- Corresponding Author:
- D. R. Acharya
Center for Health Science Studies, Ganpat University, Ganpat Vidyanagar, Mehsana–384 011, India
E–mail: drupad_acharya@yahoo.co.in
| Date of Submission | 23 April 2012 |
| Date of Revision | 6 January 2013 |
| Date of Acceptance | 10 January 2013 |
| Indian J Pharm Sci 2013;75(1):31-35 |
Abstract
A simple, accurate, rapid and precise reversed-phase high-performance liquid chromatographic method has been developed and validated for simultaneous determination of cefpodoxime proxetil and dicloxacillin sodium in tablet. The chromatographic separation was carried out on kromasil C 18 analytical column (250×4.6 mm; 5 μm) with a mixture of acetonitrile:methanol:trifloroacetic acid (0.001%) with pH 6.5 (30:50:20, v/v/v) as mobile phase; at a flow rate of 1.0 ml/min. UV detection was performed at 235 nm. The dicloxacillin sodium and cefpodoxime proxetil were eluted at 1.92 and 3.35 min, respectively. The peaks were eluted with better resolution. Calibration plots were linear over the concentration range 0.5-20 μg/ml for cefpodoxime proxetil (r 2=0.9996) and 5-50 μg/ml for dicloxacillin sodium (r 2=0.9987). The method was validated for accuracy, precision, linearity and specificity. The method was very sensitive with limit of detection 0.0726, 0.3685 μg/ml and limit of quantification 0.220, 1.116 μg/ml for cefpodoxime proxetil and dicloxacillin sodium, respectively. The high recovery and low relative standard deviation confirm the suitability of the method for routine determination of cefpodoxime proxetil and dicloxacillin sodium in bulk drug and tablet dosage form.
Keywords
Cefpodoxime proxetil, dicloxacillin sodium, reversed?phase high?performance liquid chromatographic method, tablet, validation
Cefpodoxime proxetil (CEF) is chemically 1-(isopropoxy carbonyloxy) ethyl (6R,7R)-7-[2-(2- amino-4-thiazolyl)-(z)-2-(methoxyimino) acetamido]- 3-methoxymethyl-3-cephem-4-carboxylate[1], is a third generation cephalosporin anti-biotic. It is used for infections of the respiratory tract, urinary tract and skin and soft tissues. It has greater activity against Staphylococcus aureus[2]. CEF is official in IP[3] and USP[4], which describes liquid chromatography method for its estimation. Literature survey reveals the highperformance thin layer chromatography (HPTLC)[5] method for the determination of CEF individually. Literature survey also reveals the reversed-phase high-performance liquid chromatographic (RP-HPLC) [6] and spectrophotometric[7] methods for determination of CEF with other drugs. Dicloxacillin (DCX) is chemically 9 (2S,5R,6R)-6-[3-(2,6-dichlorophenyl)- 5-methyl-1,2-oxazole-4-amido]-3,3-dimethyl-7-oxo-4- thia-1azabicyclo[3.2.0] heptane-2-carboxylic acid[8], and is a penicillinase resistant penicillin, used in the treatment of bacterial infections such as pneumonia and bone, ear, skin and urinary tract infection[9]. It is official in IP[10] and USP[11], and describe RP-HPLC method for its estimation. Literature survey reveals HPLC[12] method for determination of DCX in pharmaceutical dosage forms as well as in biological fluids. Literature survey also reveals spectrofluorimetric[13] and RP-HPLC[14-16] methods for determination of DCX with other drugs.
The combined dosage form of CEF and DCX are available in the market for the treatment of infections caused by susceptible microorganisms like urinary tract infections and gonococcal urethritis. The combination of these two drugs is not official in any pharmacopoeia, hence, no official method is available for the simultaneous estimation of CEF and DCX in their combined dosage forms. Literature survey does not reveal any simple spectrophotometric or other method for simultaneous estimation of CEF and DCX in combined dosage form. The present communication describes simple, sensitive, rapid, and accurate RP-HPLC method for simultaneous estimation of both drugs in their combined tablet dosage forms.
Materials and Methods
Shimadzu, LC?2010CHT, Japan instrument provided with a kromasil C18 column (250×4.6 mm, 5 μ) and LC 20 AD Pump and Prominence SPD 20A UV?deuterium detector was employed in the study. Data acquisition was performed by using LC solution software. HPLC grade methanol (MeOH) and acetonitrile (ACN) were purchased from Finar Chemicals Ltd., Ahmedabad, India and water from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. Trifloroacetic acid (TFA) AR grade were purchased from SD Fine Chem., Mumbai, India.
