- *Corresponding Author:
- M. Y. Wang
Department of Anesthesiology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning 110042, China
E-mail: scilijiao@163.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “208-214” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effects of dexmedetomidine combined with etomidate on cellular immune function and stress response in patients undergoing radical resection of rectal cancer is the main objective of study. 100 patients with rectal cancer treated by radical resection of rectal cancer were randomly divided into observation group and control group, in which the observation group was given dexmedetomidine combined with etomidate anesthesia, while the control group was given only dexmedetomidine anesthesia. Immune function and oxidative stress response of the two groups were analyzed. There were significant differences in postoperative visual analog scale score, peripheral blood cells and T lymphocyte subsets, oxidative stress, gastrointestinal hormone levels and complication rate between two groups and the differences were statistically significant. The total incidence of complications in observation group was 8.00 %, while that in control group was 24.00 %. In addition, the stress response in observation group was lower than that in control group and the immune function of patients was greatly improved. Dexmedetomidine combined with etomidate could effectively improve cellular immune function and stress response in patients undergoing radical resection of rectal cancer. It has significant clinical effect and is worthy of wide promotion.
Keywords
Dexmedetomidine, etomidate, radical resection of rectal cancer, cellular immune function, visual analog scale score
After radical resection of rectal cancer, the pain caused by surgical incision and the common stimulation of various drugs lead to poor mental state and increased complications. These symptoms may lead to abnormal restlessness of patients after awakening and at the same time may also lead to inflammatory reaction and imbalance of internal environment, which has a great impact on the smooth recovery of patients after surgery [1]. Drugs with significant sedative effects, such as hypnotics, opioid receptor drugs, non-steroidal anti-inflammatory drugs and local anesthetics are commonly used in clinical surgery at present. Among them, etomidate is often used to relieve the restlessness of patients undergoing surgery during the recovery period, but some scholars have pointed out that etomidate has poor effects in reducing postoperative pain and infection; dexmedetomidine, as a commonly used alpha-2 (α2)-adrenoceptor agonist, can block the transmission of pain signals in the body by inhibiting the release of norepinephrine and at the same time, can inhibit sympathetic nerve activity, further achieving the effects of controlling blood pressure and resisting infection in patients [2].
General anesthesia is mostly selected for radical resection of rectal cancer, with the aim of cooperating with the surgery. In the process of general anesthesia for patients, etomidate, an anesthetic drug, is widely used in clinical practice. It can effectively relieve the restlessness of patients under general anesthesia during the awakening period, but it does not have good effect in inhibiting the pain of patients after surgery [3-5]. Dexmedetomidine, as an agonist of α2-adrenergic receptor, can not only achieve the purpose of sedation and anti-anxiety, but also effectively inhibit the release of sympathetic excitatory transmitter in central nervous system [6].
At present, there are relatively few reports on the application of dexmedetomidine combined with etomidate in radical resection of rectal cancer. In this study, 100 patients undergoing radical resection of rectal cancer were selected to explore the effects of dexmedetomidine combined with etomidate on cellular immune function and stress response of patients undergoing radical resection of rectal cancer, thus providing theoretical basis for clinical diagnosis and treatment.
Materials and Methods
General data:
From November 2019 to November 2020, 100 patients with rectal cancer were selected, including 47 male patients and 53 female patients, with an average age of (47.15±3.24) y.
Inclusion criteria: Patients have no other diseases except colon cancer, mainly manifested as abdominal discomfort; the clinical data of patients are complete and accurate, and there are no other diseases; all subjects have signed the informed consent form.
Exclusion criteria: Patients with organ injury, mental abnormality and incomplete clinical data; patients with other tumors; patients unable to cooperate with treatment due to various reasons; when dexmedetomidine is infused, the blood pressure or heart rate fluctuates excessively.
Methods:
All patients were randomly divided into observation group and control group. Patients in observation group were given dexmedetomidine combined with etomidate anesthesia, while patients in control group were given dexmedetomidine anesthesia only.
