- *Corresponding Author:
- U. Shah
Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, CHARUSAT Campus, Changa-388421, India
E-mail: umangshah.ph@ecchanga.ac.in
| Date of Submission | 31 May 2013 |
| Date of Decision | 18 March 2014 |
| Date of Acceptance | 26 March 2014 |
| Indian J Pharm Sci 2014;76(3):198-202 |
Abstract
The present study depicts the development of a validated RP-HPLC method for the determination of the pamabrom in presence of degradation products or other pharmaceutical excipients. Stress study was performed on pamabrom and it was found that it degrade sufficiently in acidic, alkali and oxidative condition but less degradation was found in thermal and photolytic condition. The separation was carried out on Enable G 120 A 0 (250×4.6 mm, 5 µ) column having particle size 5 µ using methanol: water (75:25 v/v) with pH 4.0 adjusted with ortho phosphoric acid as mobile phase at flow rate of 1 ml/min.The wavelength of the detection was 280nm. A retention time (R t ) nearly 3.9 min was observed. The calibration curve for pamabrom was linear (r2 = 0.9997) from range of 10-60 µg/ml with limit of detection and limit of quantification of 1.41 µg/ml and 4.28 µg/ml, respectively. Analytical validation parameter such as selectivity, specificity, linearity, accuracy and precision were evaluated and relative standard deviation value for all the key parameters were less than 2.0%. The recovery of the drug after standard addition was found to be 101.35%. Thus, the developed RP-HPLC method was found to be suitable for the determination of pamabrom in bulk as well as stability samples of tablets containing various excipients.
Keywords
Pamabrom, stability indicating, RP-HPLC, validation
Stability studies are an integral part of the drug development program. The need for the stability studies on a drug candidate arises from the fact that the chemical integrity of the drug substance should be maintained until the compound is delivered to the intended site of action. Any form of chemical instability may affect the bioavaibility and can further lead to toxic effects [1]. International Conference on Harmonization (ICH) has made mandatory requirement of stability-indicating assay method (SIAM) for every drug candidate. A SAIM is a validated qualitative analytical procedure that can detect the changes with time in the properties of the drug product and drug substance under defined storage condition. In this study, the drug candidate is exposed to a variety of stress condition like acidic, alkali, oxidative, thermal and photolytic [2].
As per ICH guideline stress testing of the drug substance aids in identifying degradation products and mechanism of breakdown, the condition under which this breakdown occur, and appropriate methodology for assessing stability it can also help in establishing the degradation pathway [3].
Pamabrom chemically, 2-amino-2-methyl-propanol 8-bromo theophylline is a weak diuretic (fig. 1). It is soluble in water and methanol, it is white crystalline powder. It is effective in treating dysmenorrhoea and premenstrual syndrome [4]. It is only official in United State Pharmacopoeia [5]. Literature review reveals that various analytical methods like UV Spectrophotometry [6], HPLC [7], HPTLC [8], human plasma [9] and other analytical methods have been developed for individually and combination with other drug. However, no stability indicating RP-HPLC method was developed for estimation of pamabrom in pharmaceutical dosage form. The ICH [10,11] guideline entitled “Stability testing of new drug substances and products” requires that stress testing be carried out to elucidate the inherent stability characteristics of the active substance. An ideal stability-indicating method is one that resolves the drug and its degradation products efficiently. Consequently, the implementation of an analytical methodology to determine pamabrom in presence of its degradation products is rather a challenge for pharmaceutical analyst. Hence, it was proposed to develop simple, accurate, precise and specific Stability indicating RP-HPLC method for estimation of pamabrom in pharmaceutical dosage form.
Materials and Methods
The pamabrom reference standard was kindly gifted by Suven Life Science Ltd (Hyderabad, India). Methanol and acetonitrile (ACN) were procured from Loba Chemi Pvt. Ltd., Mumbai, India as a solvent for the preparation of stock and working standard solution.
Chromatographic separation was performed on a Shimadzu HPLC system consisting of pump (LC 20AT Shimadzu), detector PDA (SPD - M20A, Shimadzu), injection system (Rheodyne System 20 μl loop), oven (CTO -10AS, Shimadzu). An ODS C18 RP-Column (Enable, 250×4.6 mm, 5 μ). The elution was carried out isocratically at flow rate of 1ml/min using methanol:water (75:25 v/v) with pH 4.0 adjusted with ortho phosphoric acid as a mobile phase. Shimadzu AUX 220 analytical balance was used for weighing.