Preparation of solutions and reagents
A solution containing acetonitrile, methanol and water in the proportion of 20:50:30 v/v/v was used as diluent for the proposed method. A mixed standard stock solution of CEF (1.0 mg/ml) and DCX (1.0 mg/ml) was prepared by accurately weighing CEF (25 mg) and DCX (25 mg) and dissolving in diluent and make up to 25 ml with diluent in the 25 ml volumetric flask. An aliquot of 1.0 ml of stock solution was transferred in 10 ml volumetric flask and adjusted up to the mark with diluent having concentration (100 µg/ml).
Preparation of sample solution
Twenty tablets were weighed and powdered. The powder equivalent to 200 mg CEF and 500 mg DCX was transferred to 100 ml volumetric flask. Diluent (50 ml) was added to it and sonicated for 20 min. The volume was adjusted with diluent after the filtration of sonicated solution. An aliquot of 1.0 ml of this solution was transferred in 100 ml volumetric flask and adjusted up to the mark with diluent CEF (20 µg/ml) and DCX (50 µg/ml). From this solution, 2.5 ml was transferred to 10 ml volumetric flask and adjusted up to the mark with diluent to achieve the concentration of CEF 5 µg/ml and DCX 12.5 µg/ml.
Methodology
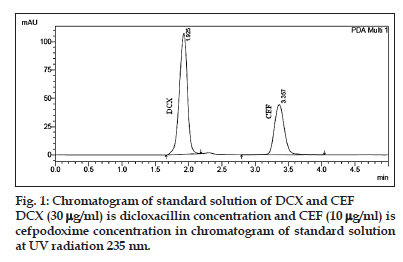
To optimize the RP?HPLC parameters, several mobile phase compositions were tried. A satisfactory separation and good peak symmetry for CEF and DCX was obtained with a mobile phase ACN:MeOH:TFA (0.001%) with pH 6.5 (30:50:20 v/v/v) at a flow rate of 1.0 ml/min to get better reproducibility and repeatability. Quantification was carried out at 235 nm based on peak area. Complete resolution of the peaks with clear baseline was obtained (fig. 1). System suitability test parameters for CEF and DCX for the proposed method are reported in Table 1.
Validation developed method
The proposed method has been validated for the simultaneous determination of CEF and DCX in tablet dosage form[17]. Calibration curves were constructed by plotting peak areas versus concentrations of CEF and DCX, and the regression equations were calculated. The calibration curves were plotted over the concentration range 0.5?20 µg/ml for CEF and 5?50 µg/ml for DCX. Accurately measured working standard solutions of CEF (0.05, 0.1, 0.5, 1.0, 1.5 and 2.0 ml) and measured working standard solution of DCX (0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 ml) were transferred to a series of 10 ml of volumetric flasks and diluted to the mark with diluent. Aliquots (20 µl) of each solution were injected and analysed under the operating chromatographic conditions described as above. Regression parameters are mentioned in Table 2.
For the repeatability, the relative standard deviation (RSD) values for CEF and DCX were found to be 0.21 and 0.38%, respectively. The RSD values were found to be <2%, which indicates that the proposed method is repeatable. The low % RSD values of interday (0.70?1.45 and 0.63?1.42%) and intraday (0.12?0.48 and 0.11?0.59%) for CEF and DCX, respectively, reveal that the proposed method is precise (Table 2). limit of detection (LOD) values for CEF and DCX were found to be 0.072 and 0.368 µg/ ml, respectively, and limit of quantification (LOQ) values for CEF and DCX were found to be 0.22 and 1.11 µg/ml, respectively (Table 2). These data show that the proposed method is sensitive for the determination of CEF and DCX.
| Parameters (units) | CEF±% RSD*(n=7)# | DCX±% RSD (n=7) |
|---|---|---|
| Retention time (min) | 3.35±0.07 | 1.92±0.21 |
| Tailing factor | 1.15±0.27 | 0.94±1.82 |
| Theoretical plates | 2323±0.33 | 2546±0.48 |
| Capacity factor | 5.74±0.83 | 2.99±0.59 |
| Resolution | 5.53±1.65 | |
| *% RSD=Relative standard deviation, #n=7-Number of observations, CEF=Cefpodoxime proxetil, DCX=Dicloxacillin | ||
Table 1: system suitability parameters.
