- *Corresponding Author:
- Hai Lian Feng
Department of Operating Room, Maternal and Child Care Center of Qinhuangdao, China
E-mail: lnage666@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “239-246” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of sustained-release growth factor biomaterials on tendon healing in rats. A total of 45 healthy adult Sprague-Dawley rats were randomly divided into 3 groups viz. blank group, vascular endothelial growth factor group and basic fibroblast growth factor group with 15 rats in each group. The rats in vascular endothelial growth factor group and basic fibroblast growth factor group were injected with 0.2 ml vascular endothelial growth factor (100 ng) and 0.2 ml basic fibroblast growth factor (100 ng) respectively. They were injected again on the 7th d, 14th d and 21st d after operation. At 2, 4 and 6 w after operation, 4 rats in each group were selected for experimental observation. The changes of vascular endothelial growth factor and basic fibroblast growth factor levels in the treated experimental animals and the control rats were compared by the Hematoxylin and Eosin staining and adhesion were observed during the observation period. The expression of both vascular endothelial growth factor and basic fibroblast growth factor in the experimental group was significantly higher than that in the control group. The concentration of the control group at each time phase had no significant change compared with that before operation. Both vascular endothelial growth factor and basic fibroblast growth factor play an important role in the healing process of muscle bonds. Vascular endothelial growth factor is better than basic fibroblast growth factor in promoting micro angiogenesis and cell proliferation in the early stage and basic fibroblast growth factor may be better than vascular endothelial growth factor in promoting collagen synthesis.
Keywords
Tendon injury, vascular endothelial growth factor, basic fibroblast growth factor, healing
Tendon injuries, especially hand flexor tendon injuries, are common in clinical practice. Postoperative tendon adhesion has a great impact on function. Clinical researchers have tried to take different methods to improve tendon adhesion, but no breakthrough progress has been made. At present, it is considered that the mechanism of tendon healing includes endogenous and exogenous healing mechanisms. It is generally believed that tendon exogenous healing plays an important role in the repair process of tendon injury, but it is also the main cause of tendon adhesion. In recent years, more and more attention has been paid to the endogenous healing of tendon, which can improve tendon adhesion[1,2]. How to increase endogenous healing and reduce exogenous healing which can improve tendon adhesion, the regulatory factors of tendon endogenous healing has been the focus of scientific research in recent years.
With the development of molecular biotechnology, more and more growth factors have been found to play a very important role in the process of tendon injury healing[3]. Literature have shown that the expression of basic Fibroblast Growth Factor (bFGF), Insulin-like Growth Factor (IGF-1), Platelet-Derived Growth Factor (PDGF), Epidermal Growth Factor (EGF), Vascular Endothelial Growth Factor (VEGF), Transforming Growth Factor Beta (TGF-β) and other growth factors and their receptors are significantly increased during tendon injury healing[4,5]. A variety of growth factors can promote cell proliferation and collagen synthesis, suggesting that growth factors may play a very important role in the regulation of the healing process. This study aimed to investigate the effects of growth factor-releasing biomaterials on tendon healing in rats.
Materials and Methods
Establishment of animal models:
45 healthy adult Sprague-Dawley (SD) rats, with an average weight of 260 g (250-310 g), which are of clean grade, aged 2-3 mo were selected.
Rat model of rotator cuff injury: After weighing, 45 SD rats were anesthetized by intraperitoneal injection of 10 % chloral hydrate (3 ml/kg). In the normal group, a 1 cm incision was made on the posterolateral side of the shoulder joint to expose the sub post tendon. The rotator cuff injury model was established in the rotator cuff injury group. The surgical approach was a 1 cm incision on the posterolateral side of the acromion of rats. 50 % of the full thickness of the tendon was excised and the width was about 5 mm. In the hyaluronic acid treatment group, 0.05 ml sodium hyaluronate was injected into the subacromial bursa. In the hormone treatment group, 0.05 ml betamethasone was injected into the subacromial bursa. The incision was closed with two stitches. There was no limitation of movement after surgery. 3 d after operation, procaine penicillin was injected into the right hind leg muscle (180 000 U/d). Drugs were added to the tendon anastomosis site on the 7th, 14th and 21st d after operation according to the experimental design.
