- *Corresponding Author:
- Rong Zhao
The First Clinical Medical College,Yunnan University of Traditional Chinese Medicine, Kunming, Yunnan 650000, P. R. China
E-mail: 1820059756@qq.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “308-317” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The purpose of this study was to evaluate the effect of preventive acupuncture combined with CMNS6-1 electroacupuncture on 7,12-dimethylbenzanthracene induced mammary tumors in Sprague-Dawley rats and to explore the effect of preventive acupuncture on mammary tumors from the perspective of apoptosis. 48 w old female Sprague-Dawley rats were randomly divided into control (10), model (10), tamoxifen (10) and preventive acupuncture (10) groups. The model group, tamoxifen group and preventive acupuncture group were administered once intragastrically with 100 mg/kg body weight of 7,12-dimethylbenzanthracene to induce spontaneous breast cancer. On d 1 after modeling, acupuncture plus electrical stimulation was administered to the preventive acupuncture group. Density wave was selected for electrical stimulation with a frequency of 2 Hz/100 Hz and an intensity of 1 mA and three acupoints, "Zu San Li", "San Yin Jiao" and "Tan Zhong", were stimulated for 20 min per treatment, once every other day, three times a week for 16 w. Tamoxifen (3 mg/kg body weight, intragastrically, every day) was started the day after 7,12-dimethylbenzanthracene administration and continued for 16 w, as positive control treatment. Apoptosis of breast tumor tissue cells was detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling assay, and expression of caspase-3 and B-cell lymphoma-2 was assessed by immunohistochemistry. During the experiment, no tumors were detected in rats from the control+sham group, all the rats in the 7,12-dimethylbenzanthracene+sham group developed tumors and the cumulative incidence of tumors was 100 %, meanwhile the cumulative incidence of tumors in the 7,12-dimethylbenzanthracene+preventive acupuncture group and the 7,12-dimethylbenzanthracene+tamox group were 60 % and 50 %, respectively. Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay showed that preventive acupuncture could significantly promote apoptosis in breast tumor cells in 7,12-dimethylbenzanthracene induced rat, while increased the expression levels of pro-apoptotic proteins such as caspase-3 and B-cell lymphoma-2 associated X. The expression level of antiapoptotic protein B-cell lymphoma-2 was down-regulated and the ratio of B-cell lymphoma-2/B-cell lymphoma-2 associated X was increased. Preventive acupuncture therapy combined with CMNS6-1 electroacupuncture instrument inhibited 7,12-dimethylbenzanthracene-induced mammary tumorigenesis in rats and the mechanism may be related to the regulation of apoptosis-related protein expression and promotion of tumor cell apoptosis.
Keywords
Preventive acupuncture therapy, CMNS6-1 electroacupuncture instrument, mammary tumorigenesis, cell apoptosis, caspase-3, B-cell lymphoma-2
According to the GLOBOCAN 2020 database, breast cancer has surpasses lung cancer and become the most common cancer in both sexes for the first time with 2 261 419 new cases worldwide, accounting for 11.7 % of the overall cancer incidence[1]. Furthermore, breast cancer caused 684 996 cases of death, accounting for 6.9 % of cancer deaths in total and ranks the 4th most common cause of cancer related death. It is estimated that by 2030, the global incidence of breast cancer will reach 2.64 million and the death toll will be 1.7 million[1]. Statistics in China has revealed about 169 000 new cases and 45 000 deaths in 2008, ranking breast cancer the second most common malignant tumor and the 6th most common cause of death in female[2]. Moreover, incidence of breast cancer in China has showed rapid increased and younger age at disease onset in recent years[3]. Current management of breast cancer emphasizes a holistic and individualized treatment strategy including surgery, chemotherapy, radiotherapy, endocrine therapy and targeted therapy[4]. Although the survival rate in breast cancer has improved, it varies in different countries distinctly[5], due to factors such as lack of early-stage screening, detection and cost effective therapy[6].
As a safe non-pharmaceutical therapy, acupuncture is an important part of Traditional Chinese Medicine (TCM). By stimulating specific meridians and acupoints of the human body, acupuncture and achieving the role of multi-system and multi-target regulation of human internal secretion and immunity, it is widely used in the prevention, health care and treatment of many diseases[7]. Clinical treatment confirms that it has a significant therapeutic effect on various cancerrelated discomforts, such as fatigue, hot flashes, sleep disorders, menopausal symptoms, lymphedema in breast cancer patients and side effects caused by treatment[8]. However, experimental evidences of effect of acupuncture on mammary tumorigenesis and tumor growth in vivo are lacking.
The occurrence of malignant breast tumors is a gradual process involving multi-factor participation. Cell apoptosis imbalance occupies an important role in its pathogenesis. Apoptosis is a key regulatory factor for tissue homeostasis, which is strictly regulated by the interaction of activation and inhibition pathways. Once the normal apoptosis process is destroyed, it will cause a series of diseases, including cancer, neurodegeneration, chronic, inflammatory and autoimmune diseases.
