- *Corresponding Author:
- Haidong Cheng
Department of Obstetrics, Obstetrics and Gynecology Hospital of Fudan University, Shanghai 200090,China
E-mail: hdcheng_2003@163.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “161-168” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Our study aimed to evaluate the effect of intake of different iron concentrations on the expression of insulin signaling pathway mediators and to investigate the role played by iron overload in the development of gestational diabetes. The expression of growth-related indicators, markers of glucose and lipid metabolism, iron metabolism-related indicators and insulin signaling pathway mediators were analyzed in insulin receptor heterozygous knockout pregnant mice and wild type pregnant mice. The expression of adenosine monophosphate-activated protein kinase and phosphorylated adenosine monophosphate-activated protein kinase in the insulin signaling pathway of the liver and placental tissue was significantly lower in the wild type pregnant mice fed with an iron-rich diet group than in the wild type pregnant mice fed with a conventional diet and insulin receptor heterozygous knockout pregnant mice fed with an iron-rich diet groups (p<0.05). In the liver tissue, insulin receptor substrate 1 concentration was significantly higher in the wild-type pregnant mice fed with a conventional diet group than in the wild-type pregnant mice fed with an iron-rich diet and pregnant mice fed with an iron-rich diet and insulin receptor heterozygous knockout pregnant mice fed with a conventional diet groups (p<0.05). The placental phosphorylated adenosine monophosphate-activated protein kinase/adenosine monophosphate-activated protein kinase ratio was significantly lower in wild type group than in the knockout mice group (p<0.05). The differential expression and activation of adenosine monophosphateactivated protein kinase in different tissues was affected by supplementation of high concentrations of iron. The effect of iron on adenosine monophosphate-activated protein kinase someway relates to the insulin receptor gene.
Keywords
Gestational diabetes mellitus, iron overload, glucose tolerance, primary hepatocytes
Iron (Fe) is a trace element essential for maternal physiological well-being as well as for fetal growth, and the highest rates of anemia are reported in pregnant women[1]. However, Fe-on account of its properties as a transitional metal and pro-oxidant may affect glucose metabolism, and its regular supplementation during pregnancy remains controversial. Increasing evidence has shown a strong association between increased maternal Fe storage and the development of Gestational Diabetes Mellitus (GDM)[2]; however, the molecular mechanisms that underlie this association are not well understood.
The pathogenesis of GDM is generally believed to be similar to type II diabetes mellitus which is associated with increased Insulin Resistance (IR) and/or insufficient functioning of islet beta (β)-cells[3]. The decrease of glucose uptake and utilization in peripheral tissues is the main cause of IR than Insulin Receptor (INSR) functioning. The transmembrane transport of glucose in muscle and adipose tissue is the major rate-limiting step in the absorption and utilization of glucose[4]. Inflammation, oxidative stress, damage of islet β-cell, thereby leading to Fe overload may result in abnormal glucose metabolism by possibly interfering with the insulin signalling pathway[5,6]. In animal models of type 2 diabetes, reducing Fe concentration via dietary Fe restriction, Fe chelation, or bloodletting has been demonstrated to improve β-cell function[7]. Oxidative stress caused by the accumulation of excess Fe can result in β-cell damage and apoptosis, subsequently leading to a reduction in insulin secretion[8]. Oxidative stress is responsible for the pathogenesis of many diseases, including GDM, and refers to the cellular imbalance between pro-oxidants and antioxidants which is a phenomenon that disrupts protein, lipid and Deoxyribonucleic Acid (DNA) homeostasis and ultimately causes cell damage[9].
We have previously reported a decrease of 54 % glucose uptake in pregnant women (in comparison to non-pregnant controls)[10]. The INSR/Phosphatidyl Inositol 3 Kinases (PI3K)/Serine/Threonine kinase (Akt) signalling pathway is the principal pathway that regulates glucose uptake. Both the Insulin Receptor Substrate-1 (IRS-1) and Adenosine Monophosphateactivated Protein Kinase (AMPK) regulate cellular energy and play key role in the maintenance of blood glucose homeostasis. AMPK is known to affect many aspects related to type 2 diabetes, including insulin secretion, pancreatic islet cell damage, glycogen synthesis and gluconeogenesis. Thus, there exists a complex relationship between AMPK and insulin signalling pathways at the molecular level[11]. For instance, while AMPK can upregulate or downregulate PI3K and Protein kinase B (Akt) bound to IRS-1. Insulin and Akt in turn can downregulate AMPK activity[12].
