- *Corresponding Author:
- Yan Zhang
Department of Gastroenterology, Wuhan University of Science and Technology Affiliated Puren Hospital, Wuhan, Hubei 430081, China
E-mail: yank1223@163.com
| This article was originally published in a special issue,“Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “173-179” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Non-alcoholic fatty liver disease is a common clinical chronic liver disease. It is a risk factor leading to liver cirrhosis, hepatocellular carcinoma and cardiovascular diseases. Decanoylacetaldehyde is the main medicinal component of Yuxingcao (Houttuynia cordata), which has specific effects in inflammatory diseases. BRL-3A cells were induced into non-alcoholic fatty liver disease cell model using oleic acid and palmitic acid. Different concentrations of decanoylacetaldehyde and p38 mitogen-activated protein kinase activator (metformin water) were used to intervene in the non-alcoholic fatty liver disease cell model. 250 μm oleic acid and 250 μm palmitic acid significantly reduced triglyceride content in cells without inhibiting the proliferation of non-alcoholic fatty liver disease cells. As the concentration of decanoylacetaldehyde intervention increased, the apoptosis rate of non-alcoholic fatty liver disease cells, the messenger ribonucleic acid level of interleukin-6, the protein levels of peroxisome proliferatoractivated receptor gamma, sterol regulatory element binding proteins-1c, p-p38mitogen-activated protein kinase and p-Jun N-terminal kinase were significantly decreased, and the cell survival rate, the messenger ribonucleic acid level of interleukin-10 and the protein level of peroxisome proliferatoractivated receptor alpha were significantly increased. Compared with the control group, there was a significant increase in the area of oil red O staining, triglyceride content, and sterol regulatory element binding proteins-1c protein levels, and a significant decrease in the protein level of peroxisome proliferator-activated receptor alpha in the model group. Compared with the model group, the area of oil red O staining, triglyceride content, peroxisome proliferator-activated receptor gamma and sterol regulatory element binding proteins-1c protein levels were significantly reduced and the protein level of peroxisome proliferator-activated receptor alpha was significantly increased in the decanoylacetaldehyde group. Compared with the metformin water group, the area of oil red O staining, total cholesterol content, peroxisome proliferator-activated receptor gamma and sterol regulatory element binding proteins-1c protein levels were significantly reduced and the protein level of peroxisome proliferatoractivated receptor alpha was significantly increased in the decanoylacetaldehyde+metformin water group. Decanoylacetaldehyde reduces adipocyte differentiation and inflammatory factor expression and alleviates non-alcoholic fatty liver disease progression by inhibiting p38 mitogen-activated protein kinase and p-Jun N-terminal pathway activation in non-alcoholic fatty liver disease cells.
Keywords
Decanoylacetaldehyde, non-alcoholic fatty liver disease, p38 mitogen-activated protein kinase and p-Jun N-terminal pathway, interleukin
Non-Alcoholic Fatty Liver Disease (NAFLD) is a disease characterized by fatty degeneration of the liver[1]. NAFLD is the leading cause of cirrhosis and hepatocellular carcinoma, and its incidence is increasing yearly[2]. NAFLD pathophysiology involves hepatic fat deposition, destruction of hepatocyte membranes by lipid peroxidation and inflammation. These changes stimulate hepatic stellate cells, leading to fibrosis. If progression continues, liver inflammation can lead to cirrhosis, portal hypertension and liver fibrosis[3]. Currently, the main treatments for NAFLD are lifestyle interventions and pharmacological treatments, but the research on lifestyle therapies is limited and pharmacological treatments have some degree of side effects, such as weight gain and increased mortality[4]. There is therefore an urgent need to find new therapeutic drugs.