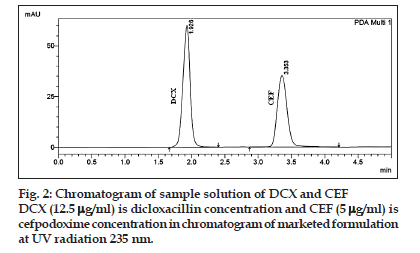
Specificity of the method was confirmed from the resolution factor and peak purity data of the analyte. The resolution factor obtained for the CEF and DCX from the nearest resolving peak was >2 in all samples. The standard chromatogram of CEF and DCX display good resolute peak (fig. 1) and no interference from excipients present in the formulation indicate specific nature of the method (fig. 2). The peak purity data of the CEF and DCX show that no other excipient is coeluted with the drug and the peak of the drug is pure in nature. The recovery experiment was performed by the standard addition method. The mean recoveries obtained were 99.77±0.89 and 99.94±0.45% for CEF and DCX, respectively (Table 2). The low value of standard deviation indicates that the proposed method is accurate. Results of recovery studies are shown in Table 3.
The robustness was studied by analysing the same samples of CEF and DCX by small but deliberate variation in the method parameters and the change in the responses of CEF and DCX were noted. Robustness of the method was studied by changing the composition of mobile phase by ±1.0 ml of organic solvent (0.001% trifloroaceticacid:meth anol:acetonitrile, composition (a) 19:51:30, (b) 21:49:30, (c) 19:50:31, (d) 21:50:29), flow rate by ±0.1 ml/min (flow rate 1.1 and 0.9 ml/min), column oven temperature by ±5° (25 and 35°) and mobile phase pH by ±0.05 unit (pH 6.45 and 6.55). None of the alterations caused a significant change in resolution between CEF and DCX, peak area % RSD, retention time % RSD, tailing factor and theoretical plates and the results are reported in Table 4.
| Parameters (units) | RP?HPLC method | |
|---|---|---|
| CEF | DCX | |
| Concentration range (µg/ml) | 0.5?20 | 5?50 |
| Regression equation (y=mx+c) |
y=44004.74x +14247.58 |
y=30461.62x +5467.59 |
| Slope | 44,004 | 30,461 |
| Intercept | 14,247 | 5467 |
| Correlation coefficient | 0.9996 | 0.9987 |
| LOD (µg/ml) | 0.0726 | 0.3685 |
| LOQ (µg/ml) | 0.22 | 1.116 |
| % Recovery±SD (accuracy, n=6) | 99.77±0.89 | 99.94±0.45 |
| Repeatability (% RSD, n=6) | 0.211 | 0.382 |
| Precision (% RSD) | ||
| Interday (n=3) | 0.70?1.45 | 0.63?1.42 |
| Intraday (n=3) | 0.12?0.48 | 0.11?0.59 |
| RP?HPLC=Reversed?phase high?performance liquid chromatography,CEF=Cefpodoxime proxetil, DCX=Dicloxacillin, LOD=Limit of detection, LOQ=Limit of quantification, RSD=Relative standard deviation |
||
Table 2: Regression analysis data andsummary of validation parameters.
| Drug | Level | Amount of sample taken (µg/ml) |
Amount of standard spiked (%) |
Mean % recovery±SD |
|---|---|---|---|---|
| CEF | I | 5 | 50 | 98.4±0.89 |
| II | 5 | 100 | 99.6±0.87 | |
| III | 5 | 150 | 100.13±0.89 | |
| DCX | I | 12.5 | 50 | 99.47±0.46 |
| II | 12.5 | 100 | 99.98±0.45 | |
| III | 12.5 | 150 | 100.38±0.45 | |
| CEF=Cefpodoxime proxetil, DCX=Dicloxacillin, SD=Standard deviation | ||||
Table 3: recovery data.
Solution stability
The difference in the initial value of percentage assay and the values obtained at 0, 12, 24 and 48 h of percentage assay should not be more than 2.0%. The assay obtained at different time intervals were compared with the initial assay values. Solution stability period for sample solution and standard solution was determined. Standard and sample solutions were stable till 48 h. Solution stability period for standard preparation and sample preparation were found to be within the acceptance criteria.