In this study, mediators of the AMPK and insulin signalling pathways were analysed at different concentrations of Fe intake in INSR+/- mice to investigate the possible mechanism of the development of gestational diabetes consequently to Fe overload.
Materials and Methods
Experimental animals:
INSR gene heterozygous Knockout (KO) (+/-) C57 black 6 (C57BL/6) Specific Pathogen Free (SPF) mice aged 6-8 w, developed by Shanghai Model Organisms Center, Inc., was utilized in our experiments. These mice were obtained via the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/ CRISPR associated protein 9 (Cas9)) technique using guide Ribonucleic Acid (RNA). All mice were housed in internal animal rooms with a controlled environment maintained at 23°±2° and 55 %±10 % humidity, 12 h light/dark cycle and unrestricted access to food and water.
The animals including INSR+/- C57BL/6 KO and Wild-Type (WT) female mice were mated with WT male mice in a cage at 2:1 from 16:00 to 17:00 PM. Pregnancy was confirmed based on the presence of a vaginal embolism at 8:00 AM the next day. Mice were dated as 0.5 d pregnant (Gestation Day (GD0.5)) at noon on the day of pregnancy confirmation and subsequent gestation day were calculated accordingly. Animals were fed either a conventional diet (100 (Parts per million (ppm)) Fe or Fe-rich diet (700 ppm Fe) from the time of pregnancy confirmation. Based on the diet, they were thus randomly divided into the WT-Conventional (WT-C), KO-C, WT-Fe, and KOFe rich diet groups.
Pregnant mice were monitored for random blood glucose levels (using caudal vein sampling method), body weight and food intake at GD5.5, GD10.5, GD15.5 and GD18.5 between 8-10 AM. Intraperitoneal Glucose Tolerance Testing (IPGTT) was performed at GD17.5. Pregnant mice at GD18.5 were anesthetized with diethyl ether and a caesarean section was used for delivery. After delivery, muscle tissue was sampled from the quadriceps femoris, liver and placenta.
Materials and chemicals:
Blood glucose meters and test strips were obtained from Roche (Basel, Switzerland). TRIzol was purchased from TransGen Biotech Co., Ltd. (Beijing, China). Antibodies against Akt, phosphorylated-Akt (p-Akt), AMPK, phosphorylated-AMPK (p-AMPK), Glucose Transporter type 4 (GLUT4), IRS-1, phosphorylated-IRS1 (p-IRS1), PI3K, phospho- Insulin, and Serum Ferritin (SF) were procured from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Recombinant Divalent Metal Transporter 1 (DMT1) specific antibody was obtained from Co., Ltd (Hangzhou, China).
IPGTT:
Mice from every group were subjected to 12 h daytime fasting and IPGTT was performed. Blood was drawn from the tail tip at 0, 30, 60 and 120 min postprandial to measure the blood glucose levels.
Biomarker measurement:
The expression of various proteins, including Akt, p-Akt, AMPK, p-AMPK, GLUT4, IRS-1, p-IRS1, PI3K, phospho insulin, SF and DMT1 was analysed by Western blotting. Briefly, 100 mg of tissue was homogenized in Radioimmunoprecipitation Assay (RIPA) buffer for 10 min, followed by centrifugation at 10 000 rpm for 5 min at 4°. Protein extracts were electrophoresed by Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride membranes which were probed with primary antibodies against the proteins of interest. Protein amounts were normalized to those of the loading control (Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)). Quantity One and Image J software were used for the quantitative analysis of each strip and the relative values were calculated, and imported to Excel for further statistics.
Statistical analysis:
All data was analysed using the Statistical Package for Social Sciences (SPSS) 21.0 version. Descriptive analysis was performed for categorical variables, medians as well as ranges of continuous variables that are represented as mean±standard deviation. T-test or Kruskal-Wallis test was used to identify significant differences between experimental groups. All tests were two-sided, and p<0.05 was considered significant.
Results and Discussion
Establishment of INSR+/- mice model was carried out using WT Polymerase Chain Reaction (PCR) amplicon, which was 2.0 kb in size and the expected theoretical band of INSR+/- mice included 2.0 kb and 1.3 kb. By screening the results INSR heterozygous KO mice have been identified.