Preparation of standard stock and working standard solution
Accurately weighed 10 mg of pamabrom was transferred to 100 ml volumetric flask. The volume was made up to the mark with mobile phase to obtain stock solution of pamabrom having concentration 100 μg/ml. From this solution prepared working range concentration. Linearity range solutions conytaining 10, 20,30,40,50 and 60 μg/ml of pamabrom were prepared.
Optimization of mobile phase
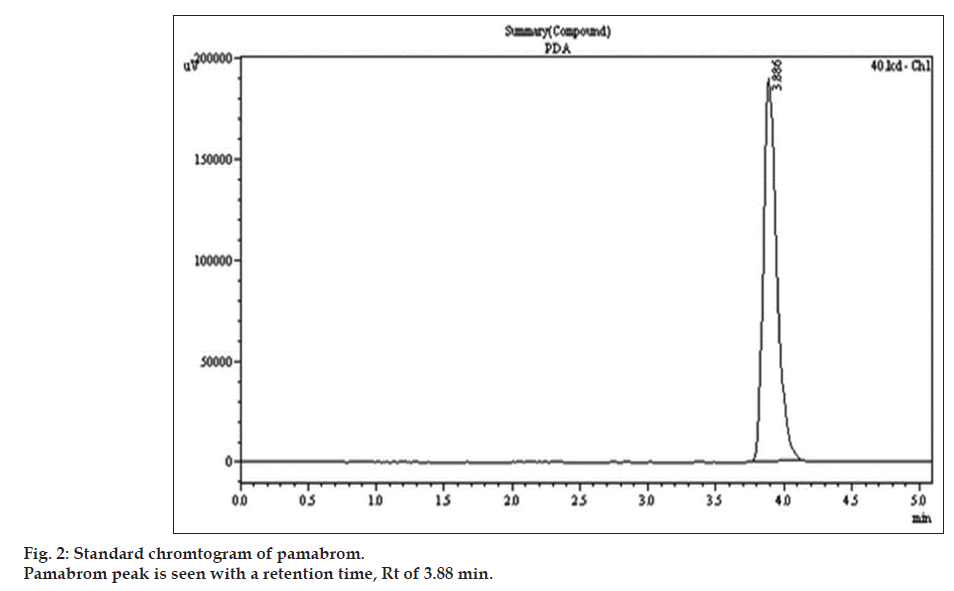
Optimization of mobile phase was performed based trial and error method. In this different mobile phase trial was taken like in methanol:water, ACN:water and methanol:ACN:water in different ratio without pH but there are different problem were observed like high tailing factor value and not optimized theoretical plate. After that trial with methanol:water (75:25) pH 4 adjusts by ortho phosphoric acid, in this pamabrom full fill all the criteria of system suitability. The mobile phase consisting of methanol:water (75:25 v/v) was selected which gave sharp, well-separated peak from pamabrom peak (fig. 2).The retention time for pamabrom was 3.888 min. So, finally methanol:water (75:25 v/v) pH 4 adjusted by ortho phosphoric acid mobile phase was selected as analysis.
Preparation of mobile phase and stock solutions
Seven hundred and fifty millilitres of methanol and 250 ml of water were mixed and pH of mixture was adjusted to 4.0 with ortho phosphoric acid. This mixture was sonicated for 10 min and filtered through 0.22 μm membrane filter and used as mobile phase.
Validation and system suitability parameters
The developed method was validated for different parameters like linearity, accuracy, precision, specificity, repeatability, limit of detection (LOD), limit of quantification (LOQ) and robustness [9]. Suitability of the chromatographic system was tested before each stage of validation. Five replicate injections of standard preparation were injected and Retention time, Tailing factor, number of theoretical plates, and relative standard deviation of peak area were determined.
