- *Corresponding Author:
- M. Y. Saka
King Fahd Medical Research Center, King Abdulaziz University, Jeddah 21589, Saudi Arabia
E-mail: myassin3@hotmail.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “168-177” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Decades of comprehensive research have resulted in a better understanding of the crucial role of phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling in different types of leukemia whereby it strongly influences the growth and survival of cancerous cells. Recently, small-molecule inhibitors have been introduced as targeted therapies that are more tolerable than conventional antineoplastic drugs for leukemia. The growth inhibition assays were performed to assess the effectiveness of phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway inhibitors like everolimus, torkinib and phosphokinase inhibitor 402 in leukemia cell lines. The cell cycle profiles, protein kinase B and S6 phosphorylations were assessed by flow cytometry. We also screened the expression of 96 cancer-associated long non-coding RNAs upon phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway inhibition. Compared to untreated cells, the smallmolecule inhibitors treated cells showed reduced viability. Phosphokinase inhibitor 402, a dual pathway inhibitor was most effective in all cell lines (56.6 %, 32.5 % and 11.4 % in Jurkat, HL-60 and K562 cells, respectively). In addition, treatment with this resulted to shift the cells to the G1 phase from the S1 phase (Jurkat, G1: 12.2 %, S1: 4.9 %; HL-60, G1: 38.4 %, S1: 44.4 %; K562 G1: 14.4 %, S1: 15.7 %), more effectively than everolimus or torkinib. It was observed that when this inhibitor was used, the long non-coding RNAs retinal non-coding RNA 3 (3-fold) and highly up-regulated in liver cancer (2-fold) levels were significantly elevated whereas antisense non-coding ribonucleic acid in the INK4 locus and zinc finger homeobox 2 were significantly decreased by at least 25 %. Our findings revealed that dualspecificity inhibitors are more potent than single-specificity inhibitors. Hence, dual-targeted therapy may be a promising therapeutic option for leukemia. In addition, long non-coding RNAs may play a key role in drug resistance and could be a potential novel therapeutic target.
Keywords
Leukemia, phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway, long non-coding ribonucleic acids, everolimus, torkinib, phosphokinase inhibitor 402
Leukemias are a group of malignant cancers of hematological stem cells involving bone marrow and other hematopoietic organs yielding high number of immature or abnormal leukocytes[1,2]. In total of around 12 types of malignant neoplasms, leukocytes are grouped under leukemias, where the most common types are Acute Myeloid Leukemia (AML) and Acute Lymphoblastic Leukemia (ALL) that accumulate cancerous cells in the bone marrow[3]. As per the recent 2021 report of Global Cancer Observatory (GLOBOCAN), it is estimated that leukemias are the 15th most diagnosed neoplasms worldwide, where the total of 311 594 death cases were reported out of 474 519 diagnosed cases[4]. Accordingly, the Saudi Cancer Registry (SCR) has categorized leukemia as the 5th most common cancer among both genders in Saudi population[5]. The pathological progression of leukemia involves various factors; however decades of comprehensive research has reported the crucial role of Phosphatidylinositol-3-Kinase (PI3K)/Protein Kinase B (Akt)/mammalian Target of Rapamycin (mTOR) pathway signaling in different types of leukemia where it strongly influences growth and survival of cancerous cells. Moreover, increased activity of mTOR Complex 1/2 (mTORC 1/2) is reported to be involved in initiation, propagation and relapse of leukemia[6]. Upon activation by the upstream receptors, PI3K initiates the conversation/transition of Phosphatidylinositol 4,5- Bisphosphate (PIP2) into Phosphatidylinositol 3,4,5 Triphosphate (PIP3). Consequently, PIP3 triggers the activation of numerous downstream products including Akt[7-11] which in turn activates mTOR. This inappropriate activation of mTOR (a crucial regulator of normal growth and division) stimulates growth of tumor cells[12]. It has been reported that mTOR phosphorylates ribosomal S6 Protein Kinase 1 (S6K1) and Eukaryotic Initiation Factor 4E-Binding Protein 1 (4E-BP1). In its hypo-phosphorylated form, 4E-BP1 attaches itself to eukaryotic Initiation Factor 4E (eIF4E) thereby preventing its interaction with eIF4G thus hinders in the recruitment of ribosomes to messenger Ribonucleic Acids (mRNAs) repressing translation. When phosphorylated, mTOR separates from eIF4E and promotes cap-dependent translation[13,14]. By doing so, mTOR increases synthesis of proteins, which are important for cell cycle regulation.
One of the recent trends in cancer treatment is molecular targeted therapies which involves preventing cancer progression and metastasis by utilizing drug molecules that interfere with a target molecule or "specific molecule"[15,16]. Targeted therapy employs its anti-cancer effects via several mechanisms, including the inhibition of cellular proliferation, inducing apoptosis, suppressing initiation of metastasis, regulation of immunological factors and reversal of multidrug resistance[17]. Several molecular targeted therapies have been approved by the Food and Drug Administration (FDA) that have established significant clinical success for the treatment of many types of cancers including leukemias, breast cancer, colorectal cancer, lungs cancer and ovarian cancer[15,18].
Long non-coding RNAs (lncRNAs), also referred to as large RNA, macroRNA, or long intergenic ncRNA are mRNA-like transcripts with lengths varying between 200 nucleotides and 100 kilobases (kb) and sequences that lack open reading frames of meaningful length[19-21]. These RNA molecules have a broad spectrum of molecular and cellular functions and they are heterogeneous, and implement diverse modes of action[22]. For cancer cells however, distinguishing key lncRNAs from noise-related molecules remain a challenge[23-25]. The role of ncRNAs and signaling of PI3K in cancer is the focus of various studies where reports are made in which lncRNA such as Colorectal Neoplasia Differentially Expressed (CRNDE) RNA cause activation of PI3K and stimulates cellular proliferation in different cancer types. Whereas some lncRNAs like Growth Arrest-Specific 5 (GAS5) can cause inhibition of PI3K signaling[26]. In fact, in breast cancer cell lines like Human Breast Tumor cell line (BT-474) and Michigan Cancer Foundation-7 (MCF7), lnc-N-Acylsphingosine Amidohydrolase (lnc-ASAH2B-2) was up-regulated following treatment with everolimus, a first-generation rapamycin analogue (rapalog), reduced its expression and inhibited the proliferation of these cell lines[27]. Recent studies have reported the role of lncRNAs in leukemias and are documented as the novel therapeutic targets[28,29].
