- *Corresponding Author:
- Baohong Xu
Department of Gastroenterology, Capital Medical University Affiliated Beijing Luhe Hospital, Tongzhou, Beijing 101100, China
E-mail: bhxu22@ccmu.edu.cn
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “79-85” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the effects of Lactobacillus acidophilus tablets based on quadruple therapy on intestinal flora and inflammatory response in patients with Helicobacter pylori-infected chronic atrophic gastritis is the objective of the study. The clinical data of Helicobacter pylori-infected patients with Helicobacter pylori-infected chronic atrophic gastritis were analyzed retrospectively and 90 patients who met the criteria of inclusion and exclusion were selected as subjects. The control group was treated with conventional quadruple therapy and the observation group was treated with compound Lactobacillus acidophilus tablets combined with quadruple therapy. The intestinal flora and inflammation of the patients were compared. The efficacy of 82 % (41/50) and 96 % (p<0.05) were significantly higher in the post-treatment observation group than in the control group; the area of gastric lesion (0.85±0.17) was significantly lower than in the control group (p<0.05) and the digestive system symptoms score (3.23±1.13) was significantly lower than in the control group (6.67 %). Gastrointestinal function score (4.63±1.39) and Helicobacter pylori eradication rate (47/50) were significantly higher (94 %) in the observation group than in the control group, p<0.05; tumor necrosis factor alpha, (23.39±6.20 µg/ml) and interleukin-6 (15.39±6.41 µg/ml) were significantly lower than that in the control group and gastrin level (1.78±0.19 nmol/l) was significantly higher. Compound Lactobacillus acidophilus tablets based on quadruple therapy for the treatment of Helicobacter pylori-infected chronic atrophic gastritis can well eradicate Helicobacter pylori, control intestinal inflammation, improve intestinal flora and reduce clinical symptoms and signs of patients.
Keywords
Compound Lactobacillus acidophilus tablets, quadruple therapy, Helicobacter pylori-infected chronic atrophic gastritis, intestinal flora, intestinal inflammation
Chronic atrophic gastritis is a common clinical disease of the digestive system in which atrophy or even loss of the intrinsic glands of the gastric mucosa is the main pathological feature and the clinical symptoms are abdominal distention, abdominal pain and belching[1]. Among the many causes of chronic atrophic gastritis, Helicobacter pylori (Hp) infection is the main cause of the disease. Hp infection not only increases the incidence of gastrointestinal diseases such as gastritis and peptic ulcer, but also increases the possibility of gastrointestinal diseases becoming cancerous, which poses a great threat to the life and health of patients, so the eradication of Hp has become the key to the clinical treatment of atrophic gastritis.
At present, the main clinical treatment option for Hp eradication is quadruple therapy, but the long-term use of quadruple therapy will significantly increase the antibiotic resistance rate, affect patient’s medication compliance and reduce the success rate of Hp eradication treatment, and when two antibiotics are used in combination therapy, it will lead to the dysbiosis of patient’s intestinal flora and cause antibiotic-related gastrointestinal adverse reactions such as diarrhea[2]. The compound Lactobacillus acidophilus is a compound prepared with the following main ingredients like Chinese and Japanese strains of Lactobacillus acidophilus powder, Streptococcus faecalis and Bacillus subtilis powder[3], and in clinical practice compound Lactobacillus acidophilus tablets are indicated for patients with intestinal flora dysbiosis and the resulting symptoms of related intestinal dysfunction. The aim of this study was to investigate the efficacy of Lactobacillus acidophilus tablets in combination with quadruple therapy in the treatment of Hp infection.
Materials and Methods
General data:
We have selected and retrospectively analyzed the clinical data of 90 patients with Hp-infected atrophic gastritis admitted in our hospital. Patients in the control group received conventional quadruple therapy and patients in the observation group received compound Lactobacillus acidophilus tablets combined with quadruple therapy.
Control group include male and female in the ratio of 25:20, age (51.23±11.35) y, Body Mass Index (BMI) (22.54±3.11) kg/m², duration of disease 6 mo~5 y and classified according to the degree of atrophy of intrinsic glands of gastric mucosa which also include 31 patients of mild, 11 patients of moderate and 3 patients of severe.