The occurrence and development of breast malignant tumor is a gradual process that goes through multiple stages and involves the participation of multiple factors. Apoptosis imbalance plays an important role in its pathogenesis[9].
Apoptosis is a key regulator of tissue homeostasis and is tightly regulated through the interaction of activating and inhibiting pathways. Characteristic manifestations of apoptosis include cell shrinking and chromatin condensation, apoptotic vesicle formation and cytoskeletal junctions. Apoptotic vesicles are catalyzed by cysteine aspartases (caspases) such as caspase-3 and caspase-7. Apoptotic caspases are usually present in cells as inactive enzymes and are activated in response to specific apoptotic stimuli. One of the pathways by which caspases activate apoptosis is the mitochondrial apoptotic pathway, which proceeds by altered permeability of the outer mitochondrial membrane leading to the release of Cytochrome-C (Cyt-C) into the cytoplasm. Cyt-C binds to Apoptosis Associated Factor-1 (APAF-1) in the presence of deoxy Adenosine Triphosphate (dATP), forming multimers and induces caspase-9 to bind to it to form apoptotic vesicles. Activated caspase-9 activates downstream caspase-3 and caspase-7, thereby inducing apoptosis.
The mitochondrial apoptotic pathway is regulated by the ratio of anti-apoptotic and pro-apoptotic members of the B-Cell Lymphoma-2 (BCL-2) protein family. Its pro-apoptotic members, Bcl-2 homologous Antagonist/ Killer (BAK) and BCL-2 Associated X (BAX), bind to the mitochondrial outer membrane during apoptosis, forming oligomeric complexes that disrupt outer membrane integrity and allow the release of Cyt-C. BAX and BAK are normally prevented from binding to the mitochondrial outer membrane by anti-apoptotic members of the BCL-2 family such as BCL-2, BCL-XL and Myeloid Cell Leukemia 1 (MCL1) which prevent their binding to the outer mitochondrial membrane. In addition, these anti-apoptotic members of the BCL-2 family can be neutralized by the only BCL-2 Homology 3 (BH3) members of the BCL-2 family (e.g. Bid, Bad and Bim), thus tilting the balance toward apoptosis. Studies have confirmed that dysregulation of cellular homeostasis due to dysregulated expression of BCL-2 and BAX genes is one of the causes of the development of carcinogenesis[10].
Studies on the regulation of apoptosis by acupuncture have gradually increased in recent years and biological and immunological results have shown that acupuncture can regulate the expression of apoptosis-related genes and proteins BCL-2/BAX, caspase family, Fas/Fas Ligand (FasL), c-fos and Tumour Necrosis Factor Alpha (TNF-α), Nuclear Factor Kappa B (NF-κB) to reduce cell death under different pathological conditions. However, few studies have been reported on the use of pre-acupuncture to explore the role and mechanism of breast tumor prevention and treatment from the perspective of promoting apoptosis.
The combined use of Zusanli (ST36) and Sanyinjiao (SP6) acupuncture points was found in acupuncture studies to significantly reduce the inflammatory response due to injury as well as reduce the release of inflammatory cytokines, especially the antiinflammatory effect was enhanced by applying Preventive Acupuncture (PA) therapy before injury occurred[11]. Danzhong (CV17) mastocytosis can be treated by moxibustion therapy[12].
Based on the above study, three meridians, ST, SP and CV, which are related to the route of mammary meridians and three acupuncture points, ST36, SP6 and CV17, which belong to the above three meridians, were selected for this experiment and 7,12-Dimethylbenzanthracene (DMBA) induced PA therapy was performed in Sprague-Dawley (SD) rats to investigate the mechanism of PA inhibiting mammary tumor growth from the perspective of cell apoptosis.
Materials and Methods
Chemicals:
DMBA (purity ≥95 %) was purchased from Sigma- Aldrich (St. Louis, MO, USA). Tamoxifen citrate tablets were purchased from Yangzijiang Pharmaceutical Group Co., Ltd., lot No: 17041311.
Animals and treatment:
Animals: Forty female SD rats (Specific Pathogen Free (SPF), 8 w old, weighing 180-220 g obtained from Hunan Lake Scenery Company Limited, license number: SCXK (Hunan) 2016-0002) were housed in polycarbonate cages in groups of five at controlled temperature 25°±2° under a 12 h light/12 h dark cycle (lights on at 7:00 am).
The animals had free access to standard chow diet and water. All experimental procedures were carried out in accordance with the recommendations of the guide for the care and use of laboratory animals. All animal studies were approved by the Kunming Animal Care and Use Committee.
Grouping: After a week of acclimatization, 40 rats were randomly divided into four groups with 10 rats in each group as follows, the control+sham group; the DMBA+sham group; the DMBA+tamox group and the DMBA+PA group.