The current study has investigated the effect of targeting the PI3K/Akt/mTOR signaling pathways in three commercially available suspension leukemic cell lines (Jurkat, Human Promyelocytic Leukemia cell line (HL-60) and Human Erythroleukemic cell line (K562)) using three inhibitors-everolimus (RAD001), an exclusive mTORC1 inhibitor; Phosphokinase Inhibitor 402 (PKI402), a second-generation mTOR inhibitor with dual-specificity that targets PI3K, mTORC1 and mTORC2; and Torkinib (PP242), a second-generation mTOR inhibitor selective for mTORC1/2[30,31]. With the use of these inhibitors, the correlation between PI3K/Akt/mTOR signaling pathways and the expression levels of lncRNAs have been explored.
Materials and Methods
Cell culture and reagents:
The Chronic Myeloid Leukemia (CML) K652, Acute Promyelocytic Leukemia (APL) HL-60 and T-cell Acute Lymphoblastic Leukemia (T-ALL) Jurkat cell lines (CLS Cell Lines Service GmbH, Eppelheim, Germany) were plated in Iscove’s Modified Dulbecco’s Media (IMDM) supplemented with 10 %-20 % Fetal Bovine Serum (FBS) and 1 % penicillin-streptomycin (Lonza, Basel, Switzerland). The cells were incubated at 37° under a 5 % Carbon dioxide (CO2) atmosphere.
Phosphorylation analysis:
The cells were counted and plated in a 6-well plate at 0.5×106 cells/well. Everolimus, PP242 and PKI402 (Selleckchem, Munich, Germany, catalogue: #S1120, S2218 and S2739, respectively) were added at a final concentration of 0.1 M into a separate well each and the remaining three wells were left untreated as control cells. The cells were incubated for 24 h at 37° under a 5 % CO2 atmosphere. Subsequently, the cells were collected, centrifuged at 4° for 5 min at 400 g, washed and transferred to Eppendorf tubes. For staining, the FlowCellectTM PI3K-mTOR signalling cascade kit (Merck Millipore, Burlington, Massachusetts, United States of America (USA), catalogue: #FCCS025210) was used as suggested by the manufacturer. Briefly, 250 μl of 1× fixation buffer was added to the cells and incubated on ice in the dark for another 20 min. Subsequently, the cells were centrifuged at 4° for 5 min at 400 g and were permeabilised on ice in the dark for 20 min with 250 μl of 1× permeabilisation buffer. The cells were centrifuged at 4° for 5 min at 400×g and stained for immuno-flow cytometry. All treated cells were stained with an anti-phospho-Akt1/PKB alpha (Ser-473)-Alexa Fluor® 488-conjugated monoclonal antibody (α-p-Akt1-488) and anti-phospho- Ribosomal Protein S6 (Ser-235)-PerCP-conjugated monoclonal antibody (α-p S6-PerCP). In addition, four untreated samples were stained with either α-p-Akt1-488+α-P S6-PerCP, only α-p-Akt1-488, only α-P S6-PerCP or they were left unstained. All antibodies were added at a final concentration of 0.5 M. All cells were incubated on ice in the dark for 1 h and were subsequently washed, centrifuged at 4° for 5 min at 400×g and re-suspended in 1 ml 1× assay buffer before flow cytometry analysis (BD FACS AriaIII, Franklin Lakes, New Jersey, USA).
Cell cycle and proliferation assays:
The cells were counted and plated in a 6-well plate at 0.5×106 cells/well. Everolimus, PP242, PKI402, adriamycin (Dabur Pharmaceuticals Ltd., Mumbai, India) and colcemid (Life Technologies, Carlsbad, California, USA) were added at a final concentration of 0.1 μM into a separate well each and the remaining well was left untreated as a control. The plate was incubated for 48 h at 37° under a 5 % CO2 atmosphere. The cells were counted using Trypan blue (catalogue: #T8154, Sigma Aldrich) to assess viability and were centrifuged at 1100 rpm for 10 min at 4°, washed twice with culture media and re-suspended in 5 ml of Phosphate-Buffered Saline (PBS, Lonza, Basel, Switzerland). The cells were fixed by adding 3 ml of 100 % ethanol drop wise under vortexing at medium speed and storage at -20° for 1 w. Prior to analysis, the cells were washed twice with PBS and stained with 0.5 ml of propidium iodide combined with RNase (Sigma, St. Louis, Missouri, USA, catalogue: #P4170). All samples were incubated at 4° in the dark for 3 h and were subsequently analyzed using flow cytometry. The results were analyzed using the Modfit LT software (Verity Software House, Topsham, Maine, USA).
