- *Corresponding Author:
- Weidong Chen
Department of Digestive Endoscopy, Yueqing People’s Hospital Affiliated to Wenzhou Medical University, Yueqing, Zhejiang Province 325600, China
E-mail: liulewei1987@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “294-298” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The number of people who get and die from pancreatic cancer, a deadly type of tumor, has been rising lately. Epidermal growth factor receptor is a receptor that adds phosphate groups to tyrosine residues and regulates many cell processes, such as growth, movement, invasion, and death. Pancreatic cancer cells have more epidermal growth factor receptor than normal cells, and this affects how they develop and survive. Erlotinib is a drug that blocks epidermal growth factor receptor activity and can treat some lung cancers with epidermal growth factor receptor mutations. The way erlotinib works against pancreatic cancer is still unknown. This research examines the impact of erlotinib on the proliferation and apoptosis of three types of pancreatic cancer cells; PANC-1, BxPC-3, and SW1990. The experiments used the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay, Annexin V-fluorescein isothiocyanate/propidium iodide staining, quantitative reverse transcription-polymerase chain reaction, and enzyme-linked immunosorbent assay. Erlotinib can stop pancreatic cancer cells from growing, make them die, and lower the levels and activity of epidermal growth factor receptor and other molecules that send signals from it. This means that erlotinib could fight pancreatic cancer by blocking the epidermal growth factor receptor pathway, and offer a new way to treat it.
Keywords
Erlotinib, pancreatic cancer, epidermal growth factor receptor, signaling pathway, mortality
Pancreatic cancer is a highly lethal malignant tumor, with its incidence and mortality rates showing an upward trend in recent years[1]. Pancreatic cancer ranks 7th in global cancer mortality, and it may rise to 2nd place by 2030[2]. Mainly early diagnosis of pancreatic cancer is difficult, and most patients are already in the late stage when diagnosed and cannot undergo surgical resection[3]. Understanding how pancreatic cancer starts and progresses, and discovering new treatments and drugs, can help patients with pancreatic cancer live longer and better[4]. Epidermal Growth Factor Receptor (EGFR) is a receptor that adds phosphate groups to tyrosine residues and regulates many cell processes, such as growth, movement, invasion, and death[5]. By interacting with its ligands, such as EGF and Transforming Growth Factor Alpha (TGF-α), EGFR induces receptor heterodimerization and autophosphorylation, triggers its own tyrosine kinase activity, and then activates its downstream signaling mediators, such as the Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (AKT) and Mitogen- Activated Protein Kinase (MAPK)/Extracellular Signal Kinase (ERK) pathways, to control cell proliferation and survival[6]. Pancreatic cancer cells have more EGFR than normal cells, and this affects how they develop and survive[7]. More EGFR and its activity can make pancreatic cancer cells grow, move, and invade more, stop them from dying, and help them resist drugs and make new blood vessels[8]. Therefore, EGFR is an important therapeutic target for pancreatic cancer, and inhibitors targeting EGFR are expected to have therapeutic effects on pancreatic cancer. Erlotinib is a drug that blocks EGFR activity and can treat some lung cancers with EGFR mutations[9]. Erlotinib binds to the EGFR domain that adds phosphates to tyrosines, stopping EGFR from being activated and sending signals, and thus preventing tumor cells from growing and living[10-12]. The mechanism of erlotinib against pancreatic cancer remains unclear. This research explores the effects of erlotinib on three types of pancreatic cancer cells; PANC-1, BxPC-3, and SW1990. This can help future research in this field. This study employed a randomized controlled trial method, dividing 60 healthy male participants into four groups; placebo+strength training group (PL+RT), methyl-1- testosterone+strength training group (MGP+RT), placebo+non-strength training group (PL+NRT), and methyl-1-testosterone+non-strength training group (MGP+NRT). The intervention period lasted for 12 w, with three sessions of strength training or nonstrength training per week, each lasting for 60 min. The dose of methyl-1-testosterone was 10 mg/d, administered orally. Before and after the intervention, the participants’ body weight, body fat percentage, muscle mass, maximal strength, blood biochemical markers, and hormone levels were measured. The Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) dual labeling method was used for measuring cell apoptosis. A 2 ml aliquot of this cell suspension was uniformly distributed into each well of a 6-well plate, with three wells allocated per group. The incubation process was continued for an additional 48 h. Upon completion of each incubation cycle, the cells were harvested, rinsed twice with Phosphate Buffered Saline (PBS), and resuspended in 1×Annexin V binding buffer, adding 100 μl per well. The fluorescence signals from the cells were captured in the FL1 and FL2 channels using a flow cytometer, and the apoptosis rate of the cells was subsequently analyzed using FlowJo software. Expression of EGFR messenger Ribonucleic Acid (mRNA) was detected by the quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) method. They are uniformly seeded in a 6-well plate, with 2 ml per well and 3 replicates per group, and cultured at 37° with 5 % Carbon dioxide (CO2) for 24 h. After that, the medium is removed, and erlotinib solutions at various concentrations (0, 5, 10, 15 μM) are added, with 2 ml per well, and the culture is extended for 48 h. After each time point, cells are harvested, total RNA is isolated with TRIzol reagent, Deoxyribonucleic Acid (DNA) contamination is eliminated with DNase I, and the RNA concentration and purity are measured with a NanoDrop 2000 instrument. Reverse transcription is carried out according to the PrimeScript RT reagent kit protocol to generate complementary DNA (cDNA). qRT-PCR is conducted according to the SYBR® Premix Ex Taq™ II protocol, and the fluorescence signal is read with the StepOnePlus real-time PCR system.