Further, the reproductive capacity was assessed, for which nulliparous INSR+/- C57BL/6 KO and WT female mice were mated with fertile WT males and determined to be in the pregnant state due to the presence of copulatory plugs (Table 1).
| Outcomes during pregnancy | WT group | KO group | p-value |
|---|---|---|---|
| Rate of successful delivery | 45.50 % | 46.10 % | 1 |
| Litter size (n) | 4.8 (6.0-3.0) | 7.5 (9.0-6.3) | 0.014* |
| Litter weight (g) | 5.19±2.34 | 7.57±2.04 | 0.052 |
| Average litter weight (g) | 1.12±0.17 | 1.02±0.10 | 0.134 |
| Placental weight (g) | 0.51±0.21 | 0.89±0.21 | 0.005* |
| Average placental weight (g) | 0.12±0.23 | 0.13±0.02 | 0.375 |
Note: *p<0.05
Table 1: Pregnancy outcomes in successful matings
While 45.5 % of WT female mice maintained successful delivery, 46.1 % of the KO female mice succeeded in delivering progeny in all considered impregnated mice. KO female mice produced significantly greater numbers of progeny than their WT counterparts, but with similar individual embryo weights (n=7.5 (9.0-6.3) vs. n=4.8(6.0-3.0), p=0.014). The average placental weight was not significantly different between the KO and WT female mice.
Body weight, glucose level and food intake were monitored during the experimental period of 18.5 d. As indicated in Table 2, body weight at GD5.5 and glucose level at GD15.5 were significantly different among all the four groups (p=0.01 and p=0.03, respectively).
| Experimental period | KO-C | KO-Fe | WT-C | WT-Fe | p-value |
|---|---|---|---|---|---|
| Body weight (g) | |||||
| GD0.5 | 23.40±1.51 | 22.03±0.71 | 20.68±1.17 | 21.14±1.62 | 0.05a |
| GD5.5 | 23.50±1.44 | 22.29±0.67 | 20.01±1.36 | 21.07±1.48 | 0.01a* |
| GD10.5 | 24.66±0.90 | 23.89±0.53 | 22.17±3.25 | 23.17±2.039 | 0.20a |
| GD15.5 | 29.30±1.27 | 29.69±1.13 | 25.97±5.50 | 28.31±1.94 | 0.16a |
| GD18.5 | 31.37±2.04 | 32.88±1.69 | 27.81±5.64 | 31.06±2.50 | 0.11a |
| GD18.5-GD0.5 | 7.97±1.56 | 10.85±1.73 | 7.14±4.47 | 9.92±1.74 | 0.08a |
| Glucose level (mmol/l) | |||||
| GD0.5 | 8.70±1.79 | 8.20±1.58 | 9.25±0.49 | 9.30±1.28 | 0.68a |
| GD5.5 | 8.15±1.17 | 8.55±1.05 | 7.6±0.71 | 8.77±0.45 | 0.56a |
| GD10.5 | 8.93±1.19 | 9.56±0.92 | 8.75±1.34 | 8.40±0.61 | 0.35a |
| GD15.5 | 7.08±0.46 | 7.99±0.71 | 9.10±0.28 | 7.77±0.84 | 0.03a* |
| IPGTT (mmol/l) | |||||
| Fasting glucose | 6.45±1.79 | 6.46±1.21 | 6.20±0.42 | 6.33±0.85 | 0.99a |
| 15 min | 12.88±8.00 | 17.11±5.22 | 18.75±13.22 | 17.87±6.36 | 0.70a |
| 30 min | 11.47±2.35 | 20.16±7.01 | 16.3±6.51 | 22.50±7.76 | 0.21a |
| 60 min | 13.85±7.96 | 13.37±6.87 | 9.55±1.06 | 13.80±4.23 | 0.87a |
| 120 min | 6.35±1.77 | 7.91±3.50 | 6.45±0.21 | 8.10±0.66 | 0.73a |
| Food intake (g) | |||||
| GD17.5-GD0.5 | 49.68±3.78 | 47.59±3.54 | 41.87±1.83 | 42.22±1.80 | 0.05b |
Note: *p<0.05, aOne-way Analysis of Variance (ANOVA) and bMann-Whitney test
Table 2: Alteration of body weight, Glucose levels and food intake
In pairwise comparisons, at GD5.5, the body weight of the WT-C group (20.01±1.36 g) was significantly lower than that of the KO-C group (23.50±1.44 g) (p=0.003).