Many Chinese herbs have medicinal and food properties, they are non-toxic and highly pharmacologically active, and in recent years research has identified a number of Chinese medicinal herbs that can be used to treat a wide range of ailments[5,6]. Yuxingcao is a traditional Chinese medicinal herb that is used in everyday life as a food and is mainly found in East and Southeast Asia[7]. Studies have reported that Yuxingcao has a variety of pharmacological effects. Aqueous extract of Yuxingcao inhibited Tumor Necrosis Factor- Alpha (TNF-α) secretion at a mass concentration of 0.125 mg/ml[8]. Dose-dependent inhibition of Lipopolysaccharide (LPS)-induced TNF-α production by methylnonanethole in Yuxingcao[9]. Alpha (α)-Pinene in Yuxingcao reduces LPS-induced Interleukin (IL)-8 secretion[10]. Ethyl acetate extract of Yuxingcao shows significant antioxidant activity in a mouse model of Carbon tetrachloride (CCl4)-induced liver injury and protects against liver damage[11]. Reduced levels of hepatic oxidizing factors after treatment with Yuxingcao water and ethanol extract in an ethanol-induced mouse model of liver injury[12]. This suggests that Yuxingcao has significant efficacy in the area of liver and inflammatory diseases. Decanoylacetaldehyde (DECA) is one of the main ingredients in Yuxingcao[13]. But there is still a virtual void of research on DECA.
Therefore, this study will preliminarily investigate the role and mechanism of DECA in NAFLD at the cellular level. By inducing NAFLD Cells (NAFLCs), the role and mechanism of DECA were verified in various aspects using oil red O staining, Cell Counting Kit 8 (CCK8), Flow cytometry, Enzyme- Linked Immunoassay Assay (ELISA), Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR), and western blotting, laying the foundation for future new therapeutic approaches for NAFLD and more in-depth research on DECA.
Materials and Methods
Cell culture:
BRL-3A cells were acquired from the American Type Culture Collection (ATCC) (Manassas, Virginia, United States of America (USA)). These cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Life Technologies) supplemented with 10 % Fetal Bovine Serum (FBS), (Hyclone Laboratories, South Logan, Utah, USA) in a humidified incubator at 37° with 5 % CO2.
Cell model and groups:
The cells were distributed into 12-well plates, with each well containing 3×105 cells. Four different final concentrations of oleic acid and palmitic acid were added to the cells. Oleic acid 50 μm and palmitic acid 50 μm, oleic acid 100 μm and palmitic acid 100 μm, oleic acid 250 μm and palmitic acid 250 μm, oleic acid 500 μm and palmitic acid 500 μm. After 24 h, the NAFLD cell model was induced in all cells, except for the control group, which consisted of normal BRL-3A cells. Next, the cells were treated with different concentrations of DECA (10 μg/ml, 50 μg/ml, 100 μg/ml) for 24 h. Additionally, the Metformin Hydrochloride (HCl) group was subjected to a 24 h intervention using 50 mg/ml Metformin HCl (Selleck).
ELISA:
The Triglyceride (TG) content was measured using an ELISA kit from Abcam. Briefly, we prepared a series of diluted standard solutions and added the samples to separate test tubes. Then, we added the enzyme to each test tube and incubated the mixtures at 37° for 10 min. After the incubation, the reaction was stopped, and the Optical Density (OD) was measured at 450 nm using a spectrophotometer.
qRT-PCR:
Total Ribonucleic Acid (RNA) from cancer cells was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA, USA), following the product instructions. Quantitative RT-PCR was then performed with Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as the control. The relative expression of S100A9 was calculated using the 2-ΔΔCT method. The primer sequences used in this study were as follows. IL-6: Forward 5’-TGGAGTTCCGTTTCTACCTGG-3’ and Reverse 5’-GGATGGTCTTGGTCCTTAGCC-3’; IL-10: Forward 5’-CGGGAAGACAATAACTGCACCC-3’ and Reverse 5’-CGGTTAGCAGTATGTTGTCCAGC-3’; GAPDH: Forward 5’-ACGGATTTGGTCGTATTGGGCG-3’ and Reverse 5’-GCTCCTGGAAGATGGTGATGGG-3’.
Western blot assay:
Total proteins were extracted using Radioimmunoprecipitation assay lysis buffer (Solarbio) and quantified with a bicinchoninic acid assay kit (Solarbio). 20 mg of proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Solarbio) and transferred onto polyvinylidene fluoride membranes (Millipore, Massachusetts, USA). Following blocking with 5 % skim milk, the membranes were incubated for 1 h at room temperature with primary antibodies against Peroxisome-Proliferator-Activated Receptor-Alpha (PPAR-α), PPAR-Gamma (γ), Sterol Regulatory Element Binding Protein (SREBP)-1c, p38 Mitogen- Activated Protein (MAP), and JNK. β-tubulin was used as a control. All antibodies were sourced from Abcam.