Establishment of animal model and treatment: Spontaneous mammary tumorigenesis was induced by a single dose of DMBA (100 mg/kg in 0.5 ml sesame oil) through intragastric gavage on d 0 in rats of the DMBA+sham group, the DMBA+tamox group and the DMBA+PA group. Rats from the control+sham group were given an equal volume of sesame oil.
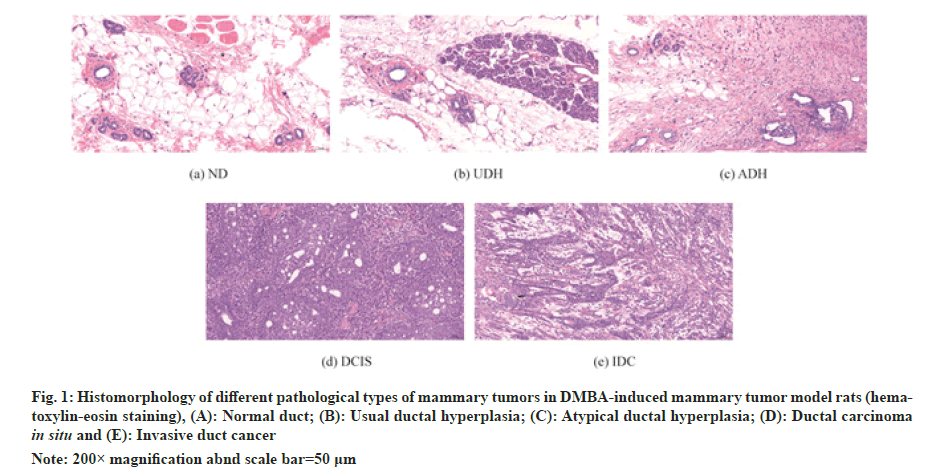
After 16 w of intervention, rats were anesthetized by intraperitoneal injection of 2 % sodium pentobarbital (0.2 ml/100 g), mammary tumors visible to the naked eye were peeled off in each group, the number of tumors was recorded and the number and volume of tumors were measured. The second pair of mammary tissues of each group of rats was taken for routine Haemotoxylin and Eosin (HE) staining to observe the histopathological changes. The Histomorphology of different pathological types of mammary tumors in DMBA-induced mammary tumor model rats was illustrated in fig. 1.
Fig. 1: Histomorphology of different pathological types of mammary tumors in DMBA-induced mammary tumor model rats (hematoxylin- eosin staining), (A): Normal duct; (B): Usual ductal hyperplasia; (C): Atypical ductal hyperplasia; (D): Ductal carcinoma in situ and (E): Invasive duct cancer
Note: 200× magnification abnd scale bar=50 μm
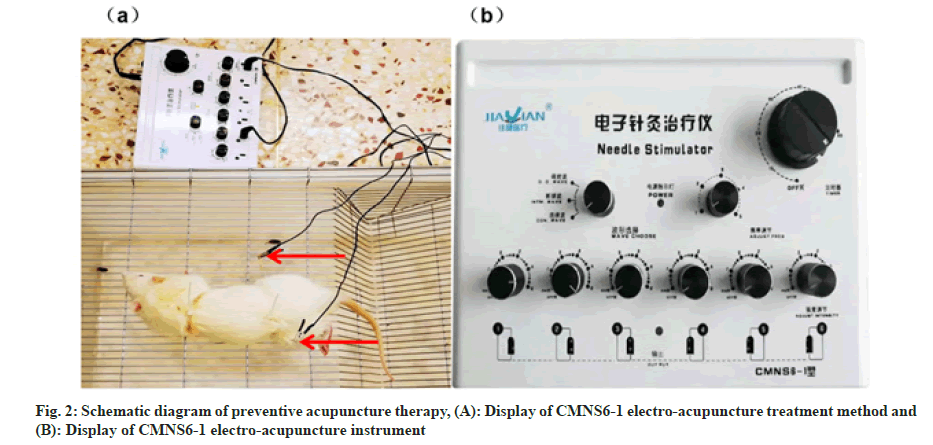
Acupoints including ST36, CV17, SP6 were located according to the atlas of acuppoints in rat according to Xu et al.[13] PA therapy combined with CMNS6-1 electrical stimulation were applied to rats of the DMBA+PA group every 3 d for 16 w while sham acupuncture at the same frequency and duration were applied to rats of the control+sham group and the DMBA+sham group. Rats of the DMBA+tamox group received tamoxifen citrate dissolved in double diluted water through intragastric gavage at a dosage of 3 mg/kg every day and lasted for 16 w. The schematic diagram of preventive acupuncture therapy was illustrated in fig. 2.
Main instruments: Stainless disposable acupuncture needles (Hwato, Suzhou Medical Appliance Factory, 0.30×25 mm), an electroacupuncture instrument (Wuxi Jiajian Medical Equipment Co., Ltd. CMNS6-1).