Furthermore, at GD15.5, the glucose levels of the WT-C group (9.10±0.28 mmol/l) and the KOFe group (7.99±0.71 mmol/l) were significantly higher than those of the KO-C group (7.08±0.46 mmol/l) (p=0.004 and p=0.042). The glucose levels of the WT-C group (9.10±0.28 mmol/l) were also significantly higher than those of the WT-Fe group (7.77±0.84 mmol/l) (p=0.046).
Although, during pregnancy the dietary intake in the WT-C group (41.87±1.83 g) and WT-Fe group (42.22±1.80 g) was slightly lower than that in the KO-C group (49.68±3.78 g) and the KO-Fe group (47.59±3.54 g), no significant differences were observed between any of the groups (p=0.05).
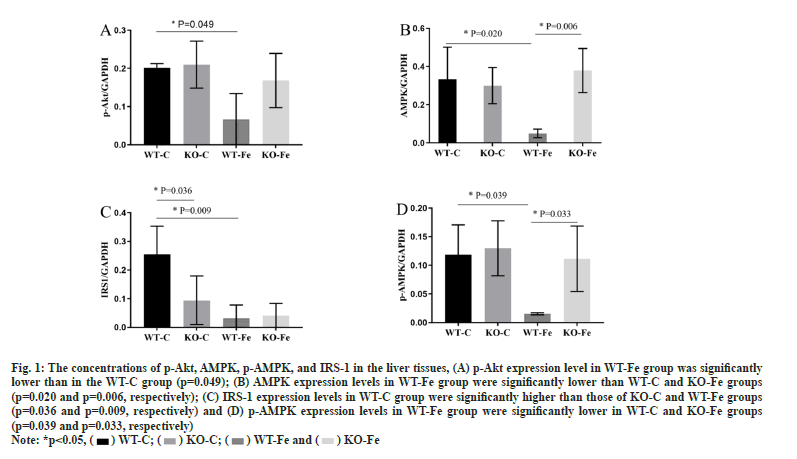
The expression of different proteins in tissues was studied. As depicted in fig. 1, the concentrations of p-Akt, AMPK, p-AMPK and IRS-1 in the liver tissues of WT-Fe pregnant mice were significantly lower than in WT-C pregnant mice (p=0.049, p=0.020, p=0.039 and p=0.009, respectively). The levels of AMPK and p-AMPK in the WT-Fe group were also significantly lower than those in the KOFe group (p=0.006 and p=0.033). IRS-1 expression levels in the WT-C group were significantly higher than those in the KO-C group (p=0.036). However, the proportion of AMPK and Akt activation in the liver tissue was not significantly different. The fulllength blots in the liver tissues were analysed.
Fig. 1: The concentrations of p-Akt, AMPK, p-AMPK, and IRS-1 in the liver tissues, (A) p-Akt expression level in WT-Fe group was significantly
lower than in the WT-C group (p=0.049); (B) AMPK expression levels in WT-Fe group were significantly lower than WT-C and KO-Fe groups
(p=0.020 and p=0.006, respectively); (C) IRS-1 expression levels in WT-C group were significantly higher than those of KO-C and WT-Fe groups
(p=0.036 and p=0.009, respectively) and (D) p-AMPK expression levels in WT-Fe group were significantly lower in WT-C and KO-Fe groups
(p=0.039 and p=0.033, respectively)
In the muscle tissue, no significant differences in the expression levels of Akt, p-Akt, AMPK, p-AMPK, DMT1, ferritin, GLUT4, IRS-1, PI3K, phospho insulin and p-IRS1 were observed among all four groups of pregnant mice.
