- *Corresponding Author:
- Y. Yongjian
Department of Anesthesiology, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong, China
E-mail: paogaiyunqin@163.com
| This article was originally published in a special issue, "XXXXXX" |
| Indian J Pharm Sci 2020:82(1)spl issue1;XX-XX |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the effects of muscarinic cholinergic receptor agonist carbachol on urinary bladder function and plasma inflammatory mediators in anesthetized rats. A total of 20 rats were randomly divided into carbachol group and solvent control group, 10 rats in each group. The detection of bladder wall thickness and histological observation were conducted by light microscopy, the changes of urinary bladder function and plasma inflammatory mediators in rats were compared between the two groups. The maximum bladder capacity, static bladder pressure, compliance, bladder wet weight and bladder wall thickness in the carbachol group were significantly higher than those in the solvent control group. Light microscopic evaluation revealed that the detrusor cells in the solvent control group appeared to be in order in terms of size, arrangement and shape, while in the carbachol group the detrusor cells exhibited hypertrophy together with disordered arrangement, diverse morphology and vacuolar degeneration of muscle fiber. The levels of TNF-α and IL-10 in plasma of the carbachol group were significantly lower than those of the solvent control group. In anesthetized rats, the stimulation of muscarinic cholinergic receptors can significantly reduce urinary frequency, increase the volume of bladder storage and decrease systemic inflammatory response.
Keywords
Bladder, urinary function, carbachol, anesthetized rats
Urinary dysfunction is a common disease characterized by urinary frequency and urgency, dysuria as well as various kind of urinary incontinence[1]. There are some clear reasons, for example, multiple sclerosis causing detrusor hyperreflexia and then gives rise to frequent urination, urinary urgency and urge incontinence, or benign prostatic hyperplasia causing bladder output obstruction[2]. However, many of the urinary disorders are idiopathic with unknown cause and conventional treatment does not control the symptoms very well[3].
Carbachol is a muscarinic cholinergic receptor (MCR) agonist with the effects of promoting gastrointestinal motility, dilating blood vessels and increasing gland secretion. In clinical trials it was previously used in the treatment of flatulence and urinary retention after surgery[4]. Recent studies have found that carbachol has obvious antiinflammatory effects[5,6]. However, there are few reports about the effect of carbachol on bladder function. In view of this, the present study was conducted to observe the effect of carbachol injected through tail vein on urinary bladder function and plasma inflammatory mediators in rats and analyze the changes seen in the process so as to provide experimental evidence for the clinical application of carbachol.
Carbachol was purchased from ABCR GmbH and Co.KG. All the drugs were dissolved in distilled water. A total of 20 male Wistar rats, weighing 35-42 g, were provided by the Hebei Experimental Animal Center. The rats were randomly divided as carbachol group and solvent control group containing 10 rats in each group. The dose of carbachol administered to the carbachol group rats was 5.5 g/kg and before the first administration, 3 constant urination cycles (6-12 min) in each rat were observed to record the maximum bladder capacity, static pressure, bladder compliance, bladder wet weight and bladder wall thickness; after each injection similar observations were made on another 3 constant urination cycles with a dosing interval of 30 min. The rats in the solvent control group were given 0.5 ml/kg saline 5 times each at an interval of 30 min.
The rats were fixed on an anatomical table and urodynamic measurements were made on each rats in both groups (figures 1-3). Rats were anesthetized by intraperitoneal injection of 25 % urethane solution (1.0 g/kg), the skin was incised in upper margin of pubic bone to expose the bladder, which was placed at the incision (to avoid the influence of abdominal pressure on pressure of detrusor). Two 24 G catheters were respectively inserted into the bladder from the top wall and after fixation one end was connected to a micro perfusion pump with intravesical instillation of saline (0.4 ml/ min), and the other end was connected to a MedLab bio-signal collection system through a pressure transducer for determination of bladder pressure. The measurement indicators included the bladder threshold capacity, residual urine, bladder voiding pressure and compliance (ml/kpa). Measurements were made 3 times from each rat at an interval of 5 min.
After the measurement of bladder pressure/volume, the rats were immediately sacrificed by cervical dislocation and the bladder was weighed. The histological changes and bladder wall thickness were observed under light microscopic, intact bladder specimen was excised, placed in Krebs buffer, the longitudinal muscle at lateral walls was cut, fixed in 10 % formaldehyde solution, and 2 h after that was cut into 2 mm segments in length to measure the thickness of bladder wall. It was then dehydrated, made to be transparent, dipped in wax, embedded and sectioned to obtain 5 μm sections These sections were baked, dewaxed, stained with hematoxylin and eosin, dehydrated and mounted and observed under an optical microscope (200X).
Immediately after sacrifice, superior mesenteric venous blood was collected in sterile tunes from each rat, plasma was separated and stored at -80°. Radioimmunoassay was used to detect the contents of TNF-α and IL-10 in the plasma of rats. The applied kits are provided by Shanghai Long Kang Biological Technology Co., Ltd., and the operation was conducted strictly according to the kit instructions.