RNA extraction and quantitative Polymerase Chain Reaction (qPCR) assay:
The cells were counted and plated in a 6-well plate at 0.5×106 cells/well. Everolimus, PP242 and PKI402 (Selleckchem, Munich, Germany, catalogue: #S1120, S2218 and S2739, respectively) were added to separate wells at final concentrations of 0.1 μM each and the remaining three wells were left untreated as negative control cells. The plated cells were incubated for 48 h at 37° under a 5 % CO2 atmosphere. RNA was extracted from the treated and untreated cell lines using mirVanaTM microRNA (miRNA) isolation kit (ThermoFisher Scientific, Waltham, Massachusetts, USA, catalogue: #AM1560). The extracted RNA was converted to complementary Deoxyribonucleic Acid (cDNA) using the standard protocol for the GoScriptTM reverse transcription system (Promega, Maddison, Wisconsin USA, catalogue: #A5000). Finally, the human and mouse LncProfilersTM qPCR array kits (System Biosciences, Palo Alto, California, USA) were used to perform the lncRNA 96-well plate assay (fig. 1); the SYBR® Green PCR master mix and SYBR Green Reverse Transcription Polymerase Chain Reaction (RT-PCR) reagents kit (ThermoFisher, Waltham, Massachusetts,, USA, catalogue: #4309155) were used to perform the qPCR experiments using the primers in Table 1.
| lncRNA | Forward primer | Reverse primer |
|---|---|---|
| ANRIL | TTGAACTAAAAGCCGCTCCG | tggtggccagaaaacagaag |
| NEAT1 | ggtctgtgtggaaggaggaa | gctggcatggacaagttgaa |
| RNCR3 | agtcagtgctgggccttatt | aagaggctcgtcaatgtgga |
| UCA1 | AAAGCTGCCCCTCTCCTATC | CAGGTGGATCTCTTCACGGA |
| HULC | ggggtggaactcatgatgga | tggaggttgaaatgtccacg |
| Zfhx2as | agatccccttgtctggtgtg | aggcagtggtcaggatcttc |
| HOXA3as | AGAAAACCACGCTTTTCCCG | CTGCTCCAAAACTCTTCGCC |
| RPL11 (+ve control) | atcctttggcatccggagaa | accacatagaagtccaggcc |
Table 1: Primer Sequences for lncRNAs
Results and Discussion
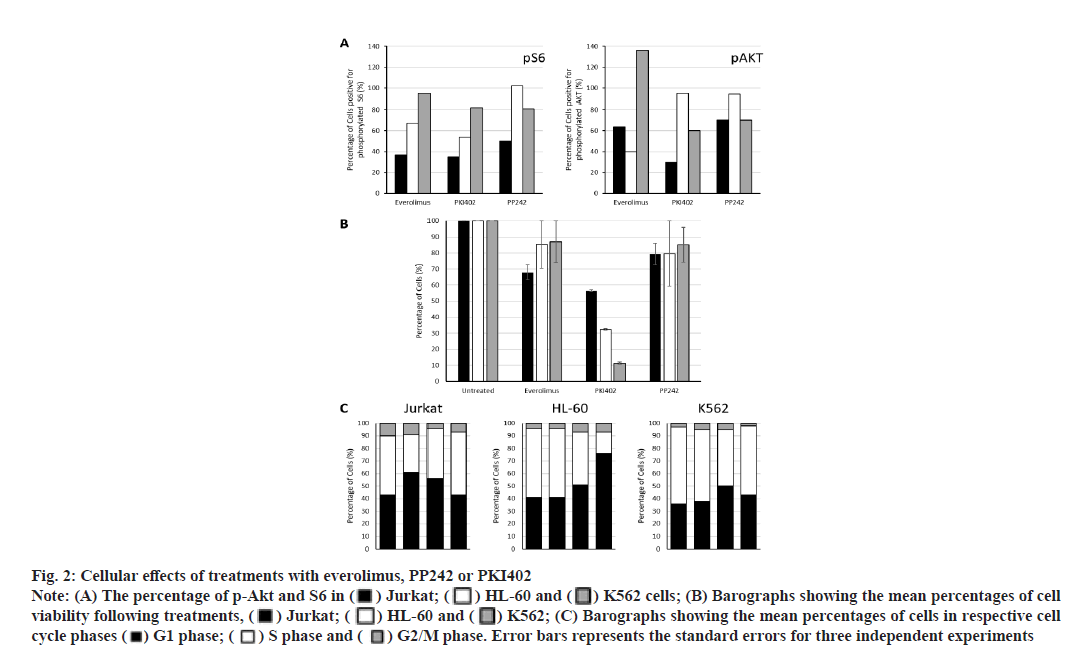
Everolimus, PP242 and PKI402 differentially affect Akt and S6 ribosomal protein (S6) phosphorylation. To measure the level of PI3K/Akt/mTOR pathway activation following drug treatment, the cells were incubated with the three targeted inhibitors separately for 24 h and the phosphorylation status of both Akt and S6 were detected by flow cytometry (fig. 2A). Everolimus, PP242 and PKI402 affected Akt and S6 phosphorylation in all three cell lines, although in a different manner. Treatment of Jurkat and HL-60 cells with everolimus reduced both p-Akt and S6. In contrast, p-Akt was increased and no significant effect on S6 phosphorylation was observed in everolimustreated K562 cells. PP242 treatment reduced p-Akt and pS6 in both Jurkat and K562 cells. No significant effect on the phosphorylation of either protein was observed in HL-60 cells. However, PKI402 caused a reduction of both p-Akt and pS6 proteins in Jurkat and K562 cells. pS6 was also reduced in PKI402-treated HL-60 cells, although the level of p-Akt was not affected.
Fig. 2:Cellular effects of treatments with everolimus, PP242 or PKI402
Note: (A) The percentage of p-Akt and S6 in ( ) Jurkat; (
) Jurkat; ( ) HL-60 and (
) HL-60 and ( ) K562 cells; (B) Barographs showing the mean percentages of cell viability following treatments, (
) K562 cells; (B) Barographs showing the mean percentages of cell viability following treatments, ( ) Jurkat; (
) Jurkat; ( ) HL-60 and (
) HL-60 and ( ) K562; (C) Barographs showing the mean percentages of cells in respective cell cycle phases (
) K562; (C) Barographs showing the mean percentages of cells in respective cell cycle phases ( ) G1 phase; (
) G1 phase; ( ) S phase and (
) S phase and ( ) G2/M phase. Error bars represents the standard errors for three independent experiments
) G2/M phase. Error bars represents the standard errors for three independent experiments
PKI402 decreases cell viability more effectively than that observed for everolimus or PP242. All three inhibitors tested reduced cell viability compared to that of untreated cells (fig. 2B). PKI402 was most effective in all cell lines tested. Interestingly, the degree of PKI402-induced cell viability reduction varied between the three cell lines with the highest effect observed in K562 cells (Jurkat, HL-60 and K562: 56.6 %, 32.5 % and 11.4 %, respectively).