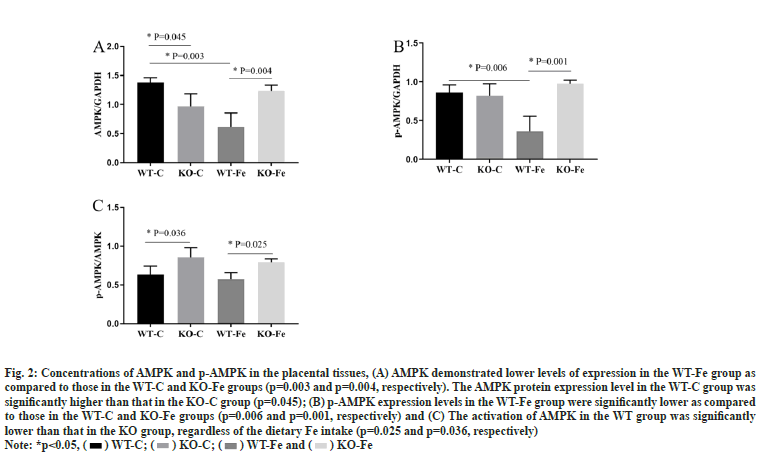
The statistical analysis of the differences among the four groups of pregnant mice in the context of AMPK and p-AMPK expression levels in the placental tissues was depicted as shown in fig. 2. The full-length blots in the placental tissues were also analysed. Both AMPK and p-AMPK demonstrated lower levels of expression in the placental tissues of WT-Fe pregnant mice as compared to those in the WT-C pregnant mice (p=0.003 and p=0.006, respectively). The levels of AMPK and p-AMPK in the WT-Fe group were also significantly lower than those in the KO-Fe group (p=0.004 and p=0.001, respectively). However, the AMPK protein expression level in the WT-C group was significantly higher than that in the KO-C group (p=0.045). This indicates that the activation of AMPK in the WT group was significantly lower than that in the KO group, regardless of the dietary Fe intake (p=0.025 and p=0.036, respectively).
Fig. 2: Concentrations of AMPK and p-AMPK in the placental tissues, (A) AMPK demonstrated lower levels of expression in the WT-Fe group as
compared to those in the WT-C and KO-Fe groups (p=0.003 and p=0.004, respectively). The AMPK protein expression level in the WT-C group was
significantly higher than that in the KO-C group (p=0.045); (B) p-AMPK expression levels in the WT-Fe group were significantly lower as compared
to those in the WT-C and KO-Fe groups (p=0.006 and p=0.001, respectively) and (C) The activation of AMPK in the WT group was significantly
lower than that in the KO group, regardless of the dietary Fe intake (p=0.025 and p=0.036, respectively)
Pregnancy is a physiological state of IR[13] and the elevation in body Fe stores has been postulated to be a risk factor for the same[14,15]. The present study evaluates the impact of low and high dietary Fe levels on blood glucose concentration, body weight, AMPK levels and insulin signalling pathway mediator levels in different tissues of INSR+/- mice to elucidate the mechanism of underlying gestational diabetes.
INSR plays a key role in the development of diabetes via insulin-related signalling pathways. The insulin signalling cascade is mediated by INSR, which results in the phosphorylation of the IRS that leads to the recruitment and activation of PI3K[16]. Furthermore, mutations in the INSR gene are involved in the development of type 2 diabetes by negatively affecting insulin function and consequently causing IR[17].
Previous studies have shown that young mice with the INSR+/- genotype demonstrate normal glucose tolerance[18]. Bruning et al. reported that 10 % of the mice heterozygous for INSR mutations had elevated insulin levels at 4-6 mo of age and eventually developed diabetes[19]. In our study, no significant differences were found in the results of the glucose tolerance screening between any of the pregnant mice groups, indicating that INSR+/- KO pregnant mice aged 8-10 w did not exhibit abnormal glucose metabolism during pregnancy. The blood glucose levels of INSR+/- pregnant mice fed on the Fe-rich diet at GD15.5 were significantly higher than those of mice in the control diet group (p=0.042) and an opposite result was observed in the WT pregnant mice group (p=0.046). This interesting change in blood glucose levels might be related to the heterozygous KO status of the INSR gene in association with different concentrations of dietary Fe intake. While certain studies state that an Fasting Blood Glucose (FBG)≥11.1 mmol/l marks the successful establishment of a GDM mouse model[20]. The mice in our study did not reach 4-6 mo of age and hence, did not achieve a gestational diabetes status since all experiments were performed at the optimum reproductive age and the most appropriate pregnancy feeding cycle was followed.