Animal grouping:
45 rats were randomly divided into 3 groups viz. Blank group, VEGF group and bFGF group, with 15 rats in each group. The rats in the blank group did not receive any treatment. The rats in VEGF group and bFGF group were injected with 0.2 ml VEGF (100 ng) and 0.2 ml bFGF (100 ng) respectively. The rats in VEGF group and bFGF group were injected again on the 7th d, 14th d and 21st d after operation. At 2, 4 and 6 w after operation, 4 rats in each group were selected for experimental observation.
Tissue specimen:
At 2, 4 and 6 w after operation, 4 rats in each group were sacrificed by excessive anesthetic, and the skin of Achilles tendon was cut and the connective tissue around Achilles tendon was separated. The degree of tendon adhesion was observed and graded immediately. The silicone tube was cut longitudinally to observe the color and texture of the broken end of Achilles tendon in the silicone tube. About 1 cm of tendon stump was cut as a tissue specimen, which was fixed with formaldehyde solution and sent to the pathology department for histological staining observation.
Index detection:
Based on the corresponding kits and double antibody sandwich Enzyme-Linked Immunosorbent Assay (ELISA) method, bFGF, VEGF and other content were determined. The operating steps of growth factor ELISA kit were as follows; the kit was equilibrated to room temperature (20°-25°) before testing; the required reaction plate (the working solution was prepared 15 min before use) was removed. 100 μl standards and 100 μl serum were added into the wells of corresponding reaction plates. The plates were mixed gently for 30 s, followed by blocking and incubation at 37° for 60 min. The liquid in the plate was poured, followed by washing using washing solution (350 μl of washing solution were added to each well) and water droplets were removed on the thick absorbent paper and repeated 5 times. 100 μl of biotin was added to each well. The mixture was gently mixed for 30 s, the plates were sealed for incubation at 37° for 60 min. Then the liquid in the plate was poured, followed by washing using washing solution and water droplets were removed on the thick absorbent paper and repeated 5 times. 100 μl of Horseradish Peroxidase (HRP) was added to each well. The mixture was gently mixed for 30 s, the plates were sealed for incubation at 37° for 30 min followed by washing which was repeated for 5 times. 100 μl 3,3′,5,5′-Tetramethylbenzidine (TMB) chromogenic solution was added into each well, followed by gentle mixing for 10 s and the plates were incubated at 37° in the dark for (15±10) min. 100 μl ELISA stop solution was added into each well, followed by mixing gently for 30 s; the Optical Density (OD) was read at 450 nm within 30 min. The standard curve was plotted with the OD value as the ordinate and the standard concentration as the abscissa. The concentration could be found on the standard curve according to the OD value of the sample.
Observation:
Four rats in each group after 2, 4 and 6 w were selected respectively to observe the wound healing, adhesion of the silicone tube to the tendon and adhesion of the tendon to the surrounding tissue. The silicone tube was cut and the general situation of tendon end was observed to verify whether it connected closely. Hitchcock adhesion classification method was adopted to carry out classification: (-) no adhesion (+) mild adhesion, there was an adhesion between thin cellulose on the tendon and surroundings. (++) moderate adhesion, a scalpel was used to separate one side to remove the tendon. (+++) severe adhesion, which required a scalpel to free the tendon at 4 w.
Hematoxylin and Eosin (HE) staining:
At 2, 4 and 6 w after the operation, the Achilles tendons were harvested from two ends of the tendon anastomosis with a length of 0.5 cm for making specimen, and fixed in 10 % formaldehyde solution for more than 24 h. After dehydration, transparency and paraffin embedding, the transverse and longitudinal sections along the broken ends of the tendon were prepared and the thickness of the sections was about 4-6 pm.
Statistical analysis:
The content of growth factors in VEGF group and bFGF group was sorted out, and the data of each group were expressed as mean±standard deviation (x±s). The mean comparison between the two groups was performed by two-sample t test and Statistical Package for the Social Sciences (SPSS) 20.0 statistical software was used for data analysis. Test criteria: p<0.05 and p<0.01 were considered statistically significant.