Linearity and repeatability
Under proposed experimental conditions, the relationship between the area and the concentration of pamabrom was studied. The calibration curve was plotted between concentrations versus area by the prepared concentration of 10-60 μg/ml of stock solution, and r2 value was found to be 0.9997 (Table 1). Six replicate of prepared 10 μg/ml solution of pamabrom taken from different stock solution and measured area. The relative standard deviation (%RSD) was found to be less than 2 %, which indicates that the proposed method is repeatable.
| Parameters | Result |
|---|---|
| Linearity range (µg/ml) | 10‑60 |
| Intraday precision (% RSD) (n=3) | 0.824‑1.403 |
| Interday precision (% RSD)(n=3) | 1.012‑1.924 |
| Linear regression equation | y=32049x+28944 |
| % recovery | 101.344 |
| LOD (µg/ml) | 1.41 |
| LOQ (µg/ml) | 4.28 |
| Robustness | Robust |
n=3 observations, LOD: limit of detection, LOQ: limit of quantification, RSD: relative standard deviation
Table 1: Summary Of Validation Parameters
Precision and specificity
Intraday and interday precision were carried out through replicating analysis (n=3) for 3 concentrations (10, 40 and 60 μg/ml). For interday precision, the analysis was carried out for three consecutive days at the same concentration level as used in intraday precision. And the intraday precision was carried out by using three concentrations at different time interval in a day [11]. The area was recorded as %RSD (Table 1). The prepared standard, sample solutions and the blank solution were injected and check any other excipients interference occurs or not.
Assay of marketed formulation
Twenty tablets were accurately weighed and finely powdered. A quantity of the powder equivalent to 10 mg of pamabrom was extracted by shaking with 20 ml of mobile phase, followed by another two extractions each with 10 ml of mobile phase. It was filtered through Whatmann filter paper no. 42 to remove insoluble materials. The volume of filtrate was diluted to 100 ml with mobile phase (100 μg/ml). It was further diluted according to the need and then analyzed following the proposed procedures. The nominal content of the tablets was calculated either from the previously plotted calibration graphs or using regression equations (Table 2).
| Name of drug | Label claim | Final concentration | Concentration* found±SD | % assay± % RSD |
|---|---|---|---|---|
| Pamabrom | 50 mg | 10 µg/ml | ±0.46 | 101.772±0.456 |
SD: standard deviation, RSD: relative standard deviation, *n=6
Table 2: Assay Of Formulation
Accuracy, LOQ, LOD and robustness
To find the accuracy of the method, the recovery experiment was carried out using the standard addition method. For the previously analyzed sample (20 μg/ml), a known amount of standard drug was added at 50, 100 and 150 % level. The contents were re-analyzed with the above described procedure (Table 3). LOD and LOQ were determined using the eqns., LOD = 3.3×σ/S and LOQ = 10×σ/S, where, σ is the standard deviation of the response and S is the slope of the calibration curve. The robustness of a method is its ability to remain unaffected by small changes in parameters like changes in Flow rate, change mobile phase composition, change in wavelength, and change in pH.
| Level (%) | Sample conc. (µg/ml) | Amt. of std. added (µg/ml) | Total conc. (µg/ml) | Found conc. (µg/ml) | % RSD | % recovery |
|---|---|---|---|---|---|---|
| 50 | 20 | 10 | 30 | 30.326 | 0.429 | 101.59 |
| 30 | 30.576 | |||||
| 30 | 30.531 | |||||
| 100 | 20 | 20 | 40 | 40.721 | 0.651 | 101.099 |
| 40 | 40.411 | |||||
| 40 | 40.185 | |||||
| 150 | 20 | 30 | 50 | 50.647 | 0.599 | 101.345 |
| 50 | 50.994 | |||||
| 50 | 50.377 |
SD: standard deviation, RSD: relative standard deviation
Table 3: Accuracy Data Of Pamabrom
Forced degradation studies
To perform the forced degradation study, 10 mg drug was subjected to acidic, alkaline, oxidizing, thermal and photolytic conditions. For acidic degradation, 10 mg drug was dissolved in 5 ml of methanol to which 5 ml of 1 M HCl was added and heated under reflux at 70º for 4 h. The mixture was neutralized by the addition of 1M NaOH. For alkaline degradation, 10 mg drug was dissolved in 5 ml of methanol to which 5 ml of 1 M NaOH was added and heated under reflux at 70º for 2 h before the mixture was neutralized by the addition 1 M HCl. For degradation under oxidizing conditions the drug was heated under reflux with 3 % H2O2(v/v) at 40º for 30 min. For thermal degradation the powdered drug was exposed at 80º for 5 days. For photolytic degradation the powdered drug was exposed to sunlight for 12 days. Pharmaceutical dosage form was also subjected to the same stress conditions to determine whether any peaks arose from the degraded excipients. After completion of the treatments, the solutions were left to return to room temperature, diluted with solvent mixture to obtain 40 μg/ml solutions. The purity of the drug peak obtained from the stressed sample was measured by using a PDA detector.