All selected patients were given 0.5 mg atropine injection (G.Y.Z.Z H32021535, Wuxi No.7 Pharmaceutical Co., Ltd.) through intramuscular injection half an hour before the surgery. After the venous access was established, all patients were supplemented with Ringer’s lactate at a dose of 3 ml/kg. Patients in the observation group were infused with etomidate (G.Y.Z.Z H32022379, Jiangsu Hengrui Pharmaceutical Company Ltd.) and dexmedetomidine (G.Y.Z.Z H20090248, Jiangsu Hengrui Pharmaceutical Company Ltd.) at a constant speed by electronic infusion pump. Patients in control group were only infused with 1 μg/kg etomidate by electronic infusion pump. In the process of infusion, if the patient had severe sinus bradycardia, 0.5 mg atropine injection was injected; if the patient’s blood pressure rose and exceeded 30 % of the basic value, the infusion was stopped [7].
Observation indicators and methods:
Comparative analysis of Visual Analog Scale (VAS) scores of patients in two groups at each time point:
VAS score method was used to score the pain degree of patients in the two groups before surgery, 6 h, 12 h, 24 h and 48 h after surgery. Among it, 0 point indicates no pain; 0-3 points indicates mild pain, which will not affect the patient’s sleep and is bearable to patients; 4-6 points indicates moderate pain, with a slight impact on sleep. Although it can be tolerated, treatment is still necessary; 7-10 points indicate severe pain, which will seriously affects the patient’s sleep [8].
Comparative analysis of peripheral blood cell values between two groups of patients: The patient’s fasting venous blood was collected and added with 3.8 % sodium citrate solution for anticoagulation. The ratio of blood to anticoagulant was kept at 4:1. They were mixed thoroughly to prevent blood coagulation. 20 μl of fluorescent labeled monoclonal antibodies Cluster of Differentiation (CD) 3-Phycoerythrin-Cyanine 5 (PE-CY5)/CD4-Fluorescein Isothiocyanate (FITC)/ CD8-PE, CD-19-FITC and CD16CD56-PE were added into the treated blood, so as to ensure the volume of the measured whole blood sample to be 100 μl and the cell concentration was adjusted to be about 10×109/l. The percentage of T cells, T helper (Th) cells, T suppressor (Ts) cells, B cells and Natural Killer (NK) cells in lymphocyte population was analyzed by flow cytometry at 488 nm and each specimen was counted as 1×105 cells.
Comparative analysis of cellular immune function between two groups of patients: Before surgery and 3 d after surgery, 4 ml of fasting venous blood was collected and centrifuged at 3500 r/min for 20 min to obtain serum. The quantity of T lymphocyte subsets CD3+, CD4+ and CD8+ was detected by flow cytometry, and CD4+/CD8+ was calculated at the same time.
Comparative analysis of oxidative stress level between two groups of patients: 10 ml of fasting venous blood of each patient was collected, centrifuged at 3500 r/min at 4° for 10 min. The treated serum was stored in an ultra-low temperature refrigerator at -80°. Oxidation of Lipid Peroxide (OLP), Glutathione S-Transferases (GST) and Catalase (CAT) were all determined according to the requirements of the kit. The standard curve was drawn and the results were calculated according to the measured values of standard reagents diluted by multiple times. Superoxide Dismutase (SOD) was determined by xanthine oxidase method and Malondialdehyde (MAD) was determined by thiobarbituric acid method.
Comparative analysis of gastrointestinal hormone level between two groups: 4 ml fasting venous blood was collected and centrifuged at 3500 r/min for 20 min and the contents of Gastrin (GAS) and Motilin (MTL) in serum were detected by automatic biochemical analyzer.
Comparative analysis of postoperative complications between the two groups: The postoperative complications such as intestinal obstruction, incision infection, pulmonary infection and anastomotic leakage were analyzed statistically.