Analysis of marketed formulation
The proposed validated method was successfully applied to determine CEF and DCX in their tablet dosage form. The amount found to be 201.1±2.62 and 501.45±2.61 mg/tablet for CEF and DCX, respectively. The result obtained for CEF and DCX was comparable with the corresponding labelled amounts (Table 5). The RP?HPLC chromatogram for CEF and DCX of sample was recorded and shown in fig. 2.
Results and Discussion
A RP?HPLC method was developed and validated for the determination of CEF and DCX in tablet dosage forms on a column (C18, 250×4.6 mm i.d., 5 µm) with variable wavelength detection at 235 nm. The retention times of DCX and CEF was 1.92 and 3.35 min, respectively. Linear correlation was obtained between area and concentrations of CEF and DCX in the concentration ranges of 0.5?20 and 5?50 µg/ml, respectively. The low RSD values of interday (0.70?1.45% for CEF and 0.63?1.42% for DCX) and intraday (0.12?0.48% for CEF and 0.11?0.59% for DCX) at 235 nm, reveal that the proposed method is precise. The LOD and the LOQ for CEF and DCX were found to be 0.07 and 0.36 μg/ml and 0.22 and 0.38 μg/ml, respectively. These data show that method is sensitive for the determination of CEF and DCX.
| Parameters altered | Values altered |
% RSD of RT | % RSD of peak area |
||
|---|---|---|---|---|---|
| CEF | DCX | CEF | DCX | ||
| Flow rate (±0.1 min/ml) | 0.9 | 0.222 | 0.407 | 0.116 | 0.451 |
| 1.1 | 0.569 | 0.626 | 0.041 | 0.022 | |
| pH change (±0.05) | 6.55 | 0.095 | 1.233 | 0.772 | 1.069 |
| 6.45 | 0.634 | 0.141 | 0.254 | 0.329 | |
| Mobile phase composition (0.001% TFA: MeOH: ACN) (20:50±1:30±1) |
19:51:30 | 0.221 | 0.271 | 0.106 | 0.018 |
| 21:49:30 | 0.12 | 0.11 | 0.004 | 0.179 | |
| 19:50:31 | 0.424 | 0.943 | 0.072 | 0.074 | |
| 21:50:29 | 0.226 | 0.303 | 0.122 | 0.49 | |
| Column temperature (±5°C) | 35°C | 1.096 | 1.384 | 0.918 | 1.41 |
| 25°C | 0.588 | 0.409 | 0.889 | 0.097 | |
| TFA=Trifloroaceticacid, ACN=Acetonitrile, MeOH=Methanol, CEF=Cefpodoxime proxetil, DCX=Dicloxacillin, RT=Room temperature, RSD=Relative standard deviation |
|||||
Table 4: Robustness data.
The recovery experiment was performed by the standard addition method. The mean recoveries were 99.77±0.89 and 99.94±0.45 for CEF and DCX, respectively (Table 2). The results of recovery studies indicate that the proposed method is highly accurate. The proposed validated method was successfully applied to determine CEF and DCX in their tablet dosage form. The results obtained for CEF and DCX were comparable with the corresponding labelled amounts (Table 3). No interference of the excipients with the absorbance of interest appeared; hence, the proposed method is applicable for the routine simultaneous estimation of CEF and DCX in pharmaceutical dosage forms.
A simple, linear, accurate, specific and selective RP?HPLC method was developed and validated for estimation of CEF and DCX in their combined dosage form. In this proposed method the linearity is observed in the concentration range of 0.5?20 µg/ml for CEF and 5?50 µg/ml for DCX with coefficient of correlation, (r2)=0.9996 and (r2)=0.9987 for CEF and DCX, respectively at 235 nm. The result of the analysis of pharmaceutical formulation by the proposed method is highly reproducible and reliable and it is in good agreement with the label claim of the drug. The method can be used for the routine analysis of the CEF and DCX in combined dosage form without any interference of excipients.