Data were expressed as mean±standard deviation and statistical analysis was performed using SPSS 21 statistical software. Significance of the differences were assessed using t test and the level of significance was set at p<0.05.
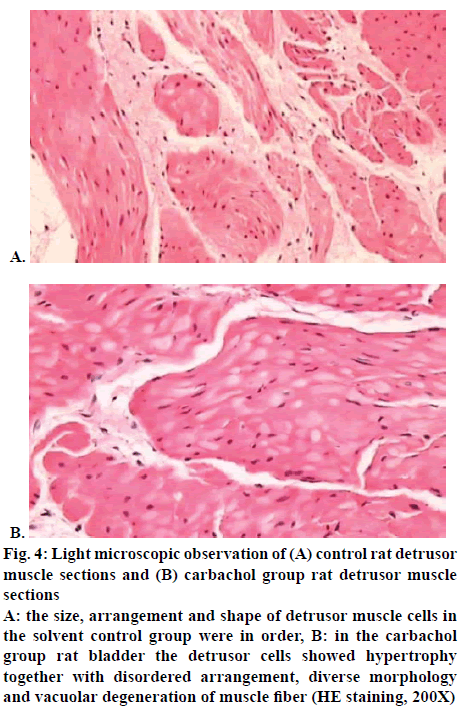
The maximum bladder capacity, static bladder pressure, compliance, bladder wet weight and bladder wall thickness in the carbachol group were significantly higher than those in the solvent control group (p<0.05), as shown in Table 1. Light microscopy revealed that the detrusor cells in the solvent control group appeared to be in order in terms of size, arrangement and shape as shown in figure 4A; while in the carbachol group eosinophilic infiltration was obviously seen in submucosa with slight mucoid degeneration in muscular layer and the detrusor cells exhibited hypertrophy with disordered arrangement, diverse morphology and vacuolar degeneration of muscle fibre; local collagen denaturation as well as fibre degradation were seen in smooth muscle tissues with obvious sponge-like changes in cytoplasm of local muscle cells and moderately more interstitial fibrous proliferation, as shown in figure 4B.
Table 1: Comparison of Urodynamic and Pathological Findings between the Two Groups
| Group | n | Maximum bladder capacity (ml) | Static bladder pressure (kpa) | Compliance (ml/kpa) | Bladder wet weight (mg) | Bladder wall thickness (mm) |
|---|---|---|---|---|---|---|
| Solvent control group | 10 | 1.75 ± 0.23 | 0.58 ± 0.13 | 0.65 ± 0.07 | 147.8 ± 3.56 | 0.91 ± 0.05 |
| Carbachol group | 10 | 3.61 ± 0.65 | 0.97 ± 0.16 | 1.43 ± 0.09 | 186.4 ± 4.35 | 1.43 ± 1.28 |
| t | 6.093 | 7.225 | 8.036 | 7.561 | 6.047 | |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Figure 4: Light microscopic observation of (A) control rat detrusor muscle sections and (B) carbachol group rat detrusor muscle sections
A: the size, arrangement and shape of detrusor muscle cells in the solvent control group were in order, B: in the carbachol group rat bladder the detrusor cells showed hypertrophy together with disordered arrangement, diverse morphology and vacuolar degeneration of muscle fiber (HE staining, 200X)
The levels of TNF-α and IL-10 in plasma of the carbachol group were significantly lower than those of the solvent control group (p<0.05), as shown in Table 2. For the bladder, when the autonomic nerves are excited, noradrenaline gets released from the nerve terminals and acts on β3 receptor of detrusor muscle causing relaxation[7]. In the bladder wall has cholinergic innervation, and the acetylcholine released stimulates the MCR on detrusor to cause contraction of bladder[8]. The experiments on isolated bladder of various animals including humans showed that the contraction of bladder under physiological conditions mainly involved the M3 choline receptor[9]. When the isolated bladder of rats with M3 cholinergic receptors knockout was studied, it was found that cholinergic agonists mediated the contractile response of bladder mainly through the M3 cholinergic receptor[10-12]. The results of this study showed that the maximum bladder capacity, static bladder pressure, compliance, bladder wet weight and bladder wall thickness in the carbachol group were significantly higher than those in the solvent control group (p<0.05), suggesting that carbachol can slow the micturition frequency and prolong the time of bladder perfusion, increase perfusion volume and increase the volume of bladder before the next time of micturition reflex.
Table 2: Comparison of Levels of Plasma Inflammatory Mediators between the Two Groups
| Group | n | TN F-α (μg/l) |
IL-10 (μg/l) |
|---|---|---|---|
| Solvent control group | 10 | 0.86 ± 0.12 | 0.75 ± 0.06 |
| Carbachol group | 10 | 0.63 ± 0.18 | 0.57 ± 0.09 |
| t | 7.392 | 7.044 | |
| p | <0.05 | <0.05 |
With the development of cell biology and molecular biology, the study of biological function on the whole has attracted more and more attention. Because of the relatively large body weight, rats and guinea pigs are currently common animal models established for analysing drug effects by cystometry in the country[13]. In this study, this approach was successfully applied to rats, observed the influence of carbachol and isoproterenol on bladder function through tail vein injection and found that the basic mechanism for micturition control of rats under anaesthesia is similar with that of human.