Results and Discussion
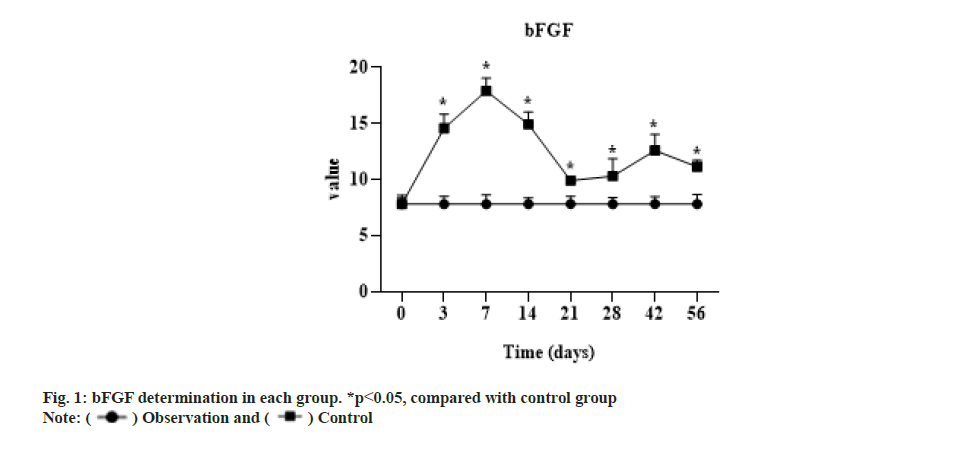
The expression of bFGF in the experimental group was significantly higher than that in the control group after 3 d (p<0.05), the expression level was lowest at 3 w, but still higher than that in the control group (p<0.05) and then rebounded. The concentration of the control group at each time phase had no significant change compared with that before operation and the t test showed no significant difference as shown in fig. 1.
The expression of VEGF in the experimental group was significantly higher than that in the control group from 3 d to 3 w (p<0.05), but there was no significant difference between the two groups after 4 w (p>0.05). The concentration of the control group at each time phase had no significant change compared with that before operation and the t test showed no significant difference as shown in fig. 2.
Normal tendon tissue was mainly composed of thick and tightly arranged collagen fibers, which were parallel to the longitudinal axis of the tendon and stained with dark red. There were a very small number of fibroblasts (tenocytes) in the middle of the collagen fibers, which were arranged longitudinally in the same direction as the collagen fibers. The tendon nuclei were stained with blue, which are small oval and long strips.
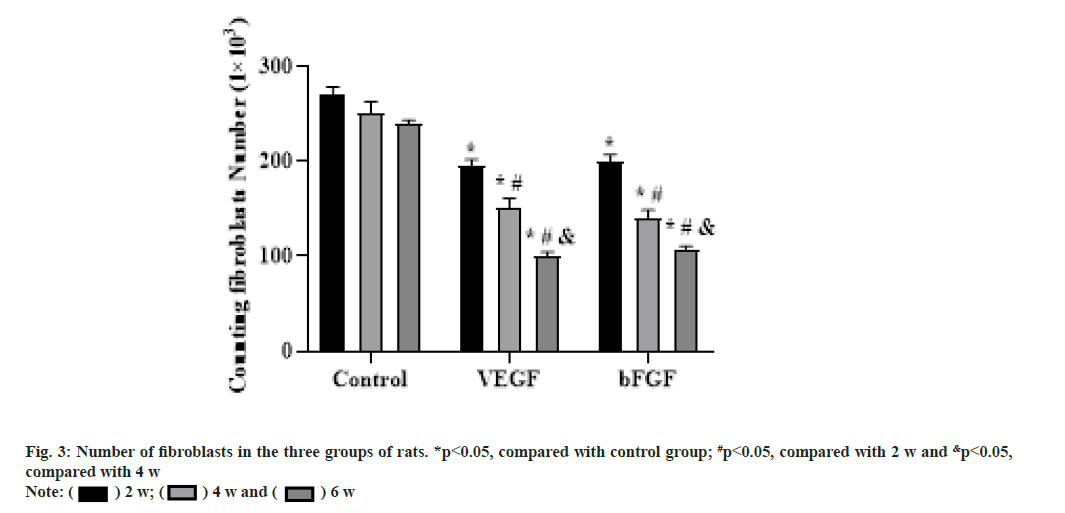
At 2 w after operation, the cells in the repair site of the tendon injury in the VEGF and bFGF groups were significantly increased, the cells were arranged disorderly, many tubular vascular tissues could be seen under low power microscope. There were some red blood cells in the middle and there were light red staining substances around the cells, which were collagen or fibers. The staining was very light. Compared with the blank group, the total number of cells and vascular tissue in the VEGF and bFGF groups was less, but there was no significant difference in cell size and shape. With the increase of time, the total number of cells was decreased significantly, the number of inflammatory cells was few and the number of fibroblasts was more. At 4 w after operation, the total number of cells in the VEGF and bFGF groups was significantly reduced compared with that at 2 w and the inflammatory cells were very few. At 6 w after operation, the number of fibroblasts in the VEGF and bFGF groups was significantly reduced compared with that at 4 w, as shown in fig. 3.