CCK8 assay:
Cells were seeded into a 96-well plate at a density of 2×104 cells per well. The proliferation of cells was evaluated using the CCK8 kit. Viable cells were quantified by measuring the absorbance at 492 nm using a microplate reader (Molecular Devices, Sunnyvale, California, USA).
Flow cytometry assay:
Flow cytometry assays were conducted using the Annexin V-Fluorescein Isothiocyante (FITC)/ Propidium Iodide (PI) apoptosis detection kit (Beyotime Biotechnology, Shanghai, China) following the manufacturer's instructions. After cells were transfected for 48 h, they were collected for apoptosis analysis and stained with FITC-Annexin V and PI. Subsequently, the apoptosis rate of the cells was measured and analyzed using the BD FACS Calibur flow cytometer (BD, USA).
Statistical analyses:
Statistical analysis was conducted using GraphPad Prism 8.0 Software. The data are expressed as the mean±Standard Deviation (SD). For comparisons between two groups, Student's t-test was employed. For calculations involving more than two independent groups, one-way Analysis of Variance (ANOVA) followed by Tukey's post hoc test was utilized. A p-value of less than 0.05 (p<0.05) was considered statistically significant. All experiments were repeated at least three times to ensure the reliability of the results.
Results and Discussion
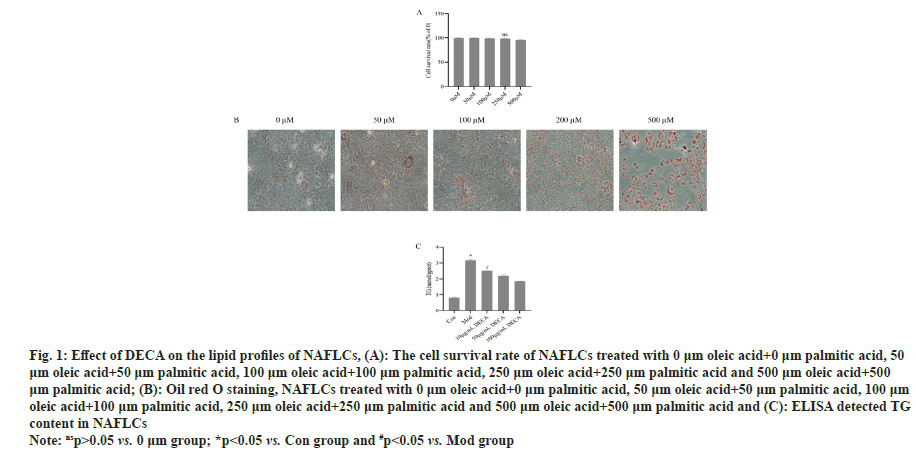
First, using BRL-3A, after induction with different concentrations of oleic acid and palmitic acid, the fat content in the cells was significantly and markedly increased after induction with 250 μm oleic acid and 250 μm palmitic acid for 24 h, (p<0.05) (fig. 1). So 250 μm oleic acid and 250 μm palmitic was used in induced cell model. Furthermore, the TG content of NAFLCs was found to decrease gradually with the increase of DECA concentration, which indicated that DECA had a concentration-dependent inhibition of TG content in NAFLCs as shown in fig. 1.