Necropsy and evaluation of mammary tumorigenesis: General status of the rats was monitored every day and Body Weight (BW) was recorded weekly. Mammary glands of rats were observed and palpated every 3 d from the 1 d of acclimatization till the end of the experiment and tumor latency (time of tumor appearance) was recorded once a new tumor was palpated. After 16 w of treatment and observation, all the rats were fasted overnight and sacrifice and autopsy of each animal were then carried out upon anesthesia with ketamine (50 mg/kg BW; intraperitoneally). All tumors were removed, counted, photographed and weighed. The length, width and height of each tumor were measured by a caliper and tumor volume was calculated using the formula
Tumor volume=0.5×tumor (length×width×height)
Mammary tumors multiplicity (i.e., average mammary tumor number/rat) in each group was also calculated. Finally, all the tumors were fixed in 10 % neutral formalin solution for at least 24 h for histomorphology.
Histopathology:
Fixed tumor samples were dehydrated by a series of ethanol solution, embedded later in paraffin blocks and cut into tissue sections in 5 μm thickness. Hematoxylin and eosin staining procedures were then carried out on tissue section of each tumor. Histomorphology was determined under an Axioskop 40 microscope connected to a computer where the image was transferred using MRGrab1.0 and Axio Vision 3.1 software (Zeiss Hallbergmoos, Germany).
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) assay for apoptosis detection:
Paraffin sections were routinely dewaxed and hydrated, then proteinase K was added drop wise, peroxidase blocker was added after 20 min of reaction, 5× reaction buffer 100 μl was added drop wise after 20 min, 50 μl of labeling reaction mixture was added drop wise after 1 h. The whole sample was covered by a sealing film and the sections were incubated in a wet box at 37° for 1 h. The film was removed and washed with Phosphate Buffered Saline (PBS), 100 μl of closure solution was added and the reaction was carried out at room temperature for 10 min. Antibody 100 μl solution was added drop wise, incubated at room temperature for 1 h under avoidance of light, washed with PBS, covered with 100 μl of closure solution drop wise, 100 μl of 3,3’-Diaminobenzidine (DAB) solution was added drop wise after 30 min, washed after 15 min, dehydrated, transparent and sealed after hematoxylin re-staining, and observed under 400× light microscope, 6 fields of view were randomly selected for counting.
Immunohistochemical analysis:
Immunohistochemical analysis of tissue sections of breast tumors was performed using standard procedures. Caspase-3, BCL-2 rabbit polyclonal antibodies were purchased from Wuhan Servicebio Technology Co. Ltd., Wuhan, China. The optical density of positive staining was quantitatively assessed by Image-Pro Plus 6.0 and expressed as mean optical density. The number of positively expressed cells was compared and the positive expression rate was calculated for each experimental group.
Western blot-assay:
Paraffin sections were routinely dewaxed and hydrated, the antigen was repaired, the relevant primary and secondary antibodies were added, the reaction was incubated, DAB stained, hematoxylin stained again, the number of caspase-3 and BCL-2 positive cells was observed under a light microscope 400× and the positive expression rate was calculated.
Statistical analysis:
Results are presented as means±Standard Deviation (SD) for each experimental group. Statistical analysis of data was performed using the one-way Analysis of Variance (ANOVA) followed by Dunnett’s post hoc test for multiple comparisons. Fisher’s exact probability test was used to compare the tumor incidence between different experiment groups. Differences in mean values for tumor multiplicities, weight and volumes between different experiment groups were evaluated by the twotailed student’s t-test. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) Statistics 19 Software (IBM). Statistical significance of differences was considered at p<0.05.
Results and Discussion
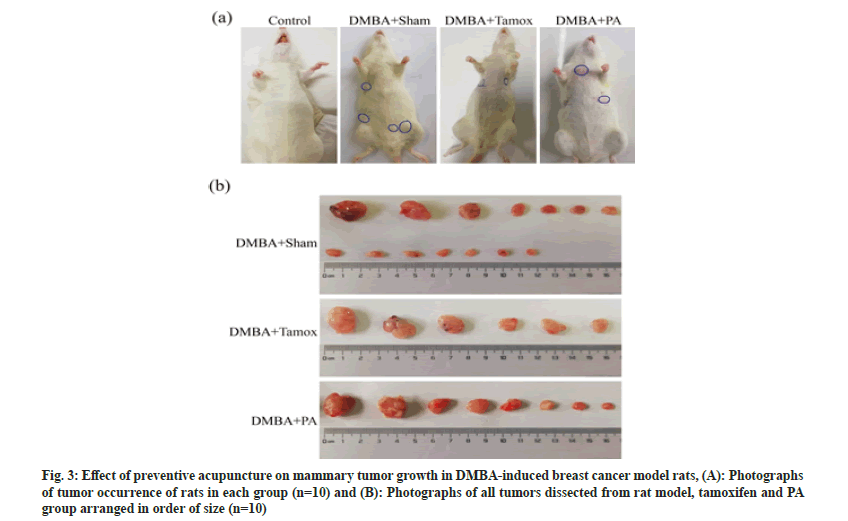
Fig. 3 is an illustration of the growth of mammary tumors in each group of rats. By the end of the experiment at 16 w, except for the control group, mammary tumors appeared in all experimental groups. The tumors of rats in the DMBA+sham group, DMBA+tamox group and DMBA+PA group were photographed in the order of tumor size as shown in fig. 3A. 14 mammary tumors were found in the DMBA+sham group, 6 mammary tumors were found in the tamox group and 8 mammary tumors were found in the PA group as shown in fig. 3B.