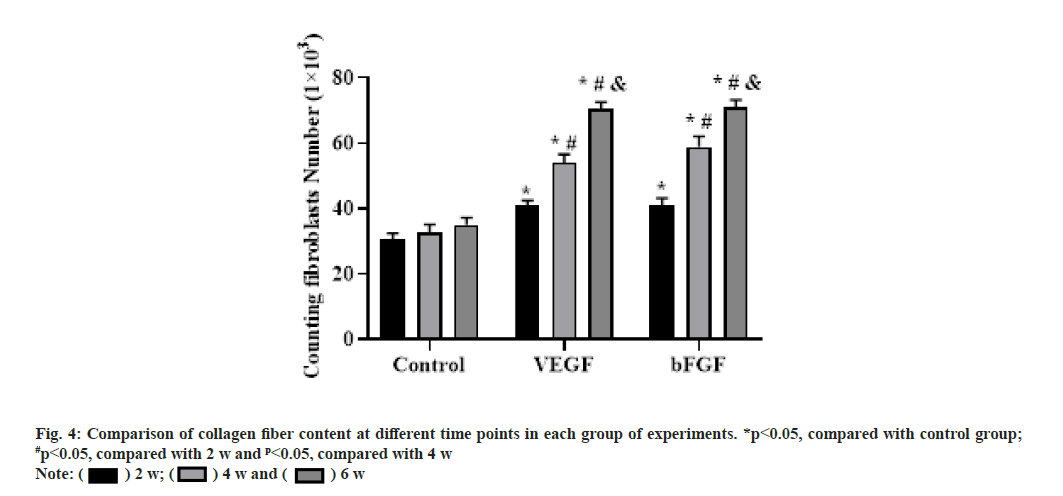
At 2 w after operation, collagen fibers were formed in all three groups and the staining was light. The collagen fibers in the repair area were arranged disorderly and poorly oriented, especially in the blank group most of the collagen fibers were arranged in transverse and longitudinal directions and the secretion of collagen fibers was less. Compared with the blank group, the content of collagen fibers in the VEGF and bFGF groups was increased and the arrangement seemed to be directional, but the collagen fibers were smaller and the whole collagen fibers were curved and relaxed.
At 4 w after operation, the collagen fibers in the three groups increased significantly, and the collagen fibers became thicker under the microscope, especially in the bFGF group. The collagen fibers in the blank group were arranged slightly disorderly and those in the VEGF group and the bFGF group were arranged orderly.
At 6 w after operation, the collagen fibers were arranged more orderly and the content was increased, forming thicker collagen fibers with a certain sense of tension. The percentage of collagen content at each time point after surgery was shown in fig. 4.
2 w after operation, the adhesion of the VEGF and bFGF group was majorly light and moderate, but the adhesion of the blank group was heavier. Adhesion of the three groups was basically separated by a scalpel dissociating one side. 4 w after operation, tendon adhesion of the two groups of severe adhesion and the wide surrounding tissues were needed to carefully separate by blade. The overall adhesion conditions of 6 w after surgery was improved compared to 4 w after surgery, which was slightly reduced and the separation was relatively easy. However, according to the adhesion grading criteria, the adhesion was still majorly serious, as shown in Table 1.