Results and Discussion
Optimizations of chromatographic conditions were performed to obtain the good peak shape and peak parameter (tailing factor, theoretical plates). For the selection of mobile phase initially methanol:water and ACN:water has been tried in different proportion but it gave poor peak shape and also poor system suitability parameters. Then ACN:methanol in different ratio have been tried but again obtained poor peak shape. Finally, methanol:water mixture was tried at pH 4 adjusted with orthophosphoric acid (75:25 v/v) at a flow rate of 1 ml/min was found to be satisfactory and good system suitability parameters. The average retention time (Rt) for pamabrom was 3.887±0.088. The tailing factor and theoretical plates for pamabrom were found to be 1.475±0.415 and 6166±0.944, respectively. The developed HPLC method was validated. The linear range, correlation coefficient, detection limit and standard deviation for pamabrom by HPLC method are shown in Table 1. Accuracy was determined by calculating the % recovery. The method was found to be accurate with % recovery between 98-102%.Precision was calculated as repeatability and intra and interday variation for pamabrom. The method was precise with % RSD less than 2%. The method was specific as no interference observed when the drugs were estimated in presence of excipients.
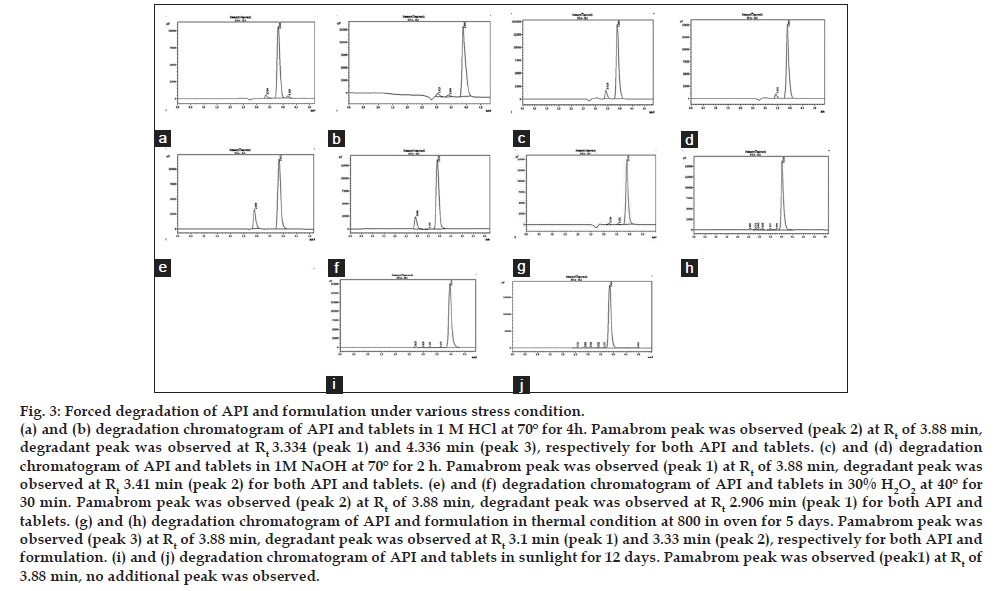
Degradation behaviour of the pamabrom under various stress condition: The drug was found to stable to thermal (80° for 5 days) and under photolytic condition (Sunlight for 12 days). The % of drug degraded after thermal and photolytic condition was 17.21% and 6.07 % respectively. In acidic condition (1M HCl, 70°, 4 h) 37.22% of drug was degraded with generation of two new peaks in addition to peak of the pamabrom. Under alkali condition (1M NaOH, 70°, 2 h) 24.76% of the drug was degraded with generation of one new peak with continuously reduction in peak area of pamabrom and in oxidative (3% H2O2, 40°, 30 min) 27.57% of the drug was degraded with the formation of one new peak (fig. 3 and Table 4).