Related studies[14] have shown that the binding of acetylcholine to nicotinic receptor can reduce the immune activity of macrophages to lipopolysaccharide and inhibit the synthesis of protein after transcription of TN and the subsequent clinical trial has confirmed that cholinergic agonist nicotine has a therapeutic effect on colitis. There is also a study finding that direct electrical stimulation of efferent pneumogastric nerve in rats with endotoxic shock can inhibit the production of inflammatory mediators in the liver and lung tissues[15], alleviate the pathological damage of lung and slow down the release of oxygen free radicals. Since this antiinflammatory pathway is involved with acetylcholine, it was refer to as the cholinergic antiinflammatory pathway, whose main mechanism is to mobilize the neuroendocrine immune system in body to modulate inflammatory response. However, there remain many problems in cholinergic antiinflammatory pathway during the process of treatment, for example direct stimulation of vagus nerve not only causes damages to the nerve itself, but also gives rise to fluctuation of breathing, heart rate and blood pressure[16]. The application of cholinergic drugs enables to achieve the same antiinflammatory effect with higher safety. Carbachol used in this experiment is a powerful cholinergic drug, which binds to M and N cholinergic receptors and has the full Ach-like function with longer duration. It has a moderately strong effect on exciting the stomach and intestine as well as bladder smooth muscle. The study results showed that the levels of TNF-α and IL-10 in the carbachol group were lower than those in the solvent control group (p<0.05), suggesting that carbachol can effectively reduce the content of TNF-α and IL-10 in plasma and inhibit release of proinflammatory factors of TNF-α and IL-10 with alleviation of inflammation and that its mechanism may be related to the fact that carbachol excites cholinergic N receptor so as to play a Ach-like effect. In summary, in anesthetized rats, the stimulation of MCR can significantly reduce urinary frequency, increase the volume of bladder storage and decrease systemic inflammatory response.
Conflict of interest
No conflict of interest between any of the authors.
References
- Gomes CM, Sammour ZM, Bessa JDJr., Barbosa ER, Lopes RI, Sallem FS, et al. Neurological status predicts response to alpha-blockers in men with voiding dysfunction and Parkinson's disease. Clinics (Sao Paulo) 2014;69:817-22.
- Elterman DS, Barkin J, Kaplan SA. Optimizing the management of benign prostatic hyperplasia. Ther Adv Urol 2012;4:77-83.
- Bushman W, Steers WD, Meythaler JM. Voiding dysfunction in patients with spastic paraplegia: Urodynamic evaluation and response to continuous intrathecal baclofen. Neurourol Urodyn 1993;12:163-70.
- Kaba M, Gromb S, Privat M, Schumann D. The adsorption of choline halides at a mercury-solution interface. J Electroanal Chem Interfacial Electrochem 1981;121:179-202.
- Treede I, Braun A, Sparla R, Kühnel M, Giese T, Turner JR, et al. Antiinflammatory effects of phosphatidylcholine. J Biol Chem 2007;282:27155-64.
- Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a rat model of postoperative pain. Br J Anaesth 2010;105:201-7.
- Chancellor MB, Chartier‐Kastler EJ. Principles of sacral nerve stimulation (sns) for the treatment of bladder and urethral sphincter dysfunctions. Neuromodulation 2000;3:16-26.
- Barbe MF, Ruggieri Sr MR. Innervation of parasympathetic postganglionic neurons and bladder detrusor muscle directly after sacral root transection and repair using nerve transfer. Neurourol Urod 2011;30:599-605.
- Han JH, Chang IH, Myung SC, Lee MY, Kim WY, Lee SY, et al. A Novel Pathway Underlying the Inhibitory Effects of Melatonin on Isolated Rat Urinary Bladder Contraction. Korean J Physiol Pharmacol 2012;16:37-42.
- Yamanishi T, Chapple CR, Yasuda K, Chess‐Williams R. The role of M2-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br J Pharmacol 2000;131:1482-8.
- Coit VA. Studies into the effects of gonadectomy on the canine urinary bladder with reference to acquired urinary incontinence in the bitch. Value Health 2009;2:189-9.
- Stengel PW, Yamada M, Wess J, Cohen ML. M3-receptor knockout rats: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol 2002;282:R1443-9.
- Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, et al. M3, muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in rats. J Physiol 2004;558:561-75.
- White CW, Short JL, Haynes JM, Matsui M, Ventura S. Contractions of the rat prostate elicited by acetylcholine are mediated by M3 muscarinic receptors. J Pharmacol Exp Ther 2011;339:870-7.
- Lai HH, Boone TB, Thompson TC, Smith CP, Somogyi GT. Using caveolin-1 knockout rat to study impaired detrusor contractility and disrupted muscarinic activity in the aging bladder. Urology 2007;69:407-11.
- Füllhase C, Campeau L, Sibaev A, Storr M, Hennenberg M, Gratzke C, et al. Bladder function in a cannabinoid receptor type 1 knockout rat. BJU Int 2014;113:144-51.