Observation group include male and female in the ratio of 26:19, age (52.58±9.13) y, BMI (21.34±3.15) kg/m², duration of disease 5 mo~6 y and classified according to the degree of atrophy of intrinsic glands of gastric mucosa which also include 27 patients of mild, 13 patients of moderate and 5 patients of severe. The general data of the two groups were not statistically significant (p>0.05) and the conditions for the study were available.
Selection of subjects:
90 patients with Hp-infected atrophic gastritis who were admitted to our hospital were selected as the study subjects.
Inclusion criteria: According to the inclusion criteria[4], patients met the diagnostic criteria of chronic atrophic gastritis and were diagnosed and the results of 13C urea breath test were Hp (+); patients were aged 18-60 y and had not used Hp eradication therapeutic drugs in the recent past; patients did not have serious cardiac, hepatic or renal insufficiency; patients did not have psychiatric diseases, no serious cognitive dysfunction and could accept the investigation.
Exclusion criteria: Patients had received drugs such as proton pump inhibitors and antibiotics within 1 mo; patients had a history of upper gastrointestinal surgery; patients were combined with serious diseases of other systems or had malignant tumors; patients had serious gastrointestinal symptoms such as pyloric obstruction, perforation and cancerous ulcers; patients were allergic to the drugs used in this study and patients had serious adverse reactions while receiving treatment[5].
Treatment methods:
Patients in the control group were treated with conventional quadruple therapy, 20 mg of rabeprazole (State drug quantifier H20110076, Zhuhai Rundu Pharmaceutical Co, Ltd, specification 20 mg/capsule)+1.0 g of amoxicillin capsule (State drug quantifier H44021351, Zhuhai Federal Pharmaceutical Co. H20040661, Hainan Wipson Pharmaceutical Biotechnology Co., Ltd., specification 250 mg/capsule)+0.6 g bismuth potassium citrate capsule (State drug quantifier H10983186, Suzhou Dongrui Pharmaceutical Co., Ltd., specification 0.3 g/capsule, containing 110 mg of bismuth)[6], orally, once in the morning and once in the evening, with warm water, for 2 w of continuous treatment.
Patients in the study group were treated with Lactobacillus acidophilus tablets (State Drug Administration H20110801, manufacturer: Tonghua Jinma Pharmaceutical Group Co., Ltd., 0.5 g/tablet) on the basis of the control group[7], 1 g of Lactobacillus acidophilus tablets, taken orally, 3 times a day, with warm water for 2 w.
Observation indexes and evaluation methods:
The patients were examined at the end of treatment and the treatment effect was evaluated in terms of inflammation, symptoms and signs, which were divided into three parts like significant effect-The active inflammation of gastric mucosa disappeared in patients and clinical symptoms and signs disappeared, and the results of 13C urea breath test were negative in patients after treatment[8]; effective-Microscopically visible gastric mucosal lesions were reduced by half compared with those before treatment and clinical symptoms and the 13C urea breath test result was negative or changed from strong positive to weak positive; invalid-The clinical symptoms and signs did not improve after treatment, and the 13C urea breath test result was still positive after treatment.
The subject patients were examined using painless gastroscopy before and after treatment, respectively and the area of the lesion was measured and counted.
Digestive symptoms and gastrointestinal function were scored by the researcher before and after treatment for both groups of patients, and the scores included the patient’s urine and stools and gastrointestinal digestion level after treatment.
According to the Guidelines for Clinical Research on Drug Use (Trial)[9] and the Expert Opinion on the Treatment of Chronic Gastritis[10], the scores of each symptom and the final total scores were compared between the two groups of patients before and after treatment, in which the scored symptoms included epigastric pain, epigastric fullness and body fatigue, and the classification and score of each symptoms were none (0 points), light (1 point), moderate (2 points) and severe (3 points).
The 13C urea breath test and clinical observations[11] were performed by the researchers on both groups before and after treatment to compare the Hp eradication rate as well as the incidence of adverse effects.
After 2 w of treatment, 4 ml elbow venous blood samples were drawn from the subject patients in fasting state and the collected samples were processed by centrifugation at 3200 r/min by laboratory personnel, and the supernatant was taken after 15 min, and the serum levels of Interleukin-6 (IL-6), Tumor Necrosis Factor alpha (TNF-α) and Gastrin (GS) levels were measured by enzyme-linked immunosorbent assay[12].