PKI402 increases the accumulation of cells in the G1 phase more effectively than that observed for everolimus or PP242. To understand the potential mechanisms underlying the observed cell death, the cell cycle profiles of all treated cells were analyzed by flow cytometry (fig. 2C). The effects were variable and dependent on the targeting molecule and the cell line tested. Treatment with everolimus accumulated cells in the G1 phase and reduced the number of cells in the S phase only in Jurkat cells, while treatment with PP242 caused a small increase in the percentage of G1 phase cells in HL-60 cells only. The effect observed following treatment with PKI402 was most notable, where the percentage of cells in the G1 has increased and those in the S phase were reduced in all cell lines.
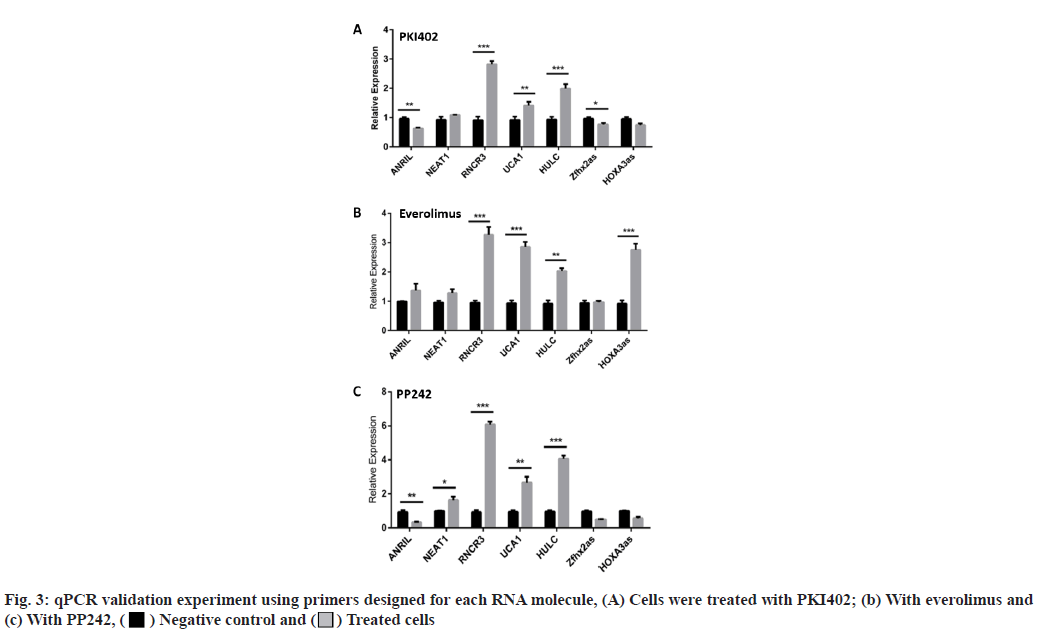
The lncRNA expression levels differed between Jurkat cells treated with dual and single molecule inhibitors. Having identified, an effect of drug treatment on the cell lines, we set out to characterize the effects of the three drugs on lncRNA expression levels. RNA was extracted from the Jurkat cell lines and was converted into cDNA using the standard protocol of GoScriptTM Reverse Transcription System (Promega). Next, the cDNA was quantified using qPCR, SYBR® green dye, SYBR green PCR master mix and SYBR green RT-PCR reagents kit (Life Technologies). The results are shown in fig. 1. In addition to the 96-well plate experiment, seven of the lncRNAs (Antisense Noncoding RNA in the INK4 Locus (ANRIL), Nuclear Enriched Abundant Transcript 1 (NEAT1), Retinal Noncoding RNA 3 (RNCR3), Urothelial Carcinoma-Associated 1 (UCA1), Highly Up-regulated in Liver Cancer (HULC), Zinc finger homeobox 2 antisense RNA (Zfhx2as) and Homeobox A3 antisense RNA (HOXA3as)) exhibiting high fold changes (fig. 3) were picked for a validation of qPCR experiment, using primers designed for each RNA. Everolimus and PP242 were also used to test for lncRNA expression differences between a singlemolecule and dual-molecule inhibitors.
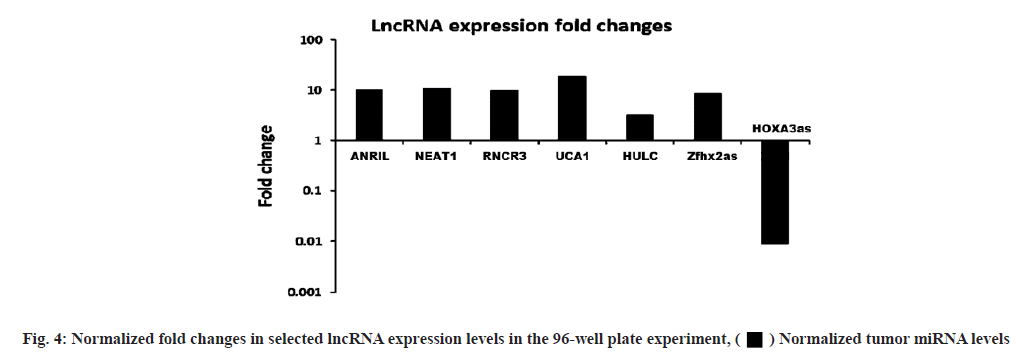
LncRNA expression levels differed between dual and single-molecule inhibitors. The validation experiment revealed that when PKI402 was used, RNCR3 and HULC levels were significantly elevated 3-fold and 2-fold increases respectively, whereas ANRIL and Zfhx2as were significantly lowered by at least 25 % (fig. 3A). Cells treated with everolimus exhibited a significant increase in the level of RNCR3 (3-fold), UCA1 (1.5-fold) and HOXA3as (3-fold) (fig. 3B). The PP242 treated cells exhibited the highest-level of increase to RNCR3 (6-fold) and HULC (4-fold) of all treated cells, whereas ANRIL levels in these cells were significantly lowered by more than 80 % (fig. 3C). The normalized fold changes in selected seven lncRNA expression levels are shown in fig. 4.