Fig. 1: Effect of DECA on the lipid profiles of NAFLCs, (A): The cell survival rate of NAFLCs treated with 0 μm oleic acid+0 μm palmitic acid, 50 μm oleic acid+50 μm palmitic acid, 100 μm oleic acid+100 μm palmitic acid, 250 μm oleic acid+250 μm palmitic acid and 500 μm oleic acid+500 μm palmitic acid; (B): Oil red O staining, NAFLCs treated with 0 μm oleic acid+0 μm palmitic acid, 50 μm oleic acid+50 μm palmitic acid, 100 μm
oleic acid+100 μm palmitic acid, 250 μm oleic acid+250 μm palmitic acid and 500 μm oleic acid+500 μm palmitic acid and (C): ELISA detected TG
content in NAFLCs
Note: nsp>0.05 vs. 0 μm group; *p<0.05 vs. Con group and #p<0.05 vs. Mod group
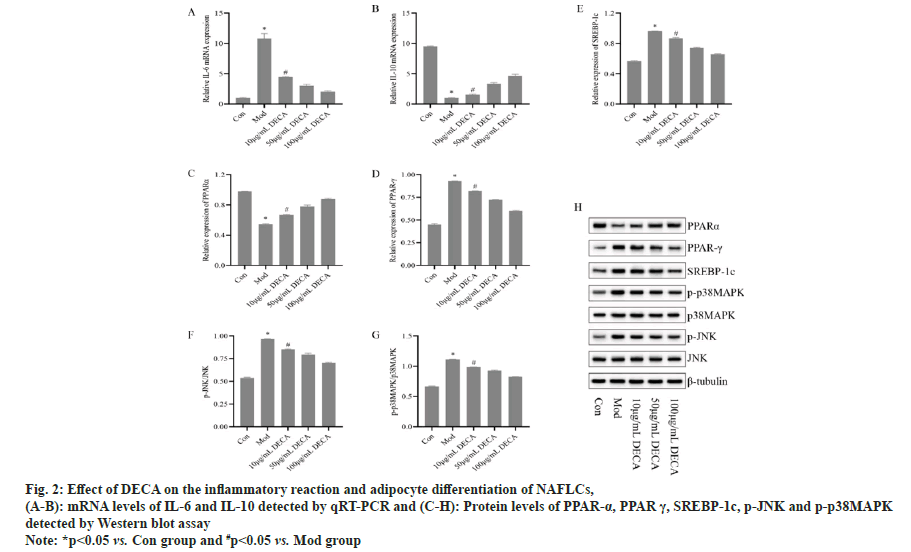
Since a necessary part of the progression of NAFLD is the accumulation of hepatic fat which leads to hepatic fat cell inflammation. DECA was found to significantly reduce TG content in the previous results. Here we will further examine the expression level of PPAR-α, PPAR-γ and SREBP-1c proteins. The results, as shown in fig. 2, showed that compared with the control group, the protein expression level of PPAR-α was significantly lower (p<0.05) and the protein expression levels of PPAR-γ and SREBP- 1c were significantly higher (p<0.05) in the model group. Compared with the model group, PPAR-α protein expression level was significantly higher (p<0.05) and PPAR-γ and SREBP-1c protein expression levels were significantly lower (p<0.05) in the 10 μg/ml DECA group. With the increase of DECA intervention concentration, PPARα protein expression level gradually increased, and PPAR-γ and SREBP-1c protein expression level gradually decreased. In addition, we identified a role for DECA in inhibiting the p38MAP/JNK pathway (fig. 2), with significantly higher levels of p-p38MAP and p-JNK protein expression in the model group compared with the control group (p<0.05). Compared with the model group, p-p38MAP and p-JNK protein expression levels were significantly reduced in the 10 μg/ml DECA group (p<0.05). With the increase of DECA intervention concentration, the p-p38MAP and p-JNK protein expression levels gradually decreased.