Statistical methods:
All the data in this study were processed by Statistical Package for the Social Sciences (SPSS) 20.0 statistical analysis software (International Business Machines Corporation, United States of America). The measurement data were expressed by mean×standard deviation (x̄±s) and the comparison between groups was made by single factor analysis of variance or repeated measurement variance analysis. The pairwise comparison between groups was made by Least Significant Difference (LSD)-t test; the counting data were expressed by percentage (%) and the comparison between groups was analyzed by X2; p<0.05 indicated statistically significant difference.
Results and Discussion
There was no difference in VAS scores before surgery between the two groups (p<0.05). With the prolongation of postoperative time, the VAS scores of patients showed a downward trend and the VAS scores of patients in observation group were lower than those in control group in all stages. Comparing the VAS scores of the two groups at each time point after surgery, the observation group was superior to the control group, with statistical significance (p<0.05) as shown in Table 1. There was no significant difference between the two groups in the data of peripheral blood cells before surgery (p>0.05). After surgery, the peripheral blood cell value of observation group patients was larger than that of control group and the observation group was superior to the control group, with significant difference (p<0.05) as shown in Table 2.
| Group | Before surgery | 6 h after surgery | 12 h after surgery | 24 h after surgery | 48 h after surgery | F value | p value |
|---|---|---|---|---|---|---|---|
| Observation group | 6.65±1.12 | 4.76±1.26 | 3.38±1.14 | 2.76±1.03 | 1.09±0.44 | 18.975 | 0.001 |
| Control group | 6.66±1.10 | 5.09±0.25 | 4.99±1.22 | 4.86±1.02 | 2.15±0.98 | 20.742 | 0.001 |
| t value | 0.244 | 3.587 | 7.256 | 9.115 | 3.922 | - | - |
| p value | 0.562 | 0.023 | 0.009 | 0.001 | 0.035 | - | - |
Table 1: Comparative Analysis of Vas Scores of Two Groups at Each Time Point (x̄±s)
| Group | Indicator | Observation group | Control group | F value | p value |
|---|---|---|---|---|---|
| Before surgery | T cells | 43.23±5.42 | 42.76±4.90 | 0.435 | 0.527 |
| B cells | 3.35±0.98 | 3.23±0.54 | 0.338 | 0.921 | |
| Th cells | 14.45±3.21 | 13.78±6.02 | 0.441 | 0.452 | |
| Ts cells | 20.23±4.62 | 19.89±5.23 | 0.582 | 0.993 | |
| NK cells | 5.53±1.24 | 5.66±2.93 | 0.431 | 0.634 | |
| After surgery | T cells | 79.56±2.19 | 73.62±4.20 | 6.564 | 0.001 |
| B cells | 13.45±3.42 | 8.13±0.94 | 7.346 | 0.002 | |
| Th cells | 34.87±2.23 | 24.22±3.49 | 6.673 | 0.001 | |
| Ts cells | 41.23±3.94 | 35.36±5.39 | 6.235 | 0.004 | |
| NK cells | 11.12±4.34 | 8.45±2.93 | 6.873 | 0.001 |
Table 2: Comparative Analysis of Peripheral Blood Cell Values Between Two Groups (x105)(x̄±s)
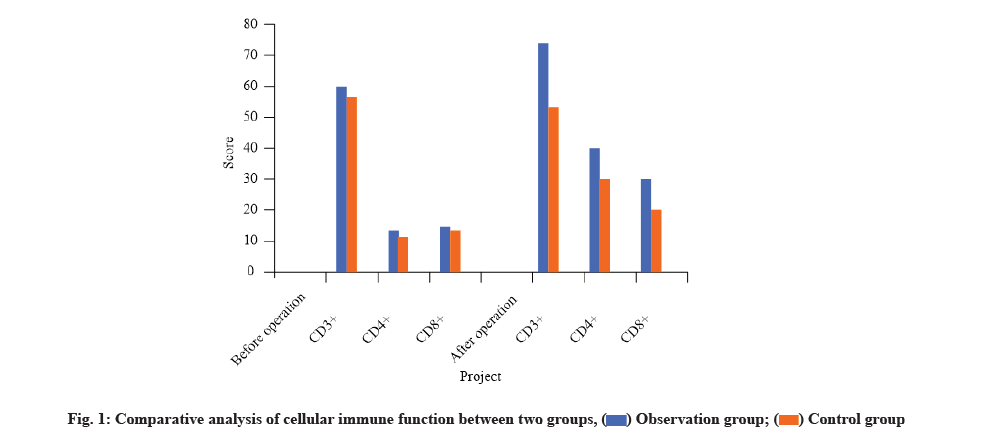
There was no significant difference between the two groups in cellular immune function before surgery (p>0.05). The observation group was superior to the control group in the comparison of T lymphocyte subsets after surgery and the difference was statistically significant (p<0.05) as shown in Table 3 and fig. 1.