Fig. 3: Forced degradation of API and formulation under various stress condition. (a) and (b) degradation chromatogram of API and tablets in 1 M HCl at 70º for 4h. Pamabrom peak was observed (peak 2) at Rt of 3.88 min, degradant peak was observed at Rt 3.334 (peak 1) and 4.336 min (peak 3), respectively for both API and tablets. (c) and (d) degradation chromatogram of API and tablets in 1M NaOH at 70º for 2 h. Pamabrom peak was observed (peak 1) at Rt of 3.88 min, degradant peak was observed at Rt 3.41 min (peak 2) for both API and tablets. (e) and (f) degradation chromatogram of API and tablets in 30% H2O2 at 40º for 30 min. Pamabrom peak was observed (peak 2) at Rt of 3.88 min, degradant peak was observed at Rt 2.906 min (peak 1) for both API and tablets. (g) and (h) degradation chromatogram of API and formulation in thermal condition at 800 in oven for 5 days. Pamabrom peak was observed (peak 3) at Rt of 3.88 min, degradant peak was observed at Rt 3.1 min (peak 1) and 3.33 min (peak 2), respectively for both API and formulation. (i) and (j) degradation chromatogram of API and tablets in sunlight for 12 days. Pamabrom peak was observed (peak1) at Rt of 3.88 min, no additional peak was observed.
| Degradation condition | % drug degraded in API | % drug degrade in tablets | Peak purity index | |
|---|---|---|---|---|
| HCl (1 M, 70°, 4 h) | 37.22 | 29.78 | 1 | |
| NaOH (1 M, 70°, 2 h) | 24.76 | 22.17 | 1 | |
| H2O2 (3%, 40°, 30 min) | 27.57 | 26.82 | 1 | |
| Thermal degradation (80°, 5 days) | 17.21 | 14.39 | 1 | |
| Sunlight (12 days) | 6.07 | 4.45 | 1 | |
| API=Active pharmaceutical ingredient |
API=Active pharmaceutical ingredient
Table 4: Forced Degradation Study Data
The intensive approach described in this manuscript was used to develop and validate a liquid chromatographic analytical method that can be used for assay of pamabrom in a pharmaceutical dosage form. Degradation products produced as a result of stress did not interfere with detection of pamabrom and the assay method can thus be regarded as stability indicating. This HPLC method for assay of pamabrom in a tablet formulation was successfully developed and validated for its intended purpose. The method was shown to specific, linear, precise, accurate, and robust. Because the method separates pamabrom and all the degradation products formed under variety of stress conditions it can be regarded as stability indicating. This method is recommended to the industry for quality control of drug content in pharmaceutical preparations. From this study we can conclude that there was no special precaution required during manufacturing and storage of formulation because there was no observed any degradation at room temperature in any of the condition.
Acknowledgements
The author(s) thank Suven Life Sciences Ltd. for providing gift sample of pamabrom, and Ramanbhai Patel College of Pharmacy (CHARUSAT), Changa for providing the necessary infrastructure to carry out the research.
References
- Bakshi M, Singh S. Development of validated assay methods-critical review. J Pharm Biomed Anal 2002;28:1011-40.
- ICH. Stability testing of new substances and product. Geneva: International conference on harmonization, IFPMA; 2003.
- ICH. Stability Testing: Photo stability testing of new drug substance and products. Geneva: International conference on harmonization, IFPMA; 1996.
- Drug Bank – Open data drug and drug target database; Available from: http://www.drugbank.ca/drugs/DB00218 [Last accessed on 2012 Jul 08].
- United State Pharmacopoeia/National Formulary. 24th ed. Rockville, MD: Pharmacoepial Convention; 2000. p. 2847.
- Padaliya H, Bhati M, Sanjaysinh V, Patel H, Pancholi SS. Development and validation of UV spectrophotometric methods for estimation of paracetamol and pamabrom in bulk and synthetic mixture by simultaneous equation method. Inventi Rapid: Pharm Anal QualAssu 2013;1:1-4
- Zhihong C, Wei WJ, Jianjun L, Ling-Bo Q. RP-HPLC Determination Pamabrom raw materials and Pamabrom tablets for Pamabrom content. J Zhengzhou Uni (Med Sci) 2008;1:165-6.
- Prajapati PP, Captain AD, Patel DS. Development and validation of HPTLC method for simultaneous determination of Paracetamol and Pamabrom in synthetic mixture. Int Res J Pharm 2012;11:167-71.
- Zhou L, Gu L, Wang Y, Linang J. HPLC for the determination of two constituents in compound Acetaminophen and Pamabrom tablets in human plasma. Chin J N Drug ClinRemed 2007;3:61-5.
- ICH. Q2 (R1) validation of analytical procedure, text and methodology. International conference on Harmonization. Nov, 1996.
- International conference of harmonization (ICH) of technical requirements for the registration of pharmaceuticals for human use, validation of analytical procedures; methodology adopted in Geneva. Nov, 1996.