Fig. 2: Effect of DECA on the inflammatory reaction and adipocyte differentiation of NAFLCs, (A-B): mRNA levels of IL-6 and IL-10 detected by qRT-PCR and (C-H): Protein levels of PPAR-α, PPAR γ, SREBP-1c, p-JNK and p-p38MAPK detected by Western blot assay
Note: *p<0.05 vs. Con group and #p<0.05 vs. Mod group
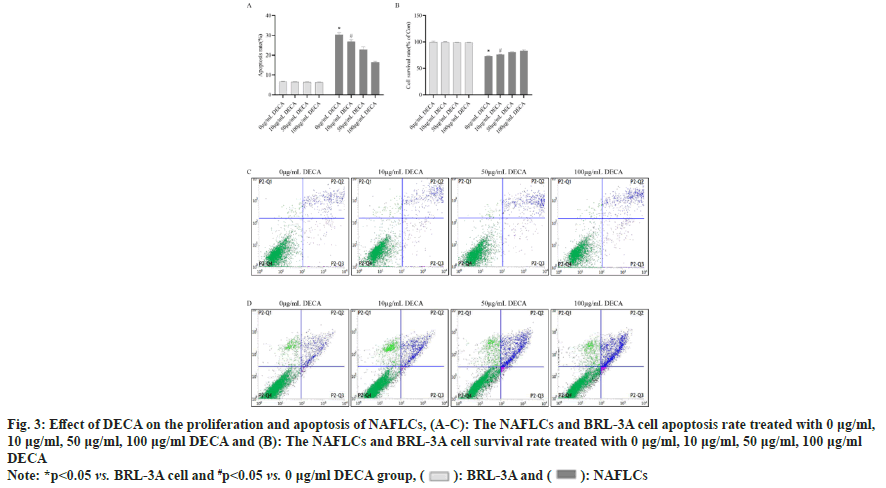
Flow cytometry and CCK-8 assays were performed to detect the survival and apoptosis levels of normal liver cells BRL-3A and NAFLCs after DECA intervention at different concentrations. As shown in fig. 3, apoptosis was significantly increased (p<0.05) and cell survival was significantly decreased (p<0.05) in NAFLCs compared to BRL-3A cells under 0 μg/ml DECA conditions. In NAFLCs, compared to the 0 μg/ml DECA group, the 10 μg/ ml DECA group showed a significant decrease in the rate of cell apoptosis (p<0.05) and a significant increase in cell survival rate (p<0.05). As the intervention concentration of DECA increased, the rate of apoptosis in NAFLCs gradually decreased, while the cell survival rate progressively increased. This indicates that after simulating the excessive accumulation of lipids in liver cells using 250 μm oleic acid and 250 μm palmitic acid, the survival of BRL-3A cells was inhibited, and apoptosis of BRL- 3A cells was promoted. In contrast, 10 μg/ml DECA not only reduced lipids in NAFLCs but also promoted the survival of BRL-3A cells. Moreover, 10 μg/ml DECA did not significantly induce apoptosis in BRL- 3A cells. Therefore, for subsequent experiments, 10 μg/ml DECA was selected as the optimal effective concentration.
Fig. 3: Effect of DECA on the proliferation and apoptosis of NAFLCs, (A-C): The NAFLCs and BRL-3A cell apoptosis rate treated with 0 μg/ml, 10 μg/ml, 50 μg/ml, 100 μg/ml DECA and (B): The NAFLCs and BRL-3A cell survival rate treated with 0 μg/ml, 10 μg/ml, 50 μg/ml, 100 μg/ml DECA
Note: *p<0.05 vs. BRL-3A cell and #p<0.05 vs. 0 μg/ml DECA group, ( ): BRL-3A and (
): BRL-3A and ( ): NAFLCs
): NAFLCs
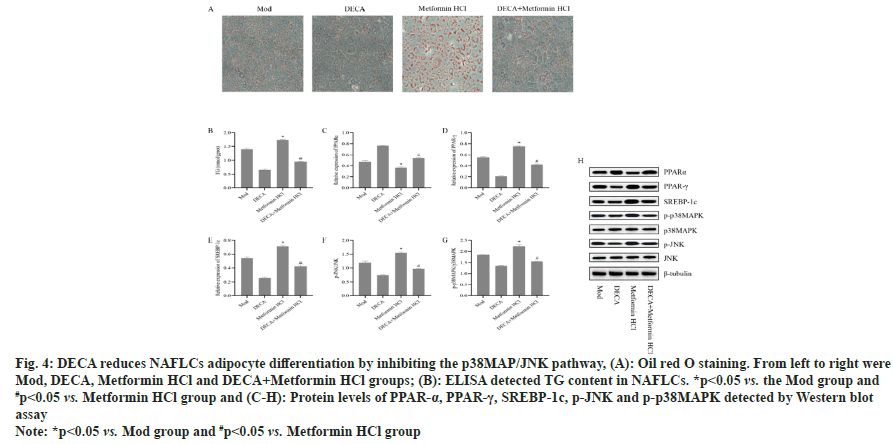
The above results revealed a dose-dependent inhibition of the p38MAP/JNK pathway and adipocyte differentiation by DECA. However, it remained unclear whether the mechanism of DECA's action involved the inhibition of the p38MAP/JNK pathway. To address this, Metformin HCl was used to activate the p38MAP/JNK pathway. As shown in fig. 4, compared to the model group, the Metformin HCl group exhibited a significant decrease in PPARα protein expression (p<0.05) and a significant increase in PPAR-γ, SREBP-1c, p-p38MAP, and p-JNK protein expressions (p<0.05). In contrast, the DECA+Metformin HCl group demonstrated a significant increase in PPAR-α protein expression (p<0.05) and a significant decrease in PPAR-γ, SREBP-1c, p-p38MAP, and p-JNK protein expressions (p<0.05) compared to the Metformin HCl group. These findings confirm that activation of the p38MAP/JNK pathway leads to increased NAFLCs adipocyte differentiation, while DECA reduces NAFLCs adipocyte differentiation by inhibiting the activation of the p38MAP/JNK pathway.