| Blank group | VEGF group | bFGF group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ |
| 0 | 0 | 3 | 1 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 |
| 0 | 0 | 2 | 2 | 0 | 1 | 2 | 1 | 0 | 0 | 3 | 1 |
| 0 | 0 | 3 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 3 | 1 |
Table 1: Adhesion in each group
Note: (-): no adhesion; (+): mild adhesion; (++): moderate adhesion; (+++): severe adhesion
Tendon adhesion is inevitable after clinical tendon injury, resulting in partial loss of tendon function. Clinicians try to improve the adhesion of tendon after healing from the surgical techniques of tendon suture, the choice of incision, the anatomy and physiological structure of tendon and the pathophysiology of the healing process. Although the function of tendon can be restored to a certain extent, the adhesion of tendon is still serious in general. In order to reduce tendon adhesion, endogenous healing should be promoted and exogenous healing should be prevented or inhibited. In recent years, many scholars have adopted different biomedical materials to form a barrier between the broken end of the tendon and the surrounding tissues to prevent the exogenous healing of the tendon. From the initial non-biological materials and bio-non-degradable materials to bio absorbable materials, they have studied the internal proliferation of the tendon and have made certain achievements[6-8]. Experiments have confirmed that growth factors play a very important role in the process of tendon healing, which can promote the proliferation of fibroblasts and secretion of collagen fibers. After tendon injury, a variety of growth factors are expressed in different parts of the tendon and regulate tendon healing at the molecular level[9,10].
VEGF can specifically act on vascular endothelial cells and plays the role of promoting angiogenesis, increasing vascular permeability and other effects. VEGF is recognized as the strongest and the highest specificity promote angiogenesis factor, can induce endothelial cells to secrete a variety of cathepsin, degrade extracellular matrix, mediate endothelial cell migration and invasion, induce angiogenesis, promote trans membrane transport of molecules inside and outside the cell and increase vascular permeability. In tumor tissue studies, VEGF and its receptor are abundantly expressed in tumor tissues[11], which confirm that VEGF promotes the massive generation of tumor neovascularization. In the protective effect on ischemic and hypoxic brain tissue, VEGF can have direct neurotropic and neuroprotective effects on various types of nerve cells, enhance cell viability and survival, and promote axonal regeneration. In cell culture, VEGF can promote the mitosis of rat Muller cells under ischemia and hypoxia. Studies have confirmed that VEGF plays an important role in embryonic development, cell proliferation and differentiation[12].
bFGF is a cytokine that induces the proliferation and differentiation of ectodermal and mesodermal derived cells, which promotes the growth of tendon cells, accelerates healing of tendon injuries and plays a key role in angiogenesis[13]. In vivo studies, the levels of endogenous growth factors and growth factor receptors were found to be significantly increased at 2 w after tendon and ligament injury by immunohistochemical methods[14]. bFGF began to increase at 3 d after injury, reached the highest level at 7 d and returned to the basic level of normal tendon stage after 2 w. A study used various technical methods to observe the changes of type I collagen messenger Ribonucleic Acid (mRNA) transcription factor, Nuclear factor kappa B (NF-kβ), mRNA and Deoxyribonucleic Acid (DNA) synthesis in tenocytes under the action of different concentrations of basic bFGF [15] and the results indicated that bFGF could increase the expression of collagen type I and NF-kβ gene and promote DNA synthesis in tenocytes. In a study of rabbit tenocytes cultured with different concentrations of bFGF, it was found to promote the proliferation of tenocytes in a dose-dependent manner. bFGF can promote the proliferation in a dose dependent way in vitro tendon cell culture, reflecting its application prospect in tendon injuries healing. Exogenous bFGF was applied to treat into the flexor tendon of the chicken toe sheath, and observed that the number of early fibroblasts and the content of collagen fibers in the experimental group were significantly improved compared with those in the control group. bFGF played an important role in promoting tendon healing, but it also aggravated the adhesion of the tendon to the surrounding tissues. Wang et al.[1] applied the defect of rabbit flexor tendon auto graft to treat into bFGF sustained-release membrane, and found there was no significant difference in the proliferation of tenocytes and the expression of collagen fibers between the transplanted tendon with bFGF and the control group. It was proved that bFGF could promote the proliferation and differentiation of tenocytes and accelerate the production of collagen fibers by tenocytes. After studying bFGF, Nakama et al.[15] found that bFGF was involved in the early repair process of tendon healing, which could promote tendon cell proliferation and increase the expression of type III collagen. Other studies have also suggested that bFGF can promote tendon cell proliferation and collagen synthesis[15].