All rats in the DMBA+sham group developed tumors at the end of the experiment with tumor incidence of 100 %. However, it was observed in the DMBA+tamox group that only half rats of the experiment group developed tumors with its tumor incidence of 50 %. Meanwhile, 6 out of 10 rats in the DMBA+PA group developed tumor with its tumor incidence at 60 % observed on the end of the experiment. The Chi-square (χ2) test further suggested that the cumulative tumor incidence was significantly different between the groups of rats (χ2 =6.106, p<0.05), as shown in Table 1.
| Group | Number of rats | Number of deaths | Number of survival | Number of tumor incidents in rats | Cumulative tumor incidence in rats (%) | χ2 | p |
|---|---|---|---|---|---|---|---|
| DMBA+sham | 10 | 1 | 9 | 9 | 1 | 6.106 | 0.047* |
| DMBA+PA | 10 | 0 | 10 | 6 | 0.6 | ||
| DMBA+tamox | 10 | 0 | 10 | 5 | 0.5 |
Table 1: Cumulative tumor incidence in ratsof each experimental group during the experiment
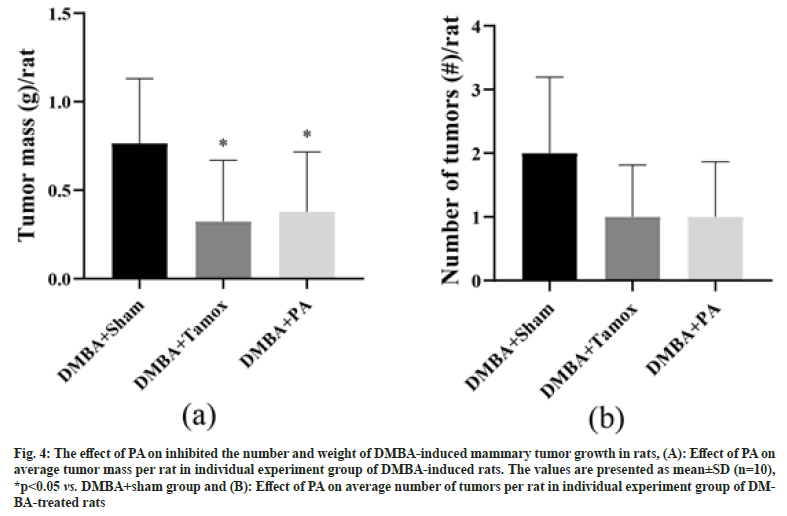
Fig. 4A demonstrated the mean tumor mass of rats in the DMBA+sham group and DMBA+tamox group was significantly lower compared with the DMBA+sham group (p<0.0001, p<0.001). Tumor multiplicity was calculated by dividing the total numbers of tumor by the number of rats in each group to obtain the average number of tumors per rat. We found that there were 2 tumors per rat in DMBA+sham group on average while treatment with PA or tamoxifen significantly reduced the tumor multiplicity to 0.9 and 0.7 tumor/rat respectively, as shown in fig. 4B.
Fig. 4: The effect of PA on inhibited the number and weight of DMBA-induced mammary tumor growth in rats, (A): Effect of PA on average tumor mass per rat in individual experiment group of DMBA-induced rats. The values are presented as mean±SD (n=10), *p<0.05 vs. DMBA+sham group and (B): Effect of PA on average number of tumors per rat in individual experiment group of DMBA- treated rats
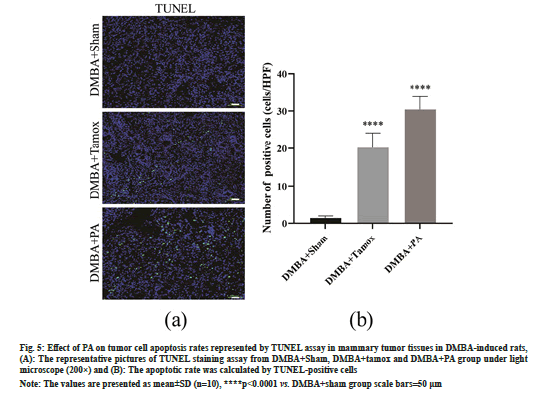
As shown in fig. 5A and fig. 5B, few TUNEL positive staining cells were found in the mammary tissues of the DMBA+sham group, whereas sporadic TUNEL staining positive apoptotic cells appeared in the mammary tumor tissues of the rats in the DMBA+tamox and DMBA+PA groups. High magnification counts of TUNEL positive staining cells showed that the number of TUNEL positive cells in mammary tissues was significantly increased in the DMBA+PA and DMBA+tamox groups compared with the DMBA+sham group (p<0.01).