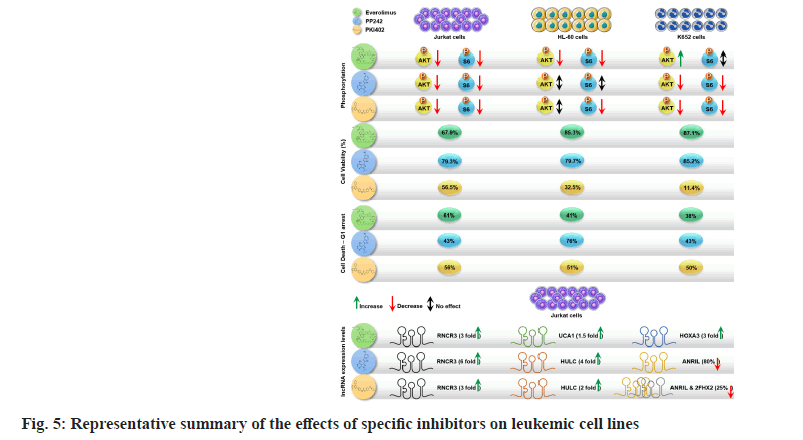
Our study, has evaluated the role of three mTORC pathway inhibitors (everolimus, PP242 and PKI402) in different leukaemic cell lines namely CML cell line (K652), APL cell line (HL-60) and T-ALL (Jurkat) cell lines. We have shown that the selected inhibitors have differential anti-proliferative effects on the tested cell lines; however, the dual pathway inhibitor P1K402 exhibited more potent effects on cell cycle arrest and decreasing the overall cell survival. We have also demonstrated that P1K402 resulted in downregulation of cancer associated lncRNAs and upregulation of tumour suppressor lncRNAs, suggesting a more complex role of this dual specific pathway inhibitor (fig. 5).
Everolimus is one of the earliest first-generation rapalog mTORC1 inhibitor approved by the United States FDA for advanced/metastatic renal cell carcinoma[32,33]. Everolimus treatment showed moderate pS6 reduction (27 %) in Jurkat cells as it is shown to specifically affect mTORC1 dependent pS6 rather than Akt[34-36]. Similarly, cell cycle arrest of Jurkat cells in G1 phase can be explained by PI3K/Akt/mTOR/p70 ribosomal S6 Kinase 1 (p70S6K1) inhibition as previously shown in breast cancer cell line i.e. human Mammary Epithelial cell line, (MCF-10)[37]. Although the cell survival and cell cycle of everolimus-treated HL-60 cells were not significantly changed, Akt and S6 phosphorylation was inhibited, suggesting that everolimus was active in these cells. These findings are in contrast with those of a study by Mallon et al. who found that everolimus could inhibit cell proliferation and induce apoptosis of HL-60 cells via inhibition of the PI3K/Akt/mTOR signaling pathway[31]. The paradoxical increase in Akt phosphorylation on everolimus treated K562 cells was unexpected. The lack of inhibition of both Akt and S6 phosphorylation suggests that everolimus did not inhibit mTORC1/2. K-562 is a relative Breakpoint Cluster Region-Abelson Murine Leukemia (BCRABL1) positive blast crisis leukaemia cell line and although everolimus has been shown to be effective in CML, it has little effect on blast crisis cells[38].
The second generation mTOR inhibitor PP242 effect the Akt feedback loop and therefore has a dual function of suppressing both complexes 1 and 2 of mTOR[39]. PP242-treated Jurkat cells showed reduced viability and proliferation, arrest at G1 phase reduced phosphorylation of S6 and Akt. Treatment of HL-60, a promyelocytic leukaemia cell line showed little to no effect on cell viability, pS6 and Akt phosphorylation or arrest at G1 phase. These might be due to short treatment durations with the drug. Alternatively, this reduced activity in these cells might be due to weak inhibition of mTOR phosphorylation in promyelocytic cells, or activation of parallel signaling pathways in these cells[40]. PP242 had no effect on K-562 cell viability or cell cycle despite reduction in phosphorylation of pS6 and Akt is downstream of mTORC1, by 18.8 % only, largely confirming our results[41].