| Group | Indicator | Observation group | Control group | F value | p value |
|---|---|---|---|---|---|
| Before surgery | CD3+ | 59.43±7.02 | 58.66±8.33 | 0.532 | 0.912 |
| CD4+ | 13.34±4.39 | 12.27±3.41 | 0.447 | 0.374 | |
| CD8+ | 14.24±3.92 | 13.78±2.56 | 0.512 | 0.463 | |
| CD4+/CD8+ | 0.45±0.14 | 0.47±0.29 | 0.983 | 0.527 | |
| After surgery | CD3+ | 72.445±3.45 | 56.77±1.87 | 7.442 | 0.002 |
| CD4+ | 42.45±4.12 | 26.33±3.65 | 7.512 | 0.001 | |
| CD8+ | 26.25±3.15 | 20.35±2.88 | 6.421 | 0.004 | |
| CD4+/CD8+ | 2.34±0.14 | 1.33±0.53 | 6.976 | 0.005 |
Table 3: Comparative Analysis of Cellular Immune Function Between Two Groups (x̄±s)
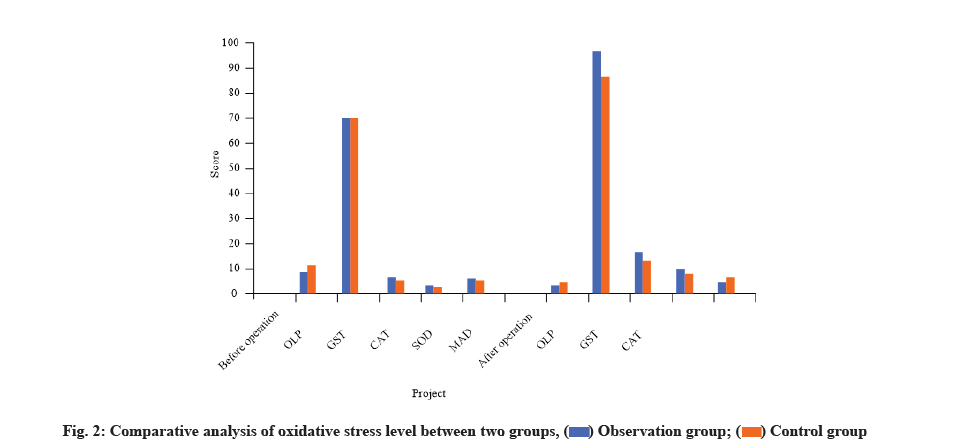
The levels of OLP and MAD in patients of observation group were higher than those in patients of control group, but the levels of GST, CAT and SOD were relatively lower. There was no significant difference between the two groups in oxidative stress level before surgery (p>0.05). However, the observation group was superior to the control group in the comparative analysis of the indexes of oxidative stress level after surgery and the difference was statistically significant (p<0.05) as shown in Table 4 and fig. 2.