Fig. 5: Effect of PA on tumor cell apoptosis rates represented by TUNEL assay in mammary tumor tissues in DMBA-induced rats, (A): The representative pictures of TUNEL staining assay from DMBA+Sham, DMBA+tamox and DMBA+PA group under light microscope (200×) and (B): The apoptotic rate was calculated by TUNEL-positive cells
Note: The values are presented as mean±SD (n=10), ****p<0.0001 vs. DMBA+sham group scale bars=50 μm
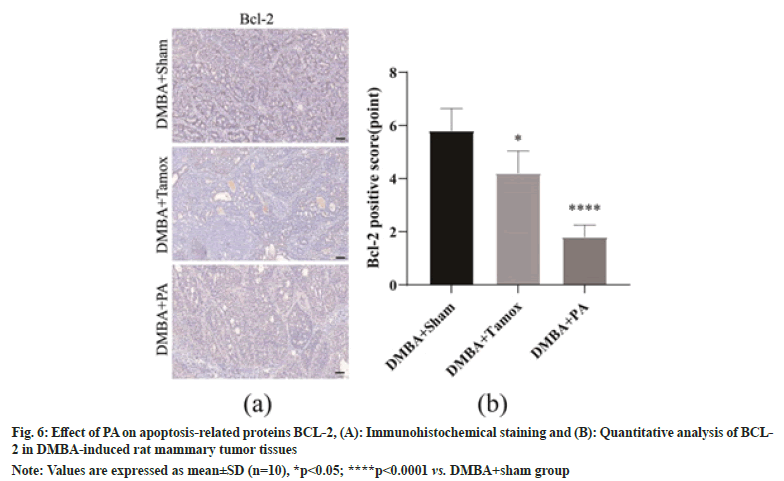
As shown in fig. 6A, the percentage of BCL-2 positive cells in tumor tissues was decreased in the DMBA+tamox and DMBA+PA groups compared to the DMBA+sham group. We further enumerated BCL-2 positive cells under High Magnification Microscopy (HPM) and found that the proportion of BCL-2 positive cells was significantly decreased in both the DMBA+tamox group (p<0.05)and the DMBA+PA group (p<0.0001) compared with the DMBA+sham group, as shown in fig. 6B.
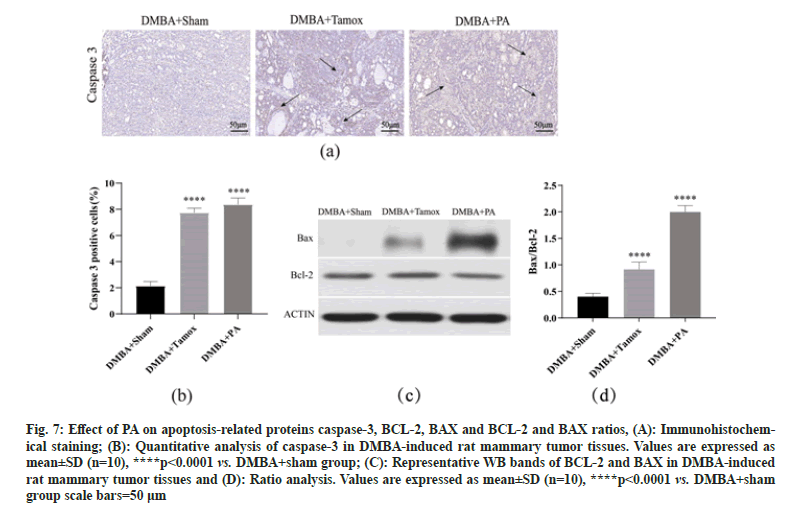
By immunohistochemical detection, we found a small amount of caspase-3 positive expression in the breast tissue of the DMBA+sham group, while the number of caspase-3 positive cells in the breast tissue of the DMBA+tamox and DMBA+PA groups was significantly increased. The caspase-3 positive expression rate was analyzed by Image-Pro Plus 6.0 software and it was found that the caspase-3 positive expression rate in breast tissues of both the DMBA+PA and DMBA+tamox groups was significantly higher than that of the DBMA+sham group (p<0.01), indicating that PA therapy may promote caspase-3 expression by inducing apoptosis of tumor cells-3 expression by inducing apoptosis in tumor cells as shown in fig. 7A and fig. 7B.