Liver, being the main target organ of insulin plays a crucial role in its degradation and thereby regulates glucose homeostasis. Additionally, liver is also the main Fe storage site in the body, where Fe metabolism is primarily regulated by hepcidin[21]. Oxidative stress due to Fe overload can damage pancreatic β-cells and directly damage islets functioning[14]. AMPK is considered as a major therapeutic target for metabolic diseases, including type 2 diabetes and obesity, owing to its central role in the regulation of bioenergy metabolism, maintenance of glycolipid balance, insulin signalling and mitochondrial biogenesis[22]. Inhibition of AMPK signalling has been found to correlate with IR, inflammation and oxidative stress[23-25]. Furthermore, AMPK activation has also been demonstrated to be attenuated in GDM mice (p<0.01)[26]. Thus, GDM is characterized by the suppression of AMPK signalling in the liver. Varghese et al. reported a dual effect of Fe on primary hepatocytes in mice, in connection with the insulin signalling pathway. Increased intracellular Fe levels resulted in enhanced basal activation of the Akt pathway in a ligand-independent manner; however, downregulation of this pathway, in response to insulin consequent to elevated Fe concentrations, reduced the expression levels of IRS-1 and IRS-2[27]. In our study, the IRS-1 levels in the liver tissue of the Fe-rich diet-fed group were significantly lower than those in the liver tissue of the control diet-fed group (p=0.009) in the WT pregnant mice. However, no significant difference was observed in the INSR +/- pregnant mice. Furthermore, in the control diet-fed groups, the IRS-1 levels in WT pregnant mice were significantly higher than those in the INSR+/- pregnant mice (p=0.036), while there was no significant difference between the Fe-rich diet-fed groups. These results indicate that Fe supplementation at a high concentration significantly reduces the expression of IRS-1 in the WT group, while the expression of the same after heterozygous INSR KO does not result in a significant difference. Therefore, the downregulation of IRS-1 expression might be induced by the high Fe intake and regulated by INSR. This is consistent with the findings reported by Varghese et al. who demonstrated a Fe-induced progressive decline in IRS-1 and IRS-2 levels. As both IRS-1 and IRS-2 function downregulates, upstream of Akt occurs in the insulin signaling pathway, so upregulation of their levels could potentially reduce downstream Akt activation in response to insulin. The hepatic expression of p-Akt in the Fe-rich diet-fed WT pregnant mice group was significantly lower than that in the control diet-fed WT pregnant mice group (p=0.049). We also observed that among the WT groups, the expression of AMPK in the Fe-rich dietfed subgroup was significantly lower than that in the control diet-fed subgroup (p=0.020), which suggests that intake of high concentrations of Fe may reduce AMPK expression. In the Fe-rich diet-fed group, the expression levels of AMPK in WT pregnant mice were significantly lower than those in INSR+/- pregnant mice group (p=0.006), which contradicts the findings of Varghese et al. but are in concordance with those of Chen et al. who reported a lowered expression of AMPK Alpha (α) in response to Ferric chloride (FeCl3) treatment in primary rat hepatocytes[28]. Our results demonstrated a Fe-induced decline in IRS-1 and IRS-2 levels as well as reduced activation of Akt via phosphorylation, resulting in the downregulation of AMPK.
Pregnancy results show an increase in basic oxygen consumption and elevated sensitization to oxidative stress. Moreover, the placenta has very high energy requirements and is extremely susceptible to mitochondrial damage. Placental mitochondrial defects could lead to increased oxidative stress, decreased energy consumption and subsequently poor metabolism in the offspring[29,30]. Inhibition of AMPK in the placenta during diabetic pregnancy might be an important contributor to impaired placental mitochondrial biogenesis in maternal diabetes[31]. However, the mechanisms underlying this phenomenon remain unclear. The relative messenger RNA (mRNA) expression levels of AMPK, PI3K and Akt were lower in the uterus of a Polycystic Ovarian Syndrome (PCOS) rat model than in the control group (p<0.001)[32]. Activation of AMPK is known to inhibit the phosphorylation of IRS-1, thereby activating the INSR/PI3K/Akt signalling pathway[33]. In our study, the expression of AMPK and p-AMPK in the placental tissues of pregnant mice showed significant differences among the four groups. Among the WT pregnant mice groups, the Fe-rich diet-fed group had significantly lower AMPK levels than the control diet-fed group (p=0.003). In contrast, the opposite trend was observed in the INSR+/- pregnant mice groups (p=0.004). Therefore, the effect of Fe supplementation on the placental expression of AMPK in WT and INSR+/- pregnant mice was not found to be consistent. Moreover, in WT mice, AMPK expression in the placental tissue demonstrated a trend that was consistent with the pattern observed in the liver tissue. A comparison of the p-AMPK/AMPK ratio in the placenta revealed that WT pregnant mice groups had significantly lower ratios than the INSR+/- pregnant mice groups (p=0.025 and p=0.036, respectively). It is therefore possible to speculate that potential oxidative damage in the placenta of INSR KO pregnant mice may be aggravated by Fe due to its properties as an oxidant. The Fe-mediated oxidative injury may be compensated by the placenta in mice with the WT genotype.