Statistical analysis:
In this study, patients with incomplete clinical data were treated as missing values. Count data are expressed as percentage of frequency (%) and measurement data are expressed as mean±standard deviation (x±s). According to random, double-blind method for two groups, using Kruskal-Wallis nonparametric rank and inspection, comparison between groups of different curative effect of count data and statistics was calculated. Least Significant Difference (LSD)-t method was used for pairwise comparison of measurement data between the two groups and p<0.05 was considered statistically significant.
Results and Discussion
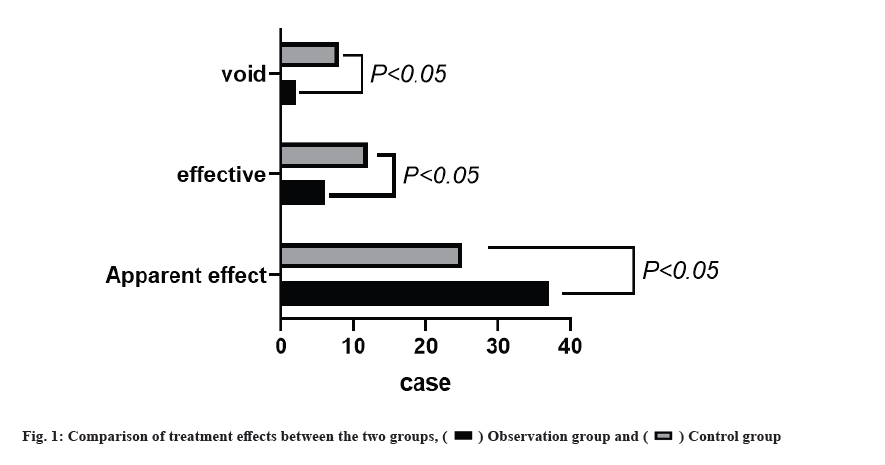
Comparison of treatment effects between the 2 groups was shown in fig. 1. We found that 82.00 % (41/50) of significant effect and 96.00 % of total effective rate in the observation group were significantly higher than those in the control group, p<0.05.
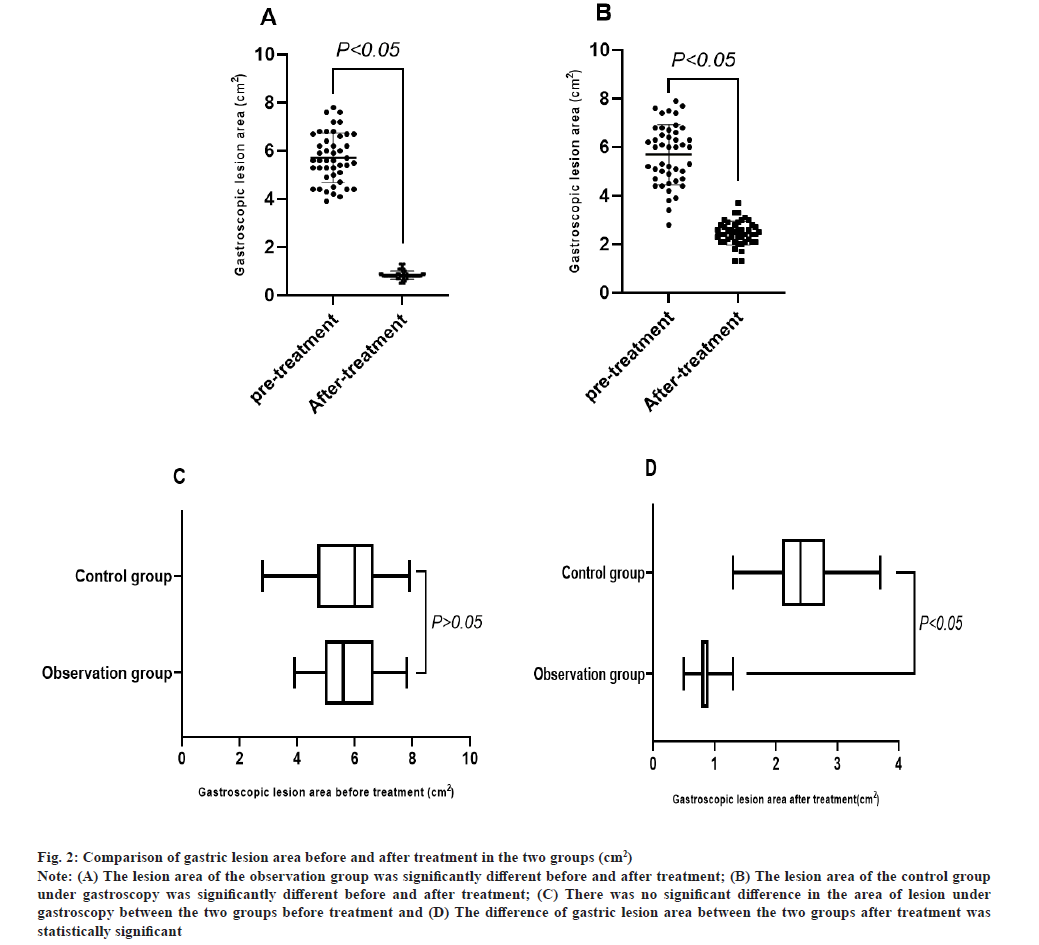
Comparison of gastric lesion area before and after treatment in 2 groups was shown in fig. 2A-fig. 2D. Before treatment, the difference between the two groups was not statistically significant, p>0.05 and after treatment, the gastric lesion area in the observation group (0.85±0.17 cm²) was significantly lower than that in the control group, t=20.824, p<0.05.