Western blot assay showed that BAX protein expression was significantly higher in mammary tumor tissues of rats in DMBA+tamox group, DMBA+PA group compared with DMBA+sham group (p<0.01), among which BAX protein expression was more significantly higher in DMBA+PA group than DMBA+tamox group (p<0.01); DMBA+tamox group had decreased BCL-2 protein expression (p<0.01) and the decrease of BCL-2 protein expression in DMBA+PA group was not significant (p>0.05). Further gray-scale analysis of the bands using the software to compare the BCL-2/BAX ratio showed that the BCL-2/BAX ratio was significantly higher in the mammary tumor tissues of rats in both the DMBA+PA and DMBA+tamox groups compared with the DMBA+sham group ass shown in fig. 7C and fig. 7D.
Fig. 7: Effect of PA on apoptosis-related proteins caspase-3, BCL-2, BAX and BCL-2 and BAX ratios, (A): Immunohistochemical staining; (B): Quantitative analysis of caspase-3 in DMBA-induced rat mammary tumor tissues. Values are expressed as mean±SD (n=10), ****p<0.0001 vs. DMBA+sham group; (C): Representative WB bands of BCL-2 and BAX in DMBA-induced rat mammary tumor tissues and (D): Ratio analysis. Values are expressed as mean±SD (n=10), ****p<0.0001 vs. DMBA+sham group scale bars=50 μm
Apoptosis is a process of cell death caused by specific signals induced by the organism to trigger an intracellular death cascade response in order to maintain the stability of the internal environment[14]. Normal cells undergo a complex series of multiple steps in the development of malignancy eventually acquiring the hallmark features of malignancy[15]. While the imbalance between cell proliferation and apoptosis is one of the important mechanisms leading to tumorigenesis, the promotion of tumor cell apoptosis is also an important target for the action of anti-tumor therapy. During apoptosis, BCL- 2 family proteins trigger endogenous mitochondrial apoptosis by regulating Mitochondrial Outer Membrane Permeabilization (MOMP) causing Cyt-C release into the cytoplasm, which is an important cell caspases belong to the class of cysteine proteases and there are more than ten members of the caspases family, which play different roles in the apoptotic process, while caspase-3 is the main terminal shear enzyme in apoptosis and plays an irreplaceable role in apoptosis, which can be blocked by BCL-2[16]. BAX is a pro-apoptotic protein that antagonizes with anti-apoptotic protein BCL-2 and maintains a relatively stable ratio to regulate apoptosis by forming homo or heterodimers. When BCL-2 protein expression exceeds BAX it has an inhibitory effect on apoptosis and conversely when BAX expression exceeds BCL-2 it has a pro-apoptotic effect on apoptosis[17].
The breast is located in the middle of the chest and is the "place of the Zongjing", where many meridians converge. The term "preventive acupuncture" first appeared in Sui-Chao Yuanfang's "treatise on the origin of diseases" and Gao Wu's "acupuncture and moxibustion" in the Ming Dynasty said "early intervention in the absence of disease is called prevention”. Preventive acupuncture means that the body uses acupuncture or moxibustion to stimulate acupuncture points before the arrival of disease, or when the disease is shallow, to stimulate the body’s positive energy, regulate yin and yang and improve the body’s immunity, so as to achieve the purpose of physical health[18]. The main acupuncture points of the meridian identification and inverse acupuncture were selected from the foot Yangming stomach meridian, the Ren meridian, the Foot Taiyin spleen meridian and the specific acupuncture points, the Zu Sanli, Tanzhong and Sanyinjiao, which are closely related to the breast circulation. It reflects the core feature of "where the meridians pass, the main treatment reaches”[19]. The combination of PA therapy and CMNS6-1 electroacupuncture is a safe and effective method to prevent and treat diseases. It can not only promote local tissue metabolism, enhance blood circulation and enhance the disease prevention effect of acupuncture, but also objectively and accurately evaluate the prevention and treatment of diseases.
At present, acupuncture intervention in apoptosis is mainly focused on neural cell repair, myocardial ischemia-reperfusion injury repair and other fields[20,21]. It was found that acupuncture has the advantages of acting on multiple systems, having multiple targets and being safe and non-toxic[22], and it is of great clinical significance to explore the mechanism of action of meridian discriminative inverse acupuncture on breast tumor prevention and treatment to carry out breast cancer prevention with TCM characteristics.
In this study, we explored the preventive and blocking effects of PA therapy combined with CMNS6-1 electroacupuncture electrostimulation on mammary tumorigenesis using a spontaneous mammary tumor model in rats induced by carcinogenic agent DMBA and further explored its possible mechanism of action from the perspective of apoptosis. The results of TUNEL staining showed that apoptotic cells were increased in mammary tumor tissues of rats after PA therapy intervention and caspase-3 was the end-shear enzyme of apoptotic cascade reaction in mammary tumor cells. Western blot further confirmed that compared with the model group, the expression of BCL-2 was downregulated, the expression of BAX protein was increased, and the BCL-2/BAX ratio was significantly higher in the PA group, suggesting that PA therapy may promote tumor cell apoptosis by regulating the expression of apoptosisrelated proteins BCL-2 and BAX, thus playing a role in preventing and treating mammary tumors. The effect of PA therapy may promote tumor cell apoptosis by regulating the expression of apoptosis-related proteins BCL-2 and BAX, thus playing a role in the prevention and treatment of breast tumors.