| Sample no. (n=6) | Label claim | Amount found | Percent of label claim | |||
|---|---|---|---|---|---|---|
| CEF (mg/tab) | DCX (mg/tab) | CEF (mg/tab) | DCX (mg/tab) | CEF (%) | DCX (%) | |
| 1 | 200 | 500 | 202.46 | 501.1 | 101.23 | 100.22 |
| 2 | 200 | 500 | 204.12 | 506.62 | 102.06 | 101.32 |
| 3 | 200 | 500 | 203.44 | 499.6 | 101.72 | 99.92 |
| 4 | 200 | 500 | 198.54 | 501.53 | 99.27 | 100.3 |
| 5 | 200 | 500 | 197.86 | 499.45 | 98.93 | 99.89 |
| 7 | 200 | 500 | 200.18 | 501.45 | 100.09 | 100.29 |
| Mean±SD | 201.1±2.62 | 501.45±2.61 | 100.55±1.31 | 100.32±0.52 | ||
| CEF=Cefpodoxime proxetil, DCX=Dicloxacillin, SD=Standard deviation | ||||||
Table 5: Analysis of formulation of cefpodoxime proxetil and dicloxacillin by reversed?phase high?performance liquid chromatographic method.
Acknowledgements
The authors thank the Indica Laboratories Ltd., Ahmedabad, Gujarat, India for providing gift sample of CEF and DCX for research. The authors also thank the Center for Health Science Studies, Ganpat University, Ganpat Vidyanagar ? 384 012, Mehsana, Gujarat, India for providing all the facilities to carry out the work.
References
- O'Neill MJ. editor. The Merck Index. An Encyclopedia of Chemicals,Drugs and Biologicals. 14th ed. Whitehouse Station, New Jersey:Merck Research Laboratories, Division of Merck and Co., Inc.; 2006.p. 319.
- Sweetman SC, editor. The Martindale: The Complete Drug Reference. 35th ed. London: Pharmaceutical Press; 2007. p. 207.
- Indian Pharmacopeia. Vol. 3. New Delhi: The Controller Publication, Govt. of India; 2010. p. 1018.
- The United State Pharmacopeia. USP28-NF23. Rockville, MD: United State Pharmacopeial Convention, Inc.; 2005. p. 397.
- Darji BH, Shah NJ, Patel AT, Patel NM. Development and validation of a HPTLC method for the estimation of cefpodoximeproxetil. Indian J Pharm Sci 2007;69:331-3.
- Singh S, Dubey N, Jain D, Tyagi L, Singh M. Spectrophotometric and RP-HPLC methods for simultaneous determination of cefpodoximeproxetil and clavulanate potassium in combined tablet dosage form. Am Eurasian J Sci Res 2010;5:88-93.
- Gandhi SV, Patil UP, Patil NG. Simultaneous spectrophotometric determination of cefpodoximeproxetil and potassium clavulanate.Merck Research Laboratories, Division of Merck and Co., Inc.; 2006. p. 523.
- O'Neill MJ. editor. The Merck Index. An Encyclopedia of Chemicals,Drugs and Biologicals. 14th ed. Whitehouse Station, New Jersey:Merck Research Laboratories, Division of Merck and Co., Inc.; 2006.p. 523.
- Sweetman SC, editor. The Martindale: The Complete Drug Reference. 35th ed. London: Pharmaceutical Press; 2007. p. 237.
- Indian Pharmacopeia. Vol. 3. New Delhi: The Controller Publication, Govt. of India; 2010. p. 1201-2.
- The United State Pharmacopeia. USP28-NF23. Rockville, MD: United States Pharmacopeial Convention, Inc.; 2005. p. 451-2.
- Alderete O, González-Esquivel DF, Del Rivero LM, Castro Torres N. Liquid chromatographic assay for dicloxacillin in plasma. J ChromatogrB AnalytTechnol Biomed Life Sci 2004;805:353-6.
- Morelli B. Second-derivative spectrophotometric assay of mixtures of dicloxacillin sodium and ampicillin sodium in pharmaceuticals. J Pharm Sci 1988;77:1042-6.
- Barot T, Patidar K, Kshartri N, Vyas N. Development and validation of LC method for the determination of ampicillin and dicloxacillin in pharmaceutical formulation using an experimental design. Eur J Chem2009;6:955-64.
- Kathiresan K, Murugan R, Hameed SM, Inimai GK, Kanyadhara T. Analytical method development and validation of cefixime and dicloxacillin tablets by RP-HPLC. Rasayana J Chem 2009;2:588-92.
- Zhang MJ, Zhang HJ, Guan X, Xiao ZH. HPLC determination of dicloxacillin and amoxicillin in human plasma. YaowuFenxiZazhi2006;26:228-31.
- The International Conference on Harmonization, Q2 (R1) Validation of Analytical Procedure: Text and Methodology, 2005. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf [Last Accessed on 2012 Dec 28].