Fig. 4: DECA reduces NAFLCs adipocyte differentiation by inhibiting the p38MAP/JNK pathway, (A): Oil red O staining. From left to right were
Mod, DECA, Metformin HCl and DECA+Metformin HCl groups; (B): ELISA detected TG content in NAFLCs. *p<0.05 vs. the Mod group and
#p<0.05 vs. Metformin HCl group and (C-H): Protein levels of PPAR-α, PPAR-γ, SREBP-1c, p-JNK and p-p38MAPK detected by Western blot assay
Note: *p<0.05 vs. Mod group and #p<0.05 vs. Metformin HCl group
NAFLD has become increasingly prevalent worldwide. This study aims to explore the potential therapeutic effects of the main component DECA from the traditional Chinese herb Houttuynia cordata on NAFLD. Through in vitro and in vivo experiments on NAFLD models, we found that DECA significantly reduced the generation of fat cells and inhibited inflammatory responses, effectively alleviating the progression of NAFLD cells. Previous studies have consistently indicated that inflammation is a key hallmark of NAFLD progression and its complications[14]. Moreover, in Coronavirus Disease (COVID-19) patients with NAFLD, the mortality rate is associated with inflammatory responses[15]. Zhou et al.[16] found that Alpinetin attenuates lipid metabolism, reduces SREBP-1c, and increases PPAR-α levels, improving high-fat diet-induced NAFLD. Onorato et al.[17] also discovered that secreted protein, acidic and rich in cysteine accelerates NAFLD by regulating hepatic lipid metabolism disorder. These findings align with the results of this study, where DECA inhibits inflammatory responses, reduces fat cell differentiation, and alleviates the progression of NAFLD.
Our experimental results demonstrate that after DECA treatment, the differentiation of fat cells in the NAFLD model was significantly inhibited. This inhibitory effect may be achieved by regulating the expression levels of key regulatory factors in fat cell differentiation, such as PPAR-α, PPAR-γ, and SREBP-1c. We observed that DECA treatment upregulated PPARα protein expression and downregulated PPAR-γ and SREBP-1c protein expression in NAFLCs, leading to a reduction in TG content. These regulatory factors are known to play crucial roles in fat cell differentiation. These findings suggest that DECA intervenes in fat cell formation and enhances lipid metabolism, providing a novel direction for the treatment of NAFLD.
Recent studies have found that activating PPARα improves hepatic steatosis in high-fat diet-induced NAFLD mice[18]. Sun et al.[19] discovered that lowering TG content and lipid accumulation by modulating the expression of SREBP-1c and PPARα is a potential strategy for treating NAFLD. Chen et al.[20] also found that a natural compound extracted from Chinese herbal medicine, diosgenin, downregulates SREBP1C gene expression in HepG2 cells exposed to free fatty acids, promoting PPARα expression to treat NAFLD. Zaiou et al.[21] pointed out that PPAR-γ is a target and regulatory factor of the epigenetic mechanisms in NAFLD. Feng et al.[22] discovered that apigenin, a compound from a natural product, inhibits the expression of PPAR-γ target genes and improves NAFLD.