PKI402 is a second-generation reversible Adenosine Triphosphate (ATP)-competitive mTOR inhibitor acting as a dual-specificity PI3K/mTOR inhibitor, which targets PI3K and both mTORC1 and mTORC2. Therefore, mTOR and PI3K dual-specificity inhibitors may be sufficient to evade PI3K pathway reactivation due to the mTOR-S6K1-Insulin Receptor Substrate-1 (IRS-1) negative feedback loop enhancing PI3K activation[30]. The deregulation of PI3K activation has downstream effects, including Akt and mTOR, which have been linked to tumor initiation and maintenance. PKI402 also inhibits cell growth[42]. The treatment of Jurkat cells with PKI402 demonstrated the inhibitor’s dual specificity and emphasized the importance of the PI3K/mTOR signaling pathway in the cell cycle. In the phosphorylation experiments, pS6 and p-Akt antibodies were used to determine the phosphorylation activity after PKI402 treatment, revealing that S6 and Akt phosphorylation was inhibited by about 66 %. PKI402 was also used to examine the effect of dual-specificity PI3K/mTOR inhibitors on the T-ALL cell cycle and to determine the stage at which the cell cycle was arrested. The results showed that 56.5 % of the PKI402-treated Jurkat cells had survived, of which 55.9 % was arrested in the G1 phase. Significant repression in the level of p-Akt by using the dual PI3K/mTOR inhibitor PKI- 402 was also reported by Hu et al.[43], who showed that inhibition in the PI3K/AKT/mTOR pathway decrease the levels of p-Akt, phosphorylated 4EBP1 (p-4EBP1) and p-P70S6K thereby suppressing cell growth and proliferation along with inducing apoptosis.
The effect of PKI402 on the S6 and Akt phosphorylation in HL-60 cells revealed that it was more effective against mTOR than against PI3K activity. In the proliferation experiments, more than 65 % decrease in cell count was observed. This may be explained by the fact that the inhibition of mTOR eventually decreases cell proliferation and may lead to cell death[41]. Furthermore, all cells were arrested in the G1 phase. These effects might have been due to the simultaneous inhibition of the PI3K and mTOR signaling pathways. PKI402- treated K562 cells showed a significant decrease in cell survival and most of the cells were arrested in the G1 phase. It has been shown that PI3K inhibitors could arrest other types of cancer cells, e.g. ovarian cancer cells, in the G1 phase[31]. The effect of PKI402 on PI3K/ mTOR activity was assessed by the degree of Akt and S6 phosphorylation and showed that PKI402 inhibited Akt phosphorylation more than S6 phosphorylation. This may be explained by the presence of two activation sites (Thr308 (T308) and Ser473 (S473)) on Akt as PKI402 is able to inhibit both sites[31]. Our study shows that dualspecificity inhibitors more efficiently reduce leukemic cell survival and proliferation compared with that of single-specificity inhibitors. On the other hand, drugs targeting a single molecule may have different effects in leukemic cell lines. However, small-molecule inhibitors had a stronger effect in ALL cell lines compared to that in AML cell lines. In addition, our data showed that the used cancer cells had an alternative mechanism to overcome some of the inhibition-induced effects. We conclude that dual-target therapy may be a promising therapy for leukemia. Although, novel inhibitors such as 1,4-Naphthoquinone (CNN1) are emerging that can overcome the multidrug resistance in leukemias[44], evaluations of dual inhibitors are expected to produce high therapeutic outcomes.
Recent genomic analysis found that only 1 %-2 % of the human genome encodes protein-coding RNA, while the remaining transcriptome is non-protein-coding[45,46]. LncRNAs (>200 base pair (bp) long) are among the most abundant of the non-coding RNAs[47]. Most lncRNAs are expressed in a tissue-specific and stress-specific manner. Long noncoding RNAs regulate a wide range of biological functions; alterations to these functions can affect genomic imprinting and transcriptional regulation, potentially leading to cancer[48]. Many lncRNAs are aberrantly expressed in a wide range of cancers. Some of these expressed lncRNAs are potential cancer biomarkers[21]. To further understand the molecular interactions associated with these therapies, we screened 96 cancer-associated lncRNAs with PKI402 on Jurkat cell lines. Interestingly, we found that many cancer-associated lncRNAs are downregulated and tumor suppresser lncRNA was either not changed or upregulated with treatment. All three drugs increased RNCR3, UCA1 and HULC levels. HOXA Cluster antisense RNA (HOXAas) is located between the HOXA clusters, HOXA3 and HOXA4 genes. We found that everolimus regulated HOXAas expression in leukemic cells. HOXAas reportedly regulates apoptosis during All-Trans Retinoic Acid (ATRA)-induced myeloid differentiation[49]. The dual inhibitor PKI402 significantly suppressed the expression of the Zfhx2as lncRNA upon treatment. Zfhx2as is transcribed on the opposite stand and downstream of the Zfhx2 polyA site. Knockout of Zfhx2as reportedly induces Zfhx2 expression in mice. However, the role of Zfhx2as in cancer has not been evaluated until now. Interestingly, we found that knocking out Zfhx2as significantly decreases cell proliferation and induces apoptosis in cancer cells. To the best of our knowledge, we are the first group to report that Zfhx2as could act as a cancerassociated lncRNA with oncogenic properties.
Our study revealed that all the tested molecules have differential anti-proliferative effects on the cell lines; however, the dual pathway inhibitor (PKI402) was shown to be more potent in cell cycle arrest and decreasing the overall cellular proliferation. PKI402 has resulted in the downregulation of cancer associated lncRNAs and upregulation of tumour suppressor lncRNAs, suggesting a more complex role of this dual specific pathway inhibitor in blood cancers. We suggest further investigations on the effect of these drugs on initiation of anti-proliferative signaling and induction of apoptosis. In addition, further evaluation of the role of these inhibitors in preclinical in vitro and in vivo trials that involves patient-derived primary cell lines, would provide significant insight.
Funding:
This research received no external funding. The research was funded by faculty’s research fund of the King Fahd Medical Research Center, King Abdulaziz University.
Acknowledgements:
The authors would like to thank the facilitators at King Fahd Medical Research Center (KFMRC) and King Abdulaziz University Hospital for their support. In addition, the author would like to extend their gratitude to the technical teams in the central labs of KFMRC.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bueno L, de Araujo WR, Paixão TR. Point of care (POC) medical biosensors for cancer detection. Medical Biosensors for Point of Care (POC) Applications. Elsevier, Woodhead Publishing; 2017. p. 183-201.