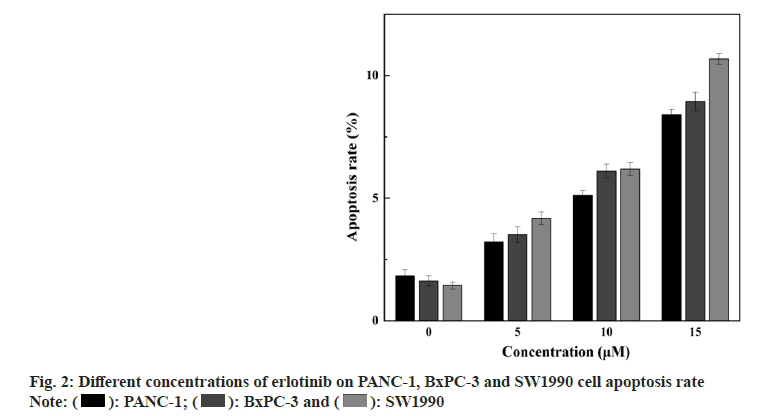
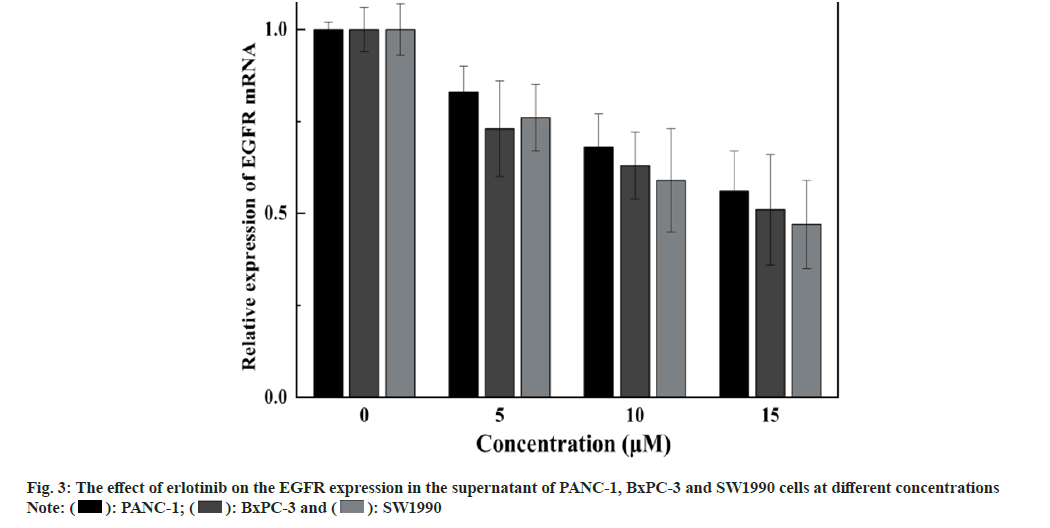
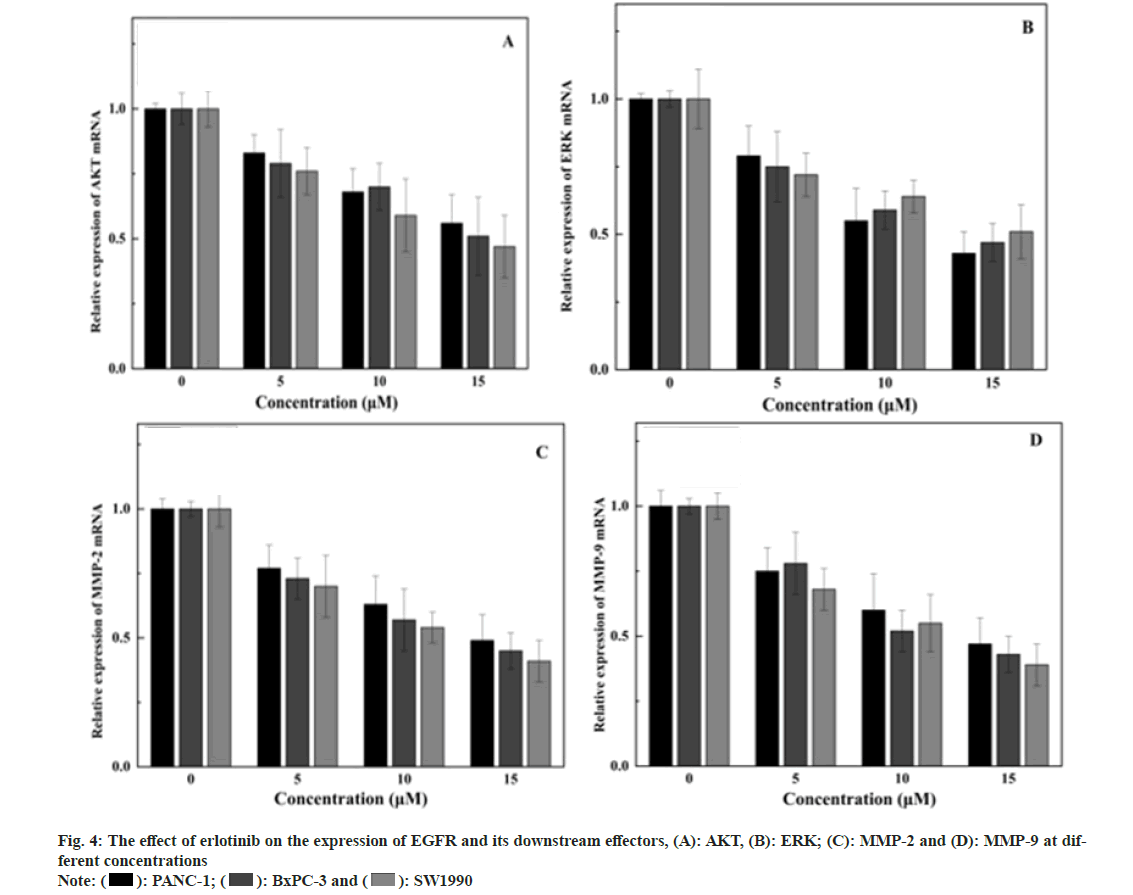
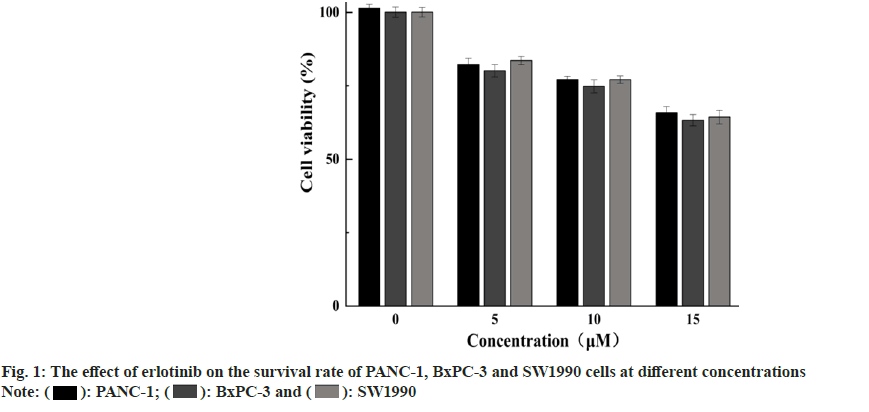
Expression of EGFR and its downstream signaling molecules was detected by the Enzyme-Linked Immunosorbent Assay (ELISA) method. The medium is removed, and erlotinib solutions at various concentrations (0, 5, 10, and 15 μM) are added, with 2 ml per well, and the culture is extended for 48 h. After each time point, cells are harvested, cells are disrupted with Radioimmunoprecipitation Assay (RIPA) reagent, and the protein concentration is measured with a Bicinchoninic Acid (BCA) reagent kit. ELISA is conducted according to the Human EGFR, AKT, ERK, Matrix Metalloproteinase (MMP)-2, MMP-9 ELISA Kit protocol, the absorbance (Optical Density (OD)) value is read at 450 nm with an enzyme label instrument, and the protein expression levels are calculated for each sample. The findings showed that erlotinib could inhibit the proliferation of pancreatic cancer cells in a dose-dependent manner. At 15 μM, the viability rates were 65.87 %±2.04 %, 63.31 %±1.86 %, and 64.34 %±2.3 % (fig. 1), respectively. The survival rate of the erlotinib group was markedly higher than that of the control group (p<0.001). These results demonstrate that erlotinib has a remarkable antigrowth effect on pancreatic cancer cells. The findings showed that erlotinib could induce the apoptosis of pancreatic cancer cells in a dose-dependent manner. At 15 μM, the apoptosis rates were 7.41 %±0.22 %, 8.94 %±0.39 %, and 10.68 %±0.23 % (fig. 2), respectively. The