In this study, non-biological non-descending medical silicone tube was used to separate the tendon stump and the surrounding connective tissue to prevent it from growing into the tendon stump and block the influence of exogenous mechanisms on tendon healing. Growth factors were added to the tendon stump to observe the endogenous healing of the tendon. After incision of the silicone tube, we did not find connective tissue growing into the stump of the tendon and there was no adhesion between the silicone tube and the tendon. The surface of the tendon wrapped by the silicone tube was smooth at 4 w and 6 w after operation, and the tendon stump healed well. Of course, silicone tube, as a non-biological inert material, also increased the inflammatory response of the local tissue around the tendon stump. There were more inflammatory cells in the early stage of tendon healing.
Different degrees of tendon adhesion occurred between the unwrapped tendon and the surrounding connective tissue, which may be related to the inflammatory reaction of the silicone tube. In the three groups of experiments, tendon adhesion occurred between the unwrapped tendon and the surrounding connective tissue and the silicone tube did not form adhesion around the tendon stump. Although the application of silicone tube may aggravate tendon adhesion, the overall experimental design basically ensured that the nutrition of the tendon stump was not affected by the surrounding connective tissues. Our experimental confirmation that the surrounding scar tissue did not grow into the broken end of the tendon helped us to investigate the role of growth factors in tendon endogenous healing. In addition, in this experiment, it was observed that the tendon tissue adhesion of the three groups was severe, the VEGF group had obvious bleeding when separating the surrounding tissue, and the amount of bleeding was more than that of the bFGF group. The connective tissue around the VEGF group was lesser, while the granulation tissue in bFGF group was denser, which may be related to the pro-angiogenic effect of VEGF and more vascular tissue formation. In the three experimental groups, the tissue adhesion was the heaviest at the 4th w. The rank sum test was used to compare the two groups and there was no significant difference between the two groups at each time point. In the experiment, the surrounding connective tissue of VEGF group was relaxed, which may be due to the fact that VEGF directly promotes the formation of peripheral vascular tissue in the experiment, while bFGF had a relatively weaker effect on angiogenesis than VEGF and does not directly promote the formation of vascular tissue in the early stage, which may be related to the upregulation of VEGF expression in the early stage and indirectly promoting the formation of vascular tissue.
The results of this study showed that the proliferation of fibroblasts around the broken end after tendon injury was obvious, and the cells were in the functional active phase. Cells in longitudinal section were ellipse, hypertrophy, moderate cytoplasmic staining, and more inflammatory cells in the surrounding. At the same time, collagen protein and fiber with reddish dye was observed in cells, the arrangement was very irregular, transverse and longitudinal staggered, and non-directional. However the mature tendon cells were prismatic, with long and deeply stained nucleus, along the long axis line of the collagen fibers. The cytoplasm was thin and wing-like covering the fiber bundle and the pterygoid process was extended into the fiber bundle and separately wrapped the collagen fibers, the collagen fibers were stained dark red. At 2, 4 and 6 w after operation, the number of fibroblasts and the content of collagen fibers in the experimental group treated with VEGF were significantly higher than those in the blank control group (p<0.05). Histological examination showed that there was a large number of micro angiogenesis around the Achilles tendon bundle. It is suggested that VEGF can promote the angiogenesis of Achilles tendon during the healing process of Achilles tendon injury, accelerate the transport of nutrients between Achilles tendon and blood vessels and provide nutrients for cell proliferation. In the direction of collagen arrangement and the quality of collagen production, the VEGF group was significantly better than the blank group. The effect of VEGF on tendon healing may be as follows; it promotes the formation of micro vessels in Achilles tendon, provides a large amount of nutrients for Achilles tendon repair, thereby promoting the proliferation of cells and promoting the secretion of collagen fibers, thereby accelerating tendon healing; it directly promotes the mitosis of fibroblasts and DNA synthesis of tendon cells, thus accelerating tendon healing.