Fig. 2: Comparison of gastric lesion area before and after treatment in the two groups (cm²).
Note: (A) The lesion area of the observation group was significantly different before and after treatment; (B) The lesion area of the control group
under gastroscopy was significantly different before and after treatment; (C) There was no significant difference in the area of lesion under
gastroscopy between the two groups before treatment and (D) The difference of gastric lesion area between the two groups after treatment was
statistically significant.
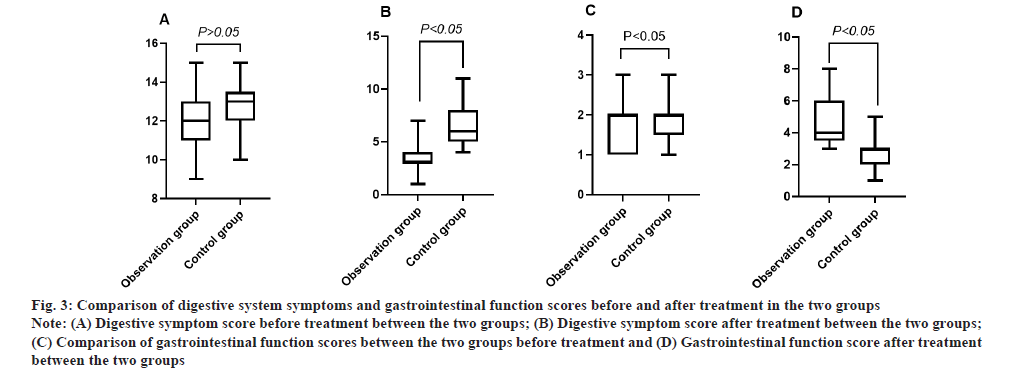
Comparison of digestive system symptoms and gastrointestinal function scores between the two groups before and after treatment was shown in fig. 3. The difference between the two groups before treatment was not statistically significant, p>0.05; the digestive system symptoms scores (3.23±1.13) in the observation group was significantly lower than that in the control group after treatment; the gastrointestinal function scores (4.63±1.39) in the observation group was significantly higher than that in the control group after treatment, t=10.768, 8.479, p<0.05.
Fig. 3: Comparison of digestive system symptoms and gastrointestinal function scores before and after treatment in the two groups.
Note: (A) Digestive symptom score before treatment between the two groups; (B) Digestive symptom score after treatment between the two groups;
(C) Comparison of gastrointestinal function scores between the two groups before treatment and (D) Gastrointestinal function score after treatment
between the two groups.
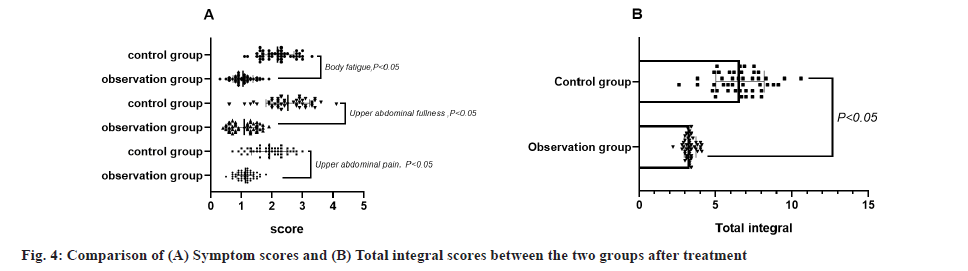
Comparison of the scores of each symptom in the 2 groups after treatment was shown in fig. 4. They include epigastric pain (1.13±0.28), epigastric fullness (1.09±0.42), body fatigue (1.10±0.33) and total scores (3.32±0.38) were significantly lower in the observation group than in the control group, p<0.05.