erlotinib group had a significantly higher apoptosis rate than the control group (p<0.001). These results demonstrate that erlotinib has a remarkable apoptosis-inducing effect on pancreatic cancer cells. The results revealed that at 15 μM, erlotinib could reduce the mRNA expression of EGFR in pancreatic cancer cells in a dosedependent way. The relative expression levels were 0.56 %±0.11 %, 0.51 %±0.15 %, and 0.47 %±0.12 % (fig. 3), respectively. The erlotinib group had a significantly lower EGFR, mRNA expression level than the control group (p<0.001). These results demonstrate that erlotinib has a remarkable inhibitory effect on the EGFR signaling pathway in pancreatic cancer cells. The results revealed that at 15 μM, erlotinib could reduce the protein expression of EGFR and its downstream targets in pancreatic cancer cells in a dose-dependent way. The relative expression levels of AKT were 0.53 %±0.1 %, 0.49 %±0.12 %, and 0.45 %±0.11 % (fig. 4A), respectively. The relative expression levels of ERK were 0.43 %±0.08 %, 0.47 %±0.08 %, and 0.51 %±0.1 % (fig. 4B), respectively. The relative expression levels of MMP-2 were 0.49 %±0.1 %, 0.45 %±0.07 %, and 0.41 %±0.08 % (fig. 4C), respectively. The relative expression levels of MMP-9 were 0.47 %±0.1 %, 0.43 %±0.07 %, and 0.39 %±0.08 % (fig. 4D), respectively. The protein expression levels of EGFR and its downstream targets in the erlotinib group were significantly lower than those in the control group (p<0.001). These results are in line with the results of mRNA expression, indicating that erlotinib has a remarkable inhibitory effect