In conclusion, PA therapy combined with CMNS6- 1 electroacupuncture exerts an inhibitory effect on tumorigenesis in a rat model of spontaneous mammary tumors and the mechanism may promote apoptosismediated tumor cell death by regulating the expression of apoptosis-related proteins BCL-2, BAX and caspase-3, suggesting that PA therapy has important applications in the prevention and treatment of mammary tumors.
Acknowledgements:
This work was supported by the National Research Foundation of China Grant funded by the China Government (81760896). Yunnan University of TMCThe First Affiliated Hospital of Yunnan University of TMC Joint Foundation Project (XYLH202006).
Conflict of interests:
The authors report no conflict of interests.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [Pub Med]
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J 2021;134(7):783-91.
[Crossref] [Google Scholar] [Pub Med]
- Qiu H, Cao S, Xu R. Cancer incidence, mortality and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun 2021;41(10):1037-48.
[Crossref] [Google Scholar] [Pub Med]
- Greenlee H, du Pont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 2017;67(3):194-232.
[Crossref] [Google Scholar] [Pub Med]
- Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the global burden of disease study. J Hematol Oncol 2019;12(1):1-21.
[Crossref] [Google Scholar] [Pub Med]
- Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017;389(10071):847-60.
[Crossref] [Google Scholar] [Pub Med]
- Li NC, Li MY, Chen B, Guo Y. A new perspective of acupuncture: The interaction among three networks leads to neutralization. Evid Based Complement Alternat Med 2019;2019:2326867.
- Jang S, Ko Y, Sasaki Y, Park S, Jo J, Kang NH, et al. Acupuncture as an adjuvant therapy for management of treatment-related symptoms in breast cancer patients: Systematic review and meta-analysis (PRISMA-compliant). Medicine 2020;99(50):e21820.
[Crossref] [Google Scholar] [Pub Med]
- Kønig SM, Rissler V, Terkelsen T, Lambrughi M, Papaleo E. Alterations of the interactome of Bcl-2 proteins in breast cancer at the transcriptional, mutational and structural level. PLoS Comput Biol 2019;15(12):e1007485.
[Crossref] [Google Scholar] [Pub Med]
- Kholoussi NM, El-Nabi SE, Esmaiel NN, El-Bary A, Mohamed N, El-Kased AF. Evaluation of Bax and Bak gene mutations and expression in breast cancer. Biomed Res Int 2014;2014:249372.
[Crossref] [Google Scholar] [Pub Med]
- Lin W, Jia D, Fu C, Zheng Y, Lin Z. Electro-acupuncture on ST36 and SP6 acupoints ameliorates lung injury via sciatic nerve in a rat model of limb ischemia-reperfusion. J Inflamm Res 2020;13:465-70.
[Crossref] [Google Scholar] [Pub Med]
- Jiamin Y, Jihui W, Xiaoyu S, Mei Z, Zhenzhen L, Dandan Q, et al. Efficacy of moxibustion by stimulating acupoints of Danzhong (CV 17) and Ganshu (BL 18) on hyperplasia of mammary gland in rats. J Tradit Chin Med 2018;38(1):76-82.
[Crossref] [Google Scholar] [Pub Med]
- Xu DS, Zhao S, Cui JJ, Ma TM, Xu B, Yu XC, et al. A new attempt of re-mapping acupoint atlas in the rat. Zhen Ci Yan Jiu 2019;44(1):62-5.
[Crossref] [Google Scholar] [Pub Med]
- Shuai L, Bindong Z. Advances in the study of apoptosis pathways. Shandong Med J 2017;57(37):103-6.
- Senga SS, Grose RP. Hallmarks of cancer-The new testament. Open Biol 2021;11(1):200358.
[Crossref] [Google Scholar] [Pub Med]
- Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem 2018;62(3):341-60.
[Crossref] [Google Scholar] [Pub Med]
- Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J 2018;285(3):416-31.
[Crossref] [Google Scholar] [Pub Med]
- Zhu S, Chen H, Li X. Exploration of PA action pattern and mechanism. Liaoning J Tradit Chin Med 2017;44(11):2450-4.
- Ding N, Li R. Traceability of meridian identification and its clinical application. Chin J Acupunct Moxibust 2014;34(3):297-9.
- Xiao Y, Ding L, Gu Y. Research progress on the effect of acupuncture on apoptotic signaling pathways in myocardial ischemia-reperfusion injury. J Acupunct Res 2017;42(5):463-6.
- Liu T, Zhang Q, Zhao F. Research progress on the mechanism of action of acupuncture for spinal cord injury based on signaling pathways. Shanghai J Acupunct Moxibustion 2016;6:639-41.
- Yang X, Xu X, Wang Z. Research progress of anti-tumor mechanism of acupuncture and moxibustion. World J Tradit Chin Med 2019;14(6):1625-8.