- Shah A, Naqvi SS, Naveed K, Salem N, Khan MA, Alimgeer KS. Automated diagnosis of leukemia: A comprehensive review. IEEE Access 2021;9:132097-124.
- Soares MR, Melanda FN, Lima Neto GS, Takagi VM, Anjos AA, Cunha LA, et al. Mortality trend and analysis of potential years of life lost due to leukemia and lymphoma in Brazil and Mato Grosso. Rev Bras Epidemiol 2022;25.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Bawazir A, Al-Zamel N, Amen A, Akiel MA, Alhawiti NM, Alshehri A. The burden of leukemia in the Kingdom of Saudi Arabia: 15 years period (1999–2013). BMC Cancer 2019;19(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Mirabilii S, Ricciardi MR, Piedimonte M, Gianfelici V, Bianchi MP, Tafuri A. Biological aspects of mTOR in leukemia. Int J Mol Sci 2018;19(8):2396.
[Crossref] [Google Scholar] [PubMed]
- Fayard E, Moncayo G, Hemmings BA, Holländer GA. Phosphatidylinositol 3-kinase signaling in thymocytes: The need for stringent control. Sci Signal 2010;3(135):re5.
[Crossref] [Google Scholar] [PubMed]
- Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol 2012;3:228.
[Crossref] [Google Scholar] [PubMed]
- Yuan TL, Cantley L. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008;27(41):5497-510.
[Crossref] [Google Scholar] [PubMed]
- Marshall JD, Whitecross DE, Mellor P, Anderson DH. Impact of p85α alterations in cancer. Biomolecules 2019;9(1):29.
[Crossref] [Google Scholar] [PubMed]
- Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol Biol Rep 2020;47(6):4587-629.
[Crossref] [Google Scholar] [PubMed]
- Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci 2020;10(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Chong WP, Sim LC, Wong KT, Yap MG. Enhanced IFNγ production in adenosine?treated CHOCells: A mechanistic study. Biotechnol Prog 2009;25(3):866-73.
[Crossref] [Google Scholar] [PubMed]
- Jossé L, Xie J, Proud CG, Smales CM. mTORC1 signalling and eIF4E/4E-BP1 translation initiation factor stoichiometry influence recombinant protein productivity from GS-CHOK1 cells. Biochem J 2016;473(24):4651-64.
[Crossref] [Google Scholar] [PubMed]
- Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol 2018;834:188-96.
[Crossref] [Google Scholar] [PubMed]
- Chi SG, Minami Y. Emerging targeted therapy for specific genomic abnormalities in acute myeloid leukemia. Int J Mol Sci 2022;23(4):2362.
[Crossref] [Google Scholar] [PubMed]
- Ke X, Shen L. Molecular targeted therapy of cancer: The progress and future prospect. Front Lab Med 2017;1(2):69-75.
- Cucchi DG, Polak TB, Ossenkoppele GJ, Groot UD, Carin A, Cloos J, et al. Two decades of targeted therapies in acute myeloid leukemia. Leukemia 2021;35(3):651-60.
[Crossref] [Google Scholar] [PubMed]
- Kapranov P, Drenkow J, Cheng J, Long J, Helt G, Dike S, et al. Examples of the complex architecture of the human transcriptome revealed by RACE and high-density tiling arrays. Genome Res 2005;15(7):987-97.
[Crossref] [Google Scholar] [PubMed]
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet 2009;10(3):155-9.
[Crossref] [Google Scholar] [PubMed]
- Gutschner T, Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol 2012;9(6):703-19.
[Crossref] [Google Scholar] [PubMed]
- Chodroff RA, Goodstadt L, Sirey TM, Oliver PL, Davies KE, Green ED, et al. Long noncoding RNA genes: Conservation of sequence and brain expression among diverse amniotes. Genome Biol 2010;11(7):1-6.
[Crossref] [Google Scholar] [PubMed]
- Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009;458(7235):223-7.
[Crossref] [Google Scholar] [PubMed]
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010;142(3):409-19.
[Crossref] [Google Scholar] [PubMed]
- Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 2007;8(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol Cancer 2019;18(1):1-28.
[Crossref] [Google Scholar] [PubMed]
- Li J, Zhang J, Jin L, Deng H, Wu J. Silencing lnc-ASAH2B-2 inhibits breast cancer cell growth via the mTOR pathway. Anticancer Res 2018;38(6):3427-34.
[Crossref] [Google Scholar] [PubMed]
- Zhu G, Luo H, Feng Y, Guryanova OA, Xu J, Chen S, et al. HOXBLINC long non-coding RNA activation promotes leukemogenesis in NPM1-mutant acute myeloid leukemia. Nat Commun 2021;12(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Hofmans M, Lammens T, Depreter B, Wu Y, Erlacher M, Caye A, et al. Long non-coding RNAs as novel therapeutic targets in juvenile myelomonocytic leukemia. Sci Rep 2021;11(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Zhou HY, Huang SL. Current development of the second generation of mTOR inhibitors as anticancer agents. Chin J Cancer 2012;31(1):8.
[Crossref] [Google Scholar] [PubMed]
- Mallon R, Hollander I, Feldberg L, Lucas J, Soloveva V, Venkatesan A, et al. Antitumor efficacy profile of PKI-402, a dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor PKI-402, a dual PI3K/mTOR inhibitor. Mol Cancer Ther 2010;9(4):976-84.
[Crossref] [Google Scholar] [PubMed]
- Meng LH, Zheng XF. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin 2015;36(10):1163-9.
[Crossref] [Google Scholar] [PubMed]
- Oleksak P, Nepovimova E, Chrienova Z, Musilek K, Patocka J, Kuca K. Contemporary mTOR inhibitor scaffolds to diseases breakdown: A patent review (2015-2021). Eur J Med Chem 2022:114498.