on the EGFR signaling pathway in pancreatic cancer cells. This study examined the effects of erlotinib on the growth and apoptosis of pancreatic cancer cell lines PANC- 1, BxPC-3, and SW1990, as well as its modulatory effect on the EGFR signaling pathway. We discovered that erlotinib could suppress the growth of pancreatic cancer cells and trigger their apoptosis, while reducing the expression and activation of EGFR and its downstream targets AKT, ERK, MMP-2, and MMP-9. The expression and activation levels of EGFR in pancreatic cancer are much higher than those in normal pancreatic tissue, and are closely associated with the progression and outcome of pancreatic cancer[13,14]. Therefore, EGFR is a key therapeutic target for pancreatic cancer, and inhibitors targeting EGFR are anticipated to have therapeutic effects on pancreatic cancer[15]. Erlotinib can attach to the tyrosine kinase of EGFR, block the phosphorylation of EGFR and the transmission of downstream signals, thereby suppressing the growth and survival of tumor cells. This study discovered that erlotinib can efficiently inhibit the expression and activation of EGFR and its downstream targets AKT, ERK, MMP-2, and MMP-9 in pancreatic cancer cells, thereby triggering the apoptosis of pancreatic cancer cells. However, this study has some limitations. Future research needs to use more experimental methods and techniques, as well as more cell lines and animal models, to verify and extend the findings of this study. This will provide stronger evidence and support for the use of erlotinib in the therapy of pancreatic cancer.