Limitations of this study include relatively small number of pregnant mice and no significant difference in the IPGTT blood glucose levels. Therefore, although our results suggest that Fe supplementation might affect the expression and activation of AMPK in different tissues, the relationship with GDM was not established.
Collectively, our results and the study limitations suggest that dietary Fe supplementation influences AMPK expression in the liver and placenta of pregnant mice and the heterozygous KO of the INSR gene affects the activation and expression of AMPK.
Ethical approval:
The animal experiment was approved by the Animal Care and Use Committee of Obstetrics and Gynecology Hospital affiliated to Fudan University (Approval number: 2015-041).
Funding:
This work was supported by the National Natural Science Foundation of China (81471469).
Conflict of interests:
The authors declared no conflict of interests.
References
- Siu AL, US Preventive Services Task Force. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: US Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163(7):529-36.
[Crossref] [Google Scholar] [PubMed]
- Khambalia AZ, Aimone A, Nagubandi P, Roberts CL, McElduff A, Morris JM, et al. High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: A prospective study and systematic review. Diabet Med 2016;33(9):1211-21.
[Crossref] [Google Scholar] [PubMed]
- Colomiere M, Permezel M, Lappas M. Diabetes and obesity during pregnancy alter insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. J Mol Endocrinol 2010;44(4):213-23.
[Crossref] [Google Scholar] [PubMed]
- Fink RI, Wallace P, Brechtel G, Olefsky JM. Evidence that glucose transport is rate-limiting for in vivo glucose uptake. Metabolism 1992;41(8):897-902.
[Crossref] [Google Scholar] [PubMed]
- Lee HJ, Choi JS, Lee HJ, Kim WH, Park SI, Song J. Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J Nutr Biochem 2015;26(12):1414-23.
[Crossref] [Google Scholar] [PubMed]
- Hansen JB, Tonnesen MF, Madsen AN, Hagedorn PH, Friberg J, Grunnet LG, et al. Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic β cell fate in response to cytokines. Cell Metab 2012;16(4):449-61.
[Crossref] [Google Scholar] [PubMed]
- Hansen JB, Moen IW, Mandrup‐Poulsen T. Iron: The hard player in diabetes pathophysiology. Acta Physiol 2014;210(4):717-32.
[Crossref] [Google Scholar] [PubMed]
- Javadian P, Alimohamadi S, Gharedaghi MH, Hantoushzadeh S. Gestational diabetes mellitus and iron supplement; effects on pregnancy outcome. Acta Med Iran 2014;52(5):385-9.
[Google Scholar] [PubMed]
- Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 2018;19(11):1-21.
[Crossref] [Google Scholar] [PubMed]
- Zuo C, Haidong C. Relationship between genotype 2, member 2 of maternal-fetal solute transport family and gestational diabetes mellitus. Chin J Perinat Med 2010;13(1):12-15.
- Xu J, Liu H, Su G, Ding M, Wang W, Lu J, et al. Purification of ginseng rare sapogenins 25-OH-PPT and its hypoglycemic, antiinflammatory and lipid-lowering mechanisms. J Ginseng Res 2021;45(1):86-97.
[Crossref] [Google Scholar] [PubMed]
- Tao R, Gong J, Luo X, Zang M, Guo W, Wen R, et al. AMPK exerts dual regulatory effects on the PI3K pathway. J Mol Signal 2010;5(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Stanley K, Fraser R, Bruce C. Physiological changes in insulin resistance in human pregnancy: Longitudinal study with the hyperinsulinaemic euglycaemic clamp technique. Br J Obstet Gynaecol 1998;105(7):756-9.
[Crossref] [Google Scholar] [PubMed]
- Le Guenno G, Chanséaume E, Ruivard M, Morio B, Mazur A. Study of iron metabolism disturbances in an animal model of insulin resistance. Diabetes Res Clin Pract 2007;77(3):363-70.