The results of this experiment showed that at 2 w after operation, the number of fibroblasts and the content of collagen fibers in the bFGF group were significantly higher than those in the blank group. At 4 w after operation, the number of fibroblasts in the blank group did not decrease, but the number of fibroblasts in the bFGF group began to decrease, mainly reflected in the quality of fibroblasts. Fibroblasts began to mature and mainly secrete extracellular matrix. The main way of tendon healing is the generation of collagen fibers and the arrangement of collagen fibers along the longitudinal axis of the tendon to achieve the purpose of tendon healing. The collagen content and collagen arrangement were significantly improved in the bFGF group and had a clear tendency of healing. The number of fibroblasts was increased in the early stage and began to decrease around 4 w after operation, but the fibroblasts were mainly close to mature fibroblasts. The morphology of fibroblasts became slightly smaller than that of 2 w after operation, the nucleus staining was uniform, and the cells arranged in a certain direction. Compared with the blank group, the fibroblasts in the bFGF group were still in a disorderly state, with uneven staining and disordered arrangement. At 6 w after operation, compared with the blank group, the bFGF group had an obvious tendency of tendon healing and the collagen fibers were arranged and the maturity of cells was significantly closer to the normal tendon tissue. The effects on tendon healing may be mainly as follows; it directly promotes the proliferation of fibroblasts; it promotes cell collagen secretion and tissue metabolism; it indirectly promotes angiogenesis.
In this study, compared with the blank group, local application of VEGF and bFGF in the broken end of the tendon could accelerate the healing of the tendon. At 2 w after operation, the number of fibrous cells in both groups was increased significantly and the cells were in the functional active phase. The longitudinal section of the cells was round and hypertrophic, with moderate cytoplasm staining. There were many inflammatory cells around and light red collagen and fibers could be observed. However, the mature tenocytes were spindleshaped, with long and dark nuclei arranged in rows along the long axis of the collagen fibers. The cytoplasm was very thin and wing-like and the pterygoid process extended into the fiber bundle to separately wrap the collagen fibers, and the collagen fibers were stained dark red. The number of fibroblasts in VEGF group was significantly higher than that in bFGF group at 2 w after operation (p<0.05). The number of fibroblasts in VEGF group was slightly higher than that in bFGF group at 4 and 6 w after operation, but the statistical analysis was not significant (p>0.05), which may be related to the sample size and error. The collagen content in bFGF group was higher than that in VEGF group and the difference was statistically significant at 4 w after operation (p<0.05). At 6 w after operation, the quality, distribution and arrangement of collagen fibers in bFGF group were more regular than those in VEGF group. Compared with the blank group, the number of fibroblasts and the content of collagen fibers in the two experimental groups were significantly increased and the difference was statistically significant. The results indicated that both VEGF and bFGF could promote the healing of tendon, but VEGF mainly promoted the proliferation of fibroblasts, while bFGF was superior in the secretion of collagen fibers. This may be related to the different roles of the two growth factors. VEGF mainly promoted the proliferation of tendon cells, but had no effect on the secretion of collagen fibers. bFGF may directly promote the proliferation of tendon cells and promote the secretion of collagen fibers. The arrangement of collagen fibers in the bFGF group was more regular, which has been confirmed by many in vitro cell culture experiments. bFGF can promote collagen secretion and improve the arrangement of collagen fibers[16], which is consistent with our experimental results.
In conclusion, the present results suggest that both VEGF and bFGF play important roles in tendon healing. VEGF is superior to bFGF in promoting micro angiogenesis and cell proliferation in tendon healing at early stage, while bFGF may be superior to VEGF in promoting collagen synthesis.
Funding:
This work was supported by the Qinhuangdao Science and Technology Research and Development Plan Task Statement (No. 202004A058).
Acknowledgement:
Wei-liang Liu and Chun-long Wang are contributed equally to this work. Hai-lian Feng and Jiao Wang are considered co-corresponding authors.
Conflict of interests
The authors declared no conflict of interests.
References
- Wang L, Gao W, Xiong K, Hu K, Liu X, He H. VEGF and BFGF expression and histological characteristics of the bone-tendon junction during acute injury healing. J Sports Sci Med 2014;13(1):15-21.
[Google Scholar] [PubMed]
- Aspenberg P. Stimulation of tendon repair: Mechanical loading, GDFs and platelets. A mini-review. Int Orthop 2007;31(6):783-9.
[Crossref] [Google Scholar] [PubMed]
- Fordham S, Garbutt G, Lopes P. Epidemiology of injuries in adventure racing athletes. Br J Sports Med 2004;38(3):300-3.
- Chan BP, Fu SC, Qin L, Lee KM, Rolf CG, Chan KM. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: A rat patellar tendon model. Acta Orthop Scand 2000;71(5):513-8.