| Group | Indicator | Observation group | Control group | F value | p value |
|---|---|---|---|---|---|
| Before surgery | OLP | 9.56±3.44 | 10.24±2.31 | 0.435 | 0.773 |
| GST | 70.87±8.43 | 71.26±9.43 | 0.521 | 0.529 | |
| CAT | 8.66±7.93 | 8.50±1.23 | 0.493 | 0.435 | |
| SOD | 4.57±1.35 | 4.59±1.12 | 0.624 | 0.732 | |
| MAD | 6.68±3.42 | 6.79±2.31 | 0.512 | 0.491 | |
| After surgery | OLP | 4.28±0.44 | 6.87±3.32 | 6.743 | 0.009 |
| GST | 92.33±9.09 | 86.33±9.34 | 5.112 | 0.011 | |
| CAT | 14.36±1.28 | 12.29±2.00 | 2.39 | 0.023 | |
| SOD | 9.15±0.77 | 8.45±3.41 | 4.356 | 0.014 | |
| MAD | 5.06±0.43 | 5.89±5.55 | 1.231 | 0.022 |
Table 4: Comparative Analysis of Oxidative Stress Level Between Two Groups (x̄±s)
There was no significant difference between the two groups in gastrointestinal hormone level before surgery (p>0.05). However, after the surgery, the observation group was superior to the control group and the difference was statistically significant (p<0.05) as shown in Table 5.
| Group | Indicator | Observation group | Control group | F value | p value |
|---|---|---|---|---|---|
| Before surgery | GAS | 324.12±36.76 | 321.90±35.61 | 0.353 | 0.658 |
| MTL | 145.86±26.76 | 146.67±30.09 | 0.765 | 0.774 | |
| After surgery | GAS | 68.79±6.57 | 89.98±5.75 | 8.978 | 0.001 |
| MTL | 54.34±7.78 | 82.23±5.65 | 8.453 | 0.001 |
Table 5: Comparative Analysis of Gastrointestinal Hormone Level Between Two Groups (pg/ml, x̄±s)
The incidence of urinary retention, incision infection, scrotal hematoma and intestinal obstruction in patients of observation group was 2.00 %, 2.00 %, 4.00 % and 0.00 % respectively, with a total incidence rate of 8.00 %. The incidence of the above complications in patients of control group was 4.00 %, 10.00 %, 8.00 % and 2.00 % respectively, with a total incidence of 24.00 %. The observation group was superior to the control group and the difference was statistically significant (p<0.05) as shown in Table 6.
| Group | Uroschesis | Incision infection | Hematoma scrotum | Intestinal obstruction | Total incidence |
|---|---|---|---|---|---|
| Observation group (n=50) | 1 (2.00) | 1 (2.00) | 2 (4.00) | 0 (0.00) | 4 (8.00) |
| Control group (n=50) | 2 (4.00) | 5 (10.00) | 4 (8.00) | 1 (2.00) | 12 (24.00) |
| X2 value | 1.331 | 6.233 | 3.937 | 1.119 | 7.738 |
| p value | 0.023 | 0.007 | 0.014 | 0.019 | 0.001 |
Table 6: Comparative Analysis of Postoperative Complications Between Two Groups [n (%)]
In this study, there were significant differences in VAS scores, peripheral blood cells and T lymphocyte subsets, oxidative stress, gastrointestinal hormone levels and complication rates between the two groups (p<0.05). The total incidence of complications in patients of observation group patients was 8.00 %, while that in patients of control group was 24.00 %. Furthermore, the stress response of patients in observation group was lower than that of patients in control group and the immune function of patients in observation group was greatly improved.