Comparison of Hp eradication rate and incidence of adverse reactions between the two groups was shown in Table 1. Hp eradication rate of 94.00 % (47/50) in the observation group was significantly higher than that in the control group; the incidence of adverse reactions in the observation group was 6.00 % significantly lower than that in the control group, χ²=7.825, p<0.05.
| Group | Patients | Hp eradication rate | Incidence of adverse reactions |
|---|---|---|---|
| Observation group | 50 | 47 (94.00) | 3 (6.00) |
| Control group | 40 | 29 (72.50) | 11 (27.50) |
| χ² | - | 7.820 | 7.820 |
| p | - | 0.005 | 0.005 |
Table 1: Comparison of Hp eradication rate and incidence of adverse reactions between the two groups [n (%)].
Comparison of inflammatory factors and GS levels in the 2 groups was shown in Table 2. TNF-α (23.39±6.20 µg/ml) and IL-6 (15.39±6.41 ng/ml) were significantly lower in the observation group than in the control group and GS (1.78±0.19) nmol/l was significantly higher in the observation group than in the control group, p=0.000.
| Group | Patients | TNF-α (µg/ml) | IL-6 (ng/ml) | GS (nmol/l) |
|---|---|---|---|---|
| Observation group | 50 | 23.39±6.20 | 15.39±6.41 | 1.78±0.19 |
| Control group | 40 | 42.69±7.71 | 33.59 ±10.59 | 1.12±0.41 |
| t | - | 13.086 | 9.863 | 9.798 |
| p | - | 0.000 | 0.000 | 0.000 |
Table 2: Comparison of inflammatory factors and GS levels between the two groups (x̄±s).
Chronic aesthetic gastritis is characterized by a high recurrence rate and long disease duration[13], and its pathological features are limited dystrophy of the intrinsic mucosal glands and a thickened mucosal layer[14], a common chronic disease of the digestive system. However, long-term application may cause antibiotic resistance in patients and it may also cause dysbiosis of intestinal flora and intestinal dysfunction in patients[15], which is not suitable for improving patient’s compliance with medication and promoting recovery from the disease. It has been shown that Lactobacillus acidophilus tablets can decompose sugars in the intestine, accelerate the production of lactic acid[16] and promote the return of intestinal acidity to normal levels, inhibit the growth of intestinal pathogenic bacteria, the control of intestinal inflammation and maintain intestinal flora in a balanced state, improving intestinal flora dysbiosis and intestinal dysfunction.
The present study showed that after using compound Lactobacillus acidophilus tablets combined with quadruple therapy for chronic atrophic gastritis, the remarkable therapeutic effect, total effective rate, Hp elimination rate and gastric lesion area were significantly high in the observation group than in the control group. It has been demonstrated that probiotics can regulate the microecological imbalance of human body and repair the damages of chemical, biochemical and immune barriers in the intestinal tract caused by Hp infection[17], helping to restore the defense function of the digestive system to normal levels and facilitating the eradication of Hp. At the very same time, compound Lactobacillus acidophilus tablets may accelerate the production of lactic acid in the intestinal tract after the decomposition of sugar substances in the intestinal tract and increase the acidity of the intestinal tract, which on the one hand can discourage the colonization and adhesion of Hp in the gastro-intestinal mucosa, discourage the growth and reproduction of Hp, protect the mucosal barrier of the intestinal tract, to improve the gastrointestinal motility and secretion function, and on its other hand, inhibit the growth and reproduction of other pathogenic bacteria in the intestinal tract to a certain extent. So compound Lactobacillus acidophilus tablets combined with quadruple therapy treatment can effectively eradicate Hp[18], control intestinal inflammation, reduce the clinical symptoms and signs of patients, and achieve good therapeutic effects.