Another crucial aspect of our research findings is that DECA effectively inhibits the expression of proinflammatory cytokines and promotes the expression of anti-inflammatory cytokines in NAFLCs. For example, DECA significantly reduces IL-6 and increases IL-10 gene levels. Further mechanistic studies revealed that DECA treatment leads to a significant decrease in the activity of the JNK/p38 MAPK inflammatory signaling pathway. The JNK/ p38 MAPK pathway is closely associated with the development of NAFLD. To verify that DECA reduces fat cell differentiation and TG content by inhibiting the JNK/p38 MAPK pathway, we activated the JNK/p38 MAPK pathway in NAFLCs and then intervened with DECA. The phosphorylation levels of JNK and p38 MAPK in the DECA intervention group were significantly lower than those in the group activated solely with the JNK/p38 MAPK pathway. In the DECA+Metformin HCl group, the protein expression level of PPAR-α was significantly higher, while the protein expression levels of PPAR-γ and SREBP-1c were significantly lower compared to the Metformin HCl group. Thus, DECA may play a critical role in the treatment of NAFLD by inhibiting the inflammatory signaling pathway. In the study by Zhu et al.[23], it was also confirmed that mitigating NAFLD involves inhibiting the phosphorylation of the JNK/p38 MAPK pathway. Huang et al.[24] found that the natural food, broccoli, inhibits IL-6 secretion and increases IL-10 secretion to improve NAFLD progression.
Based on the comprehensive experimental results, our study revealed the significant effects of DECA in reducing fat cell differentiation and inhibiting inflammatory responses, thereby alleviating the progression of NAFLD cells. These findings provide important clues for developing new treatment strategies and drugs. However, the mechanism of action of DECA and its other potential targets still require further investigation. Future preclinical studies should explore the metabolism and safety of DECA in the human body to validate its potential application value as a treatment for NAFLD. Additionally, as an active component derived from natural herbal medicine, further research should also investigate the combination of DECA with other drugs or therapeutic approaches to enhance treatment efficacy and reduce potential side effects of other medications.
In conclusion, our research findings demonstrate that DECA, the main component of the traditional Chinese herb Houttuynia cordata, exhibits significant effects in reducing fat cell differentiation and inhibiting inflammatory responses in NAFLD cells. These effects provide a potential therapeutic approach for mitigating the progression of NAFLD. DECA’s ability to regulate key factors involved in fat cell differentiation, such as PPAR-α, PPAR-γ, and SREBP-1c, as well as its suppression of the JNK/p38 MAPK inflammatory signaling pathway, suggest its crucial role in the treatment of NAFLD.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: Diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol 2015;13(12):2062-70.
[Crossref] [Google Scholar] [PubMed]
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397(10290):2212-24.
- Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, et al. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord 2022;22(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc 2015;90(9):1233-46.
[Crossref] [Google Scholar] [PubMed]
- Luo YF, Liu KY, Feng JH, Tong YR, Gao W. Research progress in synthetic biology of active compounds in Chinese medicinal plants. Zhongguo Zhong Yao Za Zhi 2021;46(22):5727-35.
[Crossref] [Google Scholar] [PubMed]
- Fan C, Jin HZ, Wu L, Zhang Y, Ye RD, Zhang W, et al. An exploration of Traditional Chinese Medicinal plants with anti-inflammatory activities. Evid Based Complement Alternat Med 2017;2017:1231820.
[Crossref] [Google Scholar] [PubMed]
- Li JJ, Chen GD, Fan HX, Hu D, Zhou ZQ, Lan KH, et al. Houttuynoid M, an anti-HSV active houttuynoid from Houttuynia cordata featuring a bis-houttuynin chain tethered to a flavonoid core. J Nat Prod 2017;80(11):3010-3.
[Crossref] [Google Scholar] [PubMed]
- Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G. Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. Am J Chin Med 2005;33(3):415-24.