Rectal cancer, as a major digestive system disease, will inevitably lead to strong physical and psychological stress response of patients, and the use of radical surgery will further aggravate the stress response of the body [9]. Acute reaction medium is the most common test index used to judge the stress response of the body, in which the reaction level of T lymphocyte subsets, the metabolism level of the body and the immune function of cells can all reflect the stress situation of the body [10]. The level of CD3+ T lymphocytes can effectively reflect the immune status of the body [11]; CD4+ cells, as a kind of counseling and inducing T lymphocytes, can play an antagonistic role in anti-tumor effect [12]; CD8+ T cells are a kind of inhibitory T lymphocytes, which can inhibit the immune response of the body. The ratio of CD4+/ CD8+ T lymphocytes is a key indicator of whether the immune regulation function is normal or not [13]. Related research results show that radical resection of rectal cancer can inhibit the cellular immune function of patients and this immunosuppression has a significant correlation with the degree of trauma of surgery [14].
Similarly, some studies have found that the stress response of patients undergoing radical resection of rectal cancer will significantly increase the synthesis and secretion level of cortisol in the body, and excessive cortisol will lead to the dysfunction of adrenal cortex in patients, which will interfere with the cellular immune function of patients and greatly reduce the postoperative survival rate of patients with rectal cancer [15]; the changes of oxidative stress and inflammatory factors in patients with rectal cancer after radical surgery are also the combined effect of trauma caused by radical surgery and the use of clinical narcotic drugs. Oxidative stress will aggravate the inflammatory reaction of the body and the inflammatory reaction will react to the oxidative stress reaction of the body through the released inflammatory mediators [16]. Etomidate can effectively relieve the restlessness symptoms of postoperative patients in recovery period and reduce the incidence of postoperative restlessness, but the anesthetic has poor effect in controlling postoperative pain and infection. Dexmedetomidine is a highly selective adrenergic receptor agonist with a distribution half-life of 6 min and a clearance half-life of about 2 h at the end stage, so it can obtain a relatively stable plasma concentration [17,18]. In addition, dexmedetomidine can relieve postoperative anxiety of patients and exert good sedative effect, with generally mild and moderate analgesic effect. It can reduce the irritation caused by endotracheal intubation and extubation and will not inhibit breathing [19].
According to the statistics of complications after radical surgery, 65 % of patients said that they had sore throat after general anesthesia and 45 % of patients had sore throat which would last for 24 h. In the application of conventional narcotic drugs, the main action site of dexmedetomidine is the locus coeruleus of brain stem, which ensures that dexmedetomidine plays a sedative and anti-anxiety role, while the analgesic effect comes from the spinal cord and above the spinal cord.
To sum up, dexmedetomidine combined with etomidate could effectively improve the cellular immune function and stress response of patients undergoing radical resection of rectal cancer, with remarkable clinical anesthesia effect. It is worthy of wide application.
Conflict of interests:
The authors declared no conflict of interest.
References
- Yu X, Xie Y. Effect of dexmedetomidine combined with etomidate on IL‑17A and S‑100β expression levels in rats with postoperative cognitive dysfunction. Exp Ther Med 2020;20(6):275-86.
[Crossref] [Google Scholar] [PubMed]
- Li Q, Pan Y, Cao Z, Zhao S. Comprehensive analysis of prognostic value and immune infiltration of chromobox family members in colorectal cancer. Front Oncol 2020;10:1901.
[Crossref] [Google Scholar] [PubMed]
- Bukovsky A. Immunology of tissue homeostasis, ovarian cancer growth and regression, and long lasting cancer immune prophylaxis-review of literature. Histol Histopathol 2021;36(1):31-46.
[Crossref] [Google Scholar] [PubMed]
- Tsukui H, Horie H, Koinuma K, Ohzawa H, Sakuma Y, Hosoya Y, et al. CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. BMC Cancer 2020;20(1):411-9.
[Crossref] [Google Scholar] [PubMed]
- Musich T, Thovarai V, Venzon DJ, Mohanram V, Tuero I, Miller-Novak LK, et al. A prime/boost vaccine regimen alters the rectal microbiome and impacts immune responses and viremia control post-simian immunodeficiency virus infection in male and female rhesus macaques. J Virol 2020;94(24):e01225-20.