[Crossref] [Google Scholar] [PubMed]
- Fu SC, Shum WT, Hung LK, Wong MW, Qin L, Chan KM. Low-intensity pulsed ultrasound on tendon healing: A study of the effect of treatment duration and treatment initiation. Am J Sports Med 2008;36(9):1742-9.
[Crossref] [Google Scholar] [PubMed]
- Hamilton B, Purdam C. Patellar tendinosis as an adaptive process: A new hypothesis. Br J Sports Med 2004;38(6):758-61.
- Hsu RW, Hsu WH, Tai CL, Lee KF. Effect of hyperbaric oxygen therapy on patellar tendinopathy in a rabbit model. J Trauma 2004;57(5):1060-4.
[Crossref] [Google Scholar] [PubMed]
- Ergen E. Sport’s injuries in children and adolescents: Etiology, epidemiology, and risk factors. Acta Orthop Traumatol Turc 2004;38:27-31.
[Google Scholar] [PubMed]
- Lu H, Qin L, Fok P, Cheung W, Lee K, Guo X, et al. Low-intensity pulsed ultrasound accelerates bone-tendon junction healing: A partial patellectomy model in rabbits. Am J Sports Med 2006;34(8):1287-96.
[Crossref] [Google Scholar] [PubMed]
- Krivic A, Majerovic M, Jelic I, Seiwerth S, Sikiric P. Modulation of early functional recovery of Achilles tendon to bone unit after transection by BPC 157 and methylprednisolone. Inflamm Res 2008;57(5):205-10.
[Crossref] [Google Scholar] [PubMed]
- Liu SH, Panossian V, Al-Shaikh R, Tomin E, Shepherd E, Finerman GA, Lane JM. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res 1997;339:253-60.
[Crossref] [Google Scholar] [PubMed]
- Lu H, Qin L, Cheung W, Lee K, Wong W, Leung K. Low-intensity pulsed ultrasound accelerated bone-tendon junction healing through regulation of vascular endothelial growth factor expression and cartilage formation. Ultrasound Med Biol 2008;34(8):1248-60.
[Crossref] [Google Scholar] [PubMed]
- Pecina M, Bojanic I, Ivkovic A, Brcic L, Smoljanovic T, Seiwerth S. Patellar tendinopathy: Histopathological examination and follow-up of surgical treatment. Acta Chir Orthop Traumatol Cech 2010;77(4):277-83.
[Google Scholar] [PubMed]
- Qin L, Lu H, Fok P, Cheung W, Zheng Y, Lee K, et al. Low-intensity pulsed ultrasound accelerates osteogenesis at bone-tendon healing junction. Ultrasound Med Biol 2006;32(12):1905-11.
[Crossref] [Google Scholar] [PubMed]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res 2005;23(5):1199-205.
[Crossref] [Google Scholar] [PubMed]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR?1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res 2006;24(3):393-400.
[Crossref] [Google Scholar] [PubMed]
 ) Observation and (
) Observation and ( ) Control
) Control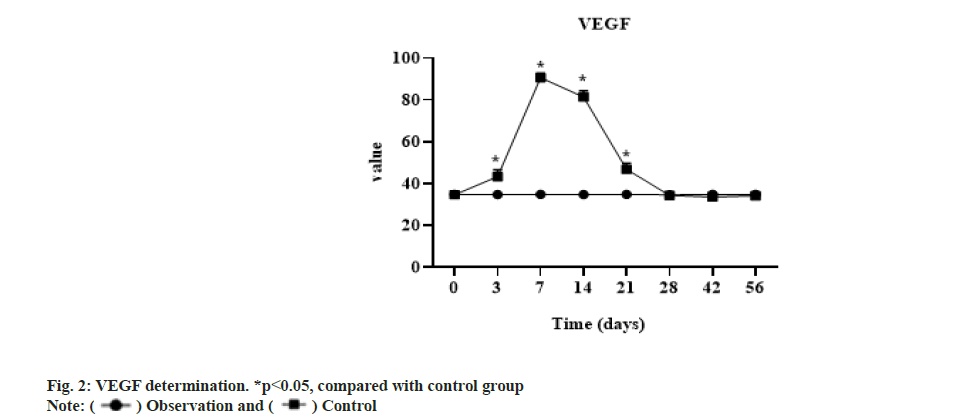
 ) 2 w; (
) 2 w; ( ) 4 w and (
) 4 w and ( ) 6 w
) 6 w