Funding:
This study was supported by National Natural Science Fund Wenzhou Science and Technology Bureau (No: Y20210882).
Conflict of interests:
The authors declared no conflict of interests.
References
- Olakowska E, Olakowski M. Treatment of type 2 diabetes mellitus and risk of pancreatic cancer. Endokrynol Pol 2021;72(4):395-401.
[Crossref] [Google Scholar] [PubMed]
- Desai D, Khandwala P, Parsi M, Potdar R. PARP inhibitors: Shifting the paradigm in the treatment of pancreatic cancer. Med Oncol 2021;38(6):61.
[Crossref] [Google Scholar] [PubMed]
- Ma X, Cui Z, Du Z, Lin H. Transforming growth factor-β signaling, a potential mechanism associated with diabetes mellitus and pancreatic cancer? J Cell Physiol 2020;235(9):5882-92.
[Crossref] [Google Scholar] [PubMed]
- Li J, Peng L, Chen Q, Ye Z, Zhao T, Hou S, et al. Integrin β1 in pancreatic cancer: Expressions, functions, and clinical implications. Cancers 2022;14(14):3377.
[Crossref] [Google Scholar] [PubMed]
- Chu X, Yang Y, Tian X. Crosstalk between pancreatic cancer cells and cancer-associated fibroblasts in the tumor microenvironment mediated by exosomal microRNAs. Int J Mol Sci 2022;23(17):9512.
[Crossref] [Google Scholar] [PubMed]
- Ranson M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br J Cancer 2004;90(12):2250-5.
[Crossref] [Google Scholar] [PubMed]
- Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells 2018;7(11):212.
[Crossref] [Google Scholar] [PubMed]
- Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer. JAMA Oncol 2018;4(5):739-42.
[Crossref] [Google Scholar] [PubMed]
- Chefrour M, Fischel JL, Formento P, Giacometti S, Ferri-Dessens RM, Marouani H, et al. Erlotinib in combination with capecitabine (5'dFUR) in resistant pancreatic cancer cell lines. J Chemother 2010;22(2):129-33.
[Crossref] [Google Scholar] [PubMed]
- Jiang J, Yuan Z, Sun Y, Bu Y, Li W, Fei Z. Ginsenoside Rg3 enhances the anti-proliferative activity of erlotinib in pancreatic cancer cell lines by downregulation of EGFR/PI3K/Akt signaling pathway. Biomed Pharmacother 2017;96:619-25.
[Crossref] [Google Scholar] [PubMed]
- Hao J, Yang X, Ding XL, Guo LM, Zhu CH, Ji W, et al. Paeoniflorin potentiates the inhibitory effects of erlotinib in pancreatic cancer cell lines by reducing ErbB3 phosphorylation. Sci Rep 2016;6(1):32809.
- Vidal RI. Antitumor activity of liposomal formulations of bis-naphthalimide derivatives and erlotinib, in vitro study in pancreatic cancer cell lines. Dissert Master Degree Pharm Technol 2013;18:21-5.
- He X, Sun Y, Fan R, Sun J, Zou D, Yuan Y. Knockdown of the DJ-1 (PARK7) gene sensitizes pancreatic cancer to erlotinib inhibition. Mol Ther Oncolytics 2021;20:364-72.
[Crossref] [Google Scholar] [PubMed]
- Czarnecka AM, Korzen P, Nowak-Dement A, Kukwa W, Korniluk J, Szczylik C. Prolonged complete response following gemcitabine-erlotinib combined therapy in advanced pancreatic cancer. Oncol Lett 2016;11(2):1101-4.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Wu X, Zhang Z, Ma C, Wu T, Tang S, et al. Monensin inhibits cell proliferation and tumor growth of chemo-resistant pancreatic cancer cells by targeting the EGFR signaling pathway. Sci Rep 2018;8(1):17914.
[Crossref] [Google Scholar] [PubMed]
 ): PANC-1; (
): PANC-1; ( ): BxPC-3 and (
): BxPC-3 and ( ): SW1990
): SW1990