After treatment, the occurrences of adverse reactions and digestive system symptoms, such as epigastric pain and epigastric fullness, as well as the overall scores were significantly lower in the observation group and the ratings of gastrointestinal function were significantly higher than those in the control group. This is due to the fact that compound Lactobacillus acidophilus tablets contain Lactobacillus acidophilus, which can attach to the intestinal mucosa and on the one hand can play a protective role in protecting the intestinal mucosa by secreting a variety of bioactive substances with immunomodulatory, antioxidant and antibacterial effects, as extracellular polysaccharides, acidic polysaccharides, surface proteins and lactic acid[19], and enhance the immunity of the intestine. On the other hand, it can promote the secretion of immunoglobulins by intestinal mucosal cells, which in turn regulates the intestinal flora and promotes the recovery of intestinal function. At the same time, compound Lactobacillus acidophilus tablets contain Bacillus subtilis and Streptococcus faecalis, both of which are aerobic bacteria, so they can reduce the amount of oxygen in the intestine[20], provide favorable environmental conditions for the growth and reproduction of anaerobic bacteria in the intestine, increase the content of the normal intestinal flora, improve the dysfunctional condition of the gastrointestinal tract and effectively solve the problems caused by the combined application of antibiotics in quadruple therapy. At the meantime, the compound Lactobacillus acidophilus tablets decompose sugar substances in the intestinal tract, promote lactic acid production and improve intestinal acidity, which is also conducive to strengthening the eradication treatment of Hp by quadruple therapy, improving the gastrointestinal function of patients and alleviating the symptoms of digestive tract.
After treatment, TNF-α and IL-6 in the observation group were markedly lower than those in the control group and GS was dramatically higher than that in the control group. It has been confirmed that Lactobacillus acidophilus in compound Lactobacillus acidophilus tablets can colonize the intestine, which can activate the activity of macrophages in the body[21], promote the destruction of pathogenic microorganisms in the gastrointestinal tract, induce non-specific and specific immunity in the body, not only improve the anti-infection and the immune function of the body, but also inhibit the generation and proliferation of cancer cells. Compound Lactobacillus acidophilus films also contain bifidobacteria, which can down-regulate the expression of Fas/Fas Ligand (FasL) in the body[16], reduce the level of free radicals, inhibit oxidative stress, increase the activity of antioxidant enzymes, reduce the release of inflammatory mediators such as IL-6 and TNF-α in the body, improve serum GS levels, reduce the inflammatory response and regulate intestinal immunity[22].
In conclusion, the treatment of Hp-infected chronic atrophic gastritis based on quadruple therapy with compound Lactobacillus acidophilus tablets can eradicate Hp very well, control intestinal inflammation, improve intestinal flora and reduce clinical signs and symptoms of patients.
Conflict of interests:
The authors declared no conflict of interest.
References
- Zhang ZS, Xu D, Yao T, Zhang M, Tang YL. A comparative study of modified dual therapy and bismuth-containing quadruple therapy in the treatment of Hp-positive chronic atrophic gastritis. J Hunan Norm Univ 2021;18(5):267-70.
- Yang HY, Zhou LP. Efficacy of compound Lactobacillus acidophilus tablets combined with "bismuth-containing quadruple" on chronic non-atrophic gastritis and the effect of intestinal flora. Chin Prim Care Med 2022;29(11):5.
- Zheng SY, Zhu L, Wu LY, Liu HR, Ma XP, Li Q, et al. Helicobacter pylori-positive chronic atrophic gastritis and cellular senescence. Helicobacter 2023;28(1):e12944.
[Crossref] [Google scholar] [PubMed]
- Tang XD. Some understanding on the diagnosis and treatment of chronic atrophic gastritis. Compilation of papers from the 18th National symposium on digestive diseases of the Chinese society of integrative medicine and the 2006 National workshop on advances in digestive diseases of integrative medicine; 2006.
- Zhao MX, Zhang XL, Zhu YX, Li R, Kong XY, Zhang LQ, et al. Application of compounded Lactobacillus acidophilus tablets combined with quadruple therapy in the remedial treatment of Helicobacter pylori eradication failure under different intervention timing. Chin J Microecol 2021;33(11):1317-20.
- Sun J. Application effect of omeprazole enteric-coated tablets combined with compound eosinophil-Lactobacillus tablets in the treatment of acute gastroenteritis. Chin J Mod Drug Appl 2021;15(23):100-2.