[Crossref] [Google Scholar] [PubMed]
- Daver N, Boumber Y, Kantarjian H, Ravandi F, Cortes J, Rytting ME, et al. A phase I/II study of the mTOR inhibitor everolimus in combination with HyperCVAD chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia. Clin Cancer Res 2015;21(12):2704-14.
[Crossref] [Google Scholar] [PubMed]
- Das A, Reis F, Maejima Y, Cai Z, Ren J. mTOR signaling in cardiometabolic disease, cancer, and aging. Oxid Med Cell Longev 2017;2017:1-4.
[Crossref] [Google Scholar] [PubMed]
- Silic-Benussi M, Sharova E, Ciccarese F, Cavallari I, Raimondi V, Urso L, et al. mTOR inhibition downregulates glucose-6-phosphate dehydrogenase and induces ROS-dependent death in T-cell acute lymphoblastic leukemia cells. Redox Biol 2022;51:102268.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Ding XF, Bouamar H, Pressley K, Sun LZ. Everolimus induces G1 cell cycle arrest through autophagy-mediated protein degradation of cyclin D1 in breast cancer cells. Am J Physiol Cell Physiol 2019;317(2):C244-52.
[Crossref] [Google Scholar] [PubMed]
- Stoklosa T, Glodkowska-Mrowka E, Hoser G, Kielak M, Seferynska I, Wlodarski P. Diverse mechanisms of mTOR activation in chronic and blastic phase of chronic myelogenous leukemia. Exp Hematol 2013;41(5):462-9.
[Crossref] [Google Scholar] [PubMed]
- Feng H, Yang Z, Bai X, Yang M, Fang Y, Zhang X, et al. Therapeutic potential of a dual mTORC1/2 inhibitor for the prevention of posterior capsule opacification: An in vitro study. Int J Mol Med 2018;41(4):2099-107.
[Crossref] [Google Scholar] [PubMed]
- Ono A, Oike R, Okuhashi Y, Takahashi Y, Itoh M, Nara N, et al. Comparative effects of PP242 and rapamycin on mTOR signalling and NOTCH signalling in leukemia cells. Anticancer Res 2013;33(3):809-13.
[Google Scholar] [PubMed]
- Li J, Xue LY, Han YX, Shang YT, Yao L, Luo JM. Inhibitory effects of rapamycin on proliferation of chronic myelogenous leukemia cells and its mechanism. Zhonghua Xue Ye Xue Za Zhi 2012;33(10):843-6.
[Google Scholar] [PubMed]
- Zeng Z, Shi YX, Tsao T, Qiu Y, Kornblau SM, Baggerly KA, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood 2012;120(13):2679-89.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Xia M, Wang J, Yu H, Chai J, Zhang Z, et al. Dual PI3K/mTOR inhibitor PKI-402 suppresses the growth of ovarian cancer cells by degradation of Mcl-1 through autophagy. Biomed Pharmacother 2020;129:110397.
[Crossref] [Google Scholar] [PubMed]
- de Sousa Portilho AJ, da Silva EL, Bezerra EC, Moraes Rego Gomes CB, Ferreira V, de Moraes ME, et al. 1, 4-naphthoquinone (CNN1) induces apoptosis through DNA damage and promotes upregulation of H2AFX in leukemia multidrug resistant cell line. Int J Mol Sci 2022;23(15):8105.
[Crossref] [Google Scholar] [PubMed]
- Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 2012;31(43):4577-87.
[Crossref] [Google Scholar] [PubMed]
- Tonge DP, Darling D, Farzaneh F, Williams GT. Whole-genome-scale identification of novel non-protein-coding RNAs controlling cell proliferation and survival through a functional forward genetics strategy. Sci Rep 2022;12(1):1-13.
- Jimeno S, Mejías-Navarro F, Prados-Carvajal R, Huertas P. Controlling the balance between chromosome break repair pathways. Adv Protein Chem Struct Biol 2019;115:95-134.
[Crossref] [Google Scholar] [PubMed]
- Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer 2011;10(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Zhao H, Zhang X, Frazão JB, Condino?Neto A, Newburger PE. HOX antisense lincRNA HOXA?AS2 is an apoptosis repressor in all trans-retinoic acid treated NB4 promyelocytic leukemia cells. J Cell Biochem 2013;114(10):2375-83.
[Crossref] [Google Scholar] [PubMed]
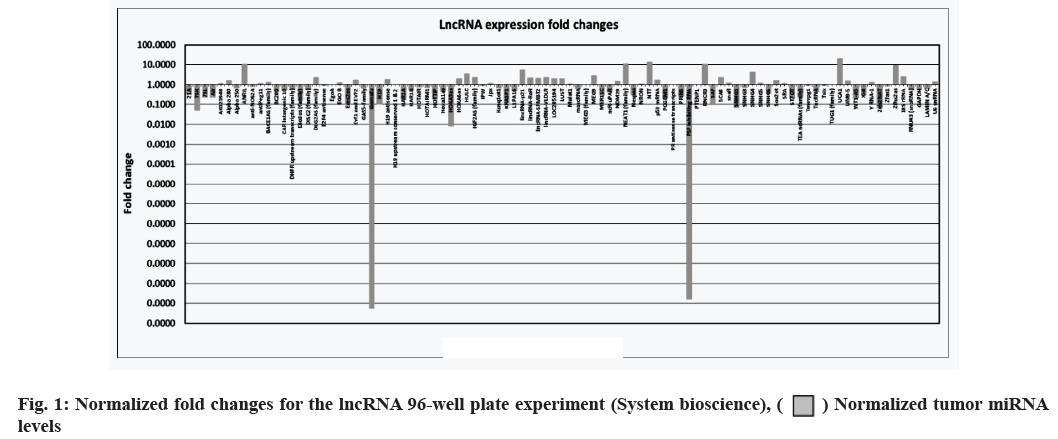
 ) Normalized tumor miRNA levels
) Normalized tumor miRNA levels