[Crossref] [Google Scholar] [PubMed]
- Wang C, He L, Wang N, Liu F. Screening anti-inflammatory components from Chinese traditional medicines using a peritoneal macrophage/cell membrane chromatography-offline-GC/MS method. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(27):3019-24.
[Crossref] [Google Scholar] [PubMed]
- Podlogar JA, Verspohl EJ. Anti-inflammatory effects of ginger and some of its components in human bronchial epithelial (BEAS‐2B) cells. Phytother Res 2012;26(3):333-6.
[Crossref] [Google Scholar] [PubMed]
- Tian L, Shi X, Yu L, Zhu J, Ma R, Yang X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. J Agric Food Chem 2012;60(18):4641-8.
[Crossref] [Google Scholar] [PubMed]
- You Y, Lee H, Yoon HG, Park J, Kim OK, Kim K, et al. A blend of extracts from Houttuynia cordata, Nelumbo nucifera, and Camellia sinensis protects against ethanol-induced liver damage in C57BL/6 mice. J Med Food 2018;21(2):203-6.
[Crossref] [Google Scholar] [PubMed]
- Lu HM, Liang YZ, Qian P. Profile-effect on quality control of Houttuynia cordata injection. Yao Xue Xue Bao 2005;40(12):1147-50.
[Google Scholar] [PubMed]
- Gómez‐Hurtado I, Gallego‐Durán R, Zapater P, Ampuero J, Aller R, Crespo J, et al. Bacterial antigen translocation and age as BMI‐independent contributing factors on systemic inflammation in NAFLD patients. Liver Int 2020;40(9):2182-93.
[Crossref] [Google Scholar] [PubMed]
- Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One 2020;15(10):e0240400.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Ding YL, Zhang JL, Zhang P, Wang JQ, Li ZH. Alpinetin improved high fat diet-induced non-alcoholic fatty liver disease (NAFLD) through improving oxidative stress, inflammatory response and lipid metabolism. Biomed Pharmacother 2018;97:1397-408.
[Crossref] [Google Scholar] [PubMed]
- Onorato MA, Fiore E, Bayo J, Casali C, Fernandez‐Tomé M, Rodríguez M, et al. SPARC inhibition accelerates NAFLD‐associated hepatocellular carcinoma development by dysregulating hepatic lipid metabolism. Liver Int 2021;41(7):1677-93.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Ma Y, Qi X, Tian J, Ma Y, Niu T. α-Lactalbumin peptide Asp-Gln-Trp ameliorates hepatic steatosis and oxidative stress in free fatty acids-treated HepG2 cells and high-fat diet-induced NAFLD mice by activating the PPARα pathway. Mol Nutr Food Res 2023;67(16):e2200499.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Zheng C, Li T, He X, Yang F, Guo W, et al. GB1a activates SIRT6 to regulate lipid metabolism in mouse primary hepatocytes. Int J Mol Sci 2023;24(11):9540.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Sun S, Feng Y, Li X, Yin G, Liang P, et al. Diosgenin attenuates nonalcoholic hepatic steatosis through the hepatic FXR-SHP-SREBP1C/PPARα/CD36 pathway. Eur J Pharmacol 2023;952:175808.
[Crossref] [Google Scholar] [PubMed]
- Zaiou M. Peroxisome proliferator-activated receptor-γ as a target and regulator of epigenetic mechanisms in nonalcoholic fatty liver disease. Cells 2023;12(8):1205.
[Crossref] [Google Scholar] [PubMed]
- Feng X, Yu W, Li X, Zhou F, Zhang W, Shen Q, et al. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem Pharmacol 2017;136:136-49.
[Crossref] [Google Scholar] [PubMed]
- Zhu M, Niu Q, Zhang J, Yu Y, Wang H, Zhu T, et al. Amorphous selenium nanodots alleviate non-alcoholic fatty liver disease via activating VEGF receptor 1 to further inhibit phosphorylation of JNK/p38 MAPK pathways. Eur J Pharmacol 2022;932:175235.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Xu J, Hu Y, Huang K, Luo Y, He X. Broccoli ameliorate NAFLD by increasing lipolysis and promoting liver macrophages polarize toward M2-type. J Functional Foods 2022;89:104898.