine and aminophylline on bacteria. J Glob Infect Dis 2011;3:133.
- Lara HH, Ixtepan-Turrent L, Treviño ENG, Singh DK. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J Nanobiotechnology 2011;9:1.
- Woolfson AD. Intravaginal drug delivery technologies. Drugs Pharm Sci 2003;126:759-74.
- Tedajo GM, Bouttier S, Fourniat J, Grossiord JL, Marty JP, Seiller M. Release of antiseptics from the aqueous compartments of aw/o/w multiple emulsions. Int J Pharm 2005;288:63-72.
- Özer Ö, Özyazici M, Tedajo M, Taner MS, Köseoglu K. W/O/W multiple emulsions containing nitroimidazole derivates for vaginal delivery. Drug Deliv 2007;14:139-45.
- Özyazįcį M, Gökçe E, Hizarcioglu SY, Taner MS, Köseoglu K, Ertan G. Dissolution and vaginal absorption characteristics of metronidazole and ornidazole. Die Pharmazie- Int J Pharm Sci 2006;61:855-61.
- Fredricsson B, Hagström B, Nord CE, Rane A. Systemic concentrations of metronidazole and its main metabolites after intravenous oral and vaginal administration. Gynecol Obstet Invest 1987;24:200-7.
- Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. J Control Release 2011;156:258-64.
- Cicinelli E, Rubini G, De Ziegler D, Barba B, Pinto V, Di Stefano MG, et al. Absorption and preferential vagina-to-uterus distribution after vaginal administration of 99m Tc-pertechnetate in postmenopausal women. Fertil Steril 2001;76:1108-12.
 ) and P60
(
) and P60
( ) form the optimized nanoemulsion in simulated vaginal
media
) form the optimized nanoemulsion in simulated vaginal
media