- Zhao F, Liu L, Sun Y. Effect of Hp infection on gastric mucosal lesions and miR-22 and NLRP3 inflammatory vesicles and intestinal flora in patients with chronic gastritis. Chin J Hosp Infect 2022;32(19):5.
- de Dieu Habimana J, Mukama O, Chen G, Chen M, Amissah OB, Wang L, et al. Harnessing enhanced CRISPR/Cas12a trans-cleavage activity with extended reporters and reductants for early diagnosis of Helicobacter pylori, the causative agent of peptic ulcers and stomach cancer. Biosens Bioelectron 2023;222:114939.
[Crossref] [Google scholar] [PubMed]
- Wang SL, Bai YN, Ni YY, Ji HY, Chen YG, Liu Zhen, et al. Study on the relationship between distribution of traditional Chinese medicine syndromes and gastric mucosal pathological changes in chronic atrophic gastritis. Chin J Basic Med Tradit Chin Med 2021;27(4):603-7.
- Zhou XJ. Clinical efficacy of levofloxacin with omeprazole and tinidazole in the treatment of Helicobacter pylori-positive chronic atrophic gastritis. Jilin Med J 2021;42(10):2462-4.
- Li H. Effect of omeprazole combined with compound Lactobacillus acidophilus on serum inflammatory factor levels in patients with acute gastroenteritis. Mod Med Health Res 2021;5(7):5-6.
- Liu S, Shu L, Yang J, Liu Y, Zhang S, Wang N, et al. Rhein exhibits anti-inflammatory effects in chronic atrophic gastritis via Nrf2 and MAPK signaling. Turk J Gastroenterol 2023;34(5):525-32.
[Crossref] [Google scholar] [PubMed]
- Lu M, Tang L. Effect of pectin bismuth on immune function and gastric function in patients with chronic atrophic gastritis. Mod Med Health Res 2021;5(18):67-9.
- Huang YC, Zhu CY, Liang HF, Lin YX, Jiang KL, Pan JL, et al. Analysis on composition principles and differentiation of prescriptions for chronic non-atrophy gastritis and chronic atrophic gastritis based on methods of data mining. Chin J Basic Med Tradit Chin Med 2021;27(3):457-61.
- Fang F, Li XL. The changes of erythrocyte immune function in patients with chronic atrophic gastritis before and after Helicobacter pylori eradication. Chin J Clin Gastroenterol 2021,33(2):83-6.
- Zhao XJ, Zhai HL, Li YM, He YN. Efficacy and safety of Bifidobacterium-Lactobacillus triple live bacteria combined with modified sequential therapy in the treatment of HP-positive chronic atrophic gastritis. Hainan Med J 2021;32(4):442-5.
- Yang XQ, Liang B, Ye LF, Wu JZ, Chen ZZ, Tan WH. Exploration of gastric mucosal morphology and IL-34 and PLR levels in patients with Hp infection in chronic atrophic gastritis. J Mol Diagn Ther 2021;13(11):1854-7.
- Wu D, Chen CC, Yu HJ. Effects of colloidal bismuth pectin combined with aluminum phosphate gel on serum GAS ET IL-6 and IL-12 in patients with chronic atrophic gastritis. Hebei Med 2021;27(5):869-73.
- Vendrami CL, Kelahan L, Escobar DJ, Goodhartz L, Hammond N, Nikolaidis P, et al. Imaging findings of eosinophilic gastrointestinal diseases in adults. Curr Probl Diagn Radiol 2023;52(2):139-47.
[Crossref] [Google scholar] [PubMed]
- Xie XC, Zhang Y, Chen SR, Zhang FL, Wang H, Zhu YD, et al. Efficacy and safety of Jinghua Weikang capsule and compound Lactobacillus acidophilus tablets combined with standard quadruple therapy in the eradication of Helicobacter pylori. Chin J Integr Tradit West Med Digest 2021;29(1):45-7.
- Chou RL, Hu W, Liu JB. Relationship between Hp infection in atrophic gastritis and the number of serum PGR, OPN and gastric mucosal G cells. Exp Lab Med 2021;39(3):620-3.
- Luo CQ, Xiao X, Shen GF. Changes and clinical significance of serum GH, PGⅠ/PGⅡ, TGF-βRⅡ, IL-6 and TNF-α levels in patients with chronic atrophic gastritis complicated with Hp infection. Hainan Med J 2021;32(18):2334-27.
 .
.