[Crossref] [Google Scholar] [PubMed]
- Martinez-Garcia MA, Luque-Ramirez M, San-Millán JL, Escobar-Morreale HF. Body iron stores and glucose intolerance in premenopausal women: Role of hyperandrogenism, insulin resistance, and genomic variants related to inflammation, oxidative stress, and iron metabolism. Diabetes Care 2009;32(8):1525-30.
[Crossref] [Google Scholar] [PubMed]
- Chang L, Chiang SH, Saltiel AR. TC10α is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology 2007;148(1):27-33.
[Crossref] [Google Scholar] [PubMed]
- Sesti G, Federici M, Lauro D, Sbraccia P, Lauro R. Molecular mechanism of insulin resistance in type 2 diabetes mellitus: Role of the insulin receptor variant forms. Diabetes Metab Res Rev 2001;17(5):363-73.
[Crossref] [Google Scholar] [PubMed]
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet 1996;12(1):106-9.
[Crossref] [Google Scholar] [PubMed]
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 1998;2(5):559-69.
[Crossref] [Google Scholar] [PubMed]
- Chen SH, Liu XN, Peng Y. MicroRNA‐351 eases insulin resistance and liver gluconeogenesis via the PI3K/AKT pathway by inhibiting FLOT2 in mice of gestational diabetes mellitus. J Cell Mol Med 2019;23(9):5895-906.
[Crossref] [Google Scholar] [PubMed]
- James JV, Varghese J, Mckie AT, Vaulont S, Jacob M. Enhanced insulin signaling and its downstream effects in iron-overloaded primary hepatocytes from hepcidin knock-out mice. Biochim Biophys Acta Mol Cell Res 2020;1867(2):1-13.
[Crossref] [Google Scholar] [PubMed]
- Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: Mechanisms of action and physiological activities. Exp Mol Med 2016;48(4):1-12.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Zhao H, Wang A. Oleuropein alleviates gestational diabetes mellitus by activating AMPK signaling. Endocr Connect 2021;10(1):45-53.
[Crossref] [Google Scholar] [PubMed]
- O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013;493(7432):346-55.
[Crossref] [Google Scholar] [PubMed]
- Wu SB, Wu YT, Wu TP, Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim Biophys Acta Gen Subj 2014;1840(4):1331-44.
[Crossref] [Google Scholar] [PubMed]
- Zou C, Zhang Q, Zhang S. Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. J Pharmacol Sci 2018;138(3):161-6.
[Crossref] [Google Scholar] [PubMed]
- Varghese J, James J, Vaulont S, Mckie A, Jacob M. Increased intracellular iron in mouse primary hepatocytes in vitro causes activation of the Akt pathway but decreases its response to insulin. Biochim Biophys Acta Gen Subj 2018;1862(9):1870-82.
[Crossref] [Google Scholar] [PubMed]
- Chen HJ, Sugiyama M, Shimokawa F, Murakami M, Hashimoto O, Matsui T, et al. Response to iron overload in cultured hepatocytes. Sci Rep 2020;10(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Sferruzzi-Perri AN, Higgins JS, Vaughan OR, Murray AJ, Fowden AL. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc Natl Acad Sci U S A 2019;116(5):1621-6.
[Crossref] [Google Scholar] [PubMed]
- Muralimanoharan S, Maloyan A, Myatt L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: Role of miR-143. Clin Sci 2016;130(11):931-41.
[Crossref] [Google Scholar] [PubMed]
- Jiang S, Teague AM, Tryggestad JB, Jensen ME, Chernausek SD. Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci Rep 2020;10(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Zuo M, Lu J, Wang Y. Adiponectin reduces embryonic loss rate and ameliorates trophoblast apoptosis in early pregnancy of mice with polycystic ovary syndrome by affecting the AMPK/PI3K/Akt/FoxO3a signaling pathway. Reprod Sci 2020;27(12):2232-41.
[Crossref] [Google Scholar] [PubMed]
- Zheng T, Yang X, Wu D, Xing S, Bian F, Li W, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria‐associated AMPK/PI3K/Akt/GSK 3β pathway. Br J Pharmacol 2015;172(13):3284-301.
[Crossref] [Google Scholar] [PubMed]