[Crossref] [Google Scholar] [PubMed]
- Martins MA, Gonzalez-Nieto L, Ricciardi MJ, Bailey VK, Dang CM, Bischof GF, et al. Rectal acquisition of simian immunodeficiency virus (SIV) SIVmac239 infection despite vaccine-induced immune responses against the entire SIV proteome. J Virol 2020;94(24):e00979-20.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Du X. Application of dexmedetomidine-assisted intravertebral anesthesia in hip replacement and its influence on T-lymphocyte subsets. Exp Ther Med 2020;20(2):1269-76.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Li Y. Application of dexmedetomidine combined with sufentanil in colon cancer resection and its effect on immune and coagulation function of patients. Oncol Lett 2020;20(2):1288-94.
[Crossref] [Google Scholar] [PubMed]
- Bai Y, He H, Zhang P, Liu W, Huang L. Effects of dexmedetomidine on immune function, renal function and inflammatory factors of patients undergoing percutaneous nephrolithotomy under general anesthesia. Exp Ther Med 2021;21(4):406-15.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Cong X, Zhang L, Sun M, Li B, Geng H, et al. Effects of thoracic nerve block on perioperative lung injury, immune function, and recovery after thoracic surgery. Clin Transl Med 2020;10(3):e38.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Du X. Application of dexmedetomidine-assisted intravertebral anesthesia in hip replacement and its influence on T-lymphocyte subsets. Exp Ther Med 2020;20(2):1269-76.
[Crossref] [Google Scholar] [PubMed]
- Cavalcanti BC, Sá LG, de Andrade Neto JB, de Sousa Silva AA, Rios ME, Barreto FS, et al. Etomidate is devoid of genotoxicty and mutagenicity in human lymphocytes and in the Salmonella typhimurium/microsomal activation test. Toxicol In Vitro 2020;68:104946.
[Crossref] [Google Scholar] [PubMed]
- Wu H, Tang J, Pan J, Han M, Cai H, Zhang H. Effects of dexmedetomidine on stress hormones in patients undergoing cardiac valve replacement: A randomized controlled trial. BMC Anesthesiol 2020;20:142-9.
[Crossref] [Google Scholar] [PubMed]
- Chai Y, Cao Z, Yu R, Liu Y, Yuan D, Lei L. Dexmedetomidine attenuates LPS-induced monocyte-endothelial adherence via inhibiting Cx43/PKC-α/NOX2/ROS signaling pathway in monocytes. Oxid Med Cell Longev 2020;2020.
[Crossref] [Google Scholar] [PubMed]
- Yang YP, Yu LY, Shi J, Li JN, Wang XY, Liu TJ. Primary signet ring cell carcinoma with tubular adenoma of the rectum: A case report and a review of the literature. Medicine 2020;99(26):e20985.
[Crossref] [Google Scholar] [PubMed]
- Ji D, Song C, Li Y, Xia J, Wu Y, Jia J, et al. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J Immunother Cancer 2020;8(2):e000826.
[Crossref] [Google Scholar] [PubMed]
- Spehner L, Kim S, Vienot A, François E, Buecher B, Adotevi O, et al. Anti-telomerase CD4+ Th1 immunity and monocytic-myeloid-derived-suppressor cells are associated with long-term efficacy achieved by docetaxel, cisplatin, and 5-fluorouracil (DCF) in advanced anal squamous cell carcinoma: Translational study of epitopes-HPV01 and 02 trials. Int J Mol Sci 2020;21(18):6838-45.
[Crossref] [Google Scholar] [PubMed]
- Hendrix A, Yeo AE, Lejeune S, Seront E. Rare case of life-threatening thrombocytopenia occurring after radiotherapy in a patient treated with immune checkpoint inhibitor. BMJ Case Rep 2020;13(6):e235249.
[Crossref] [Google Scholar] [PubMed]
- Tsukui H, Horie H, Koinuma K, Ohzawa H, Sakuma Y, Hosoya Y, et al. CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. BMC Cancer 2020;20:411-21.
[Crossref] [Google Scholar] [PubMed]