- *Corresponding Author:
- Jing Chen
Department of Medical Oncology
Shaanxi Provincial Cancer Hospital
Xi'an, Shaanxi 710000, China
E-mail: dachenjing2021@163.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “30-39” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to explore the effect of long non-coding RNA TRIM52 antisense RNA 1 on the proliferation, apoptosis and radiosensitivity of colorectal cancer cells and its possible mechanism. Real-time quantitative reverse transcription polymerase chain reaction method was used to detect the expression of long non-coding RNA TRIM52 antisense RNA 1 and microRNA-361-5p in adjacent tissues, colorectal cancer tissues and human normal colorectal mucosal cell lines, fetal human cells and human colorectal cancer cell lines. The transplanted tumor experiment in nude mice was used to detect the influence of the expression changes of long non-coding RNA TRIM52 antisense RNA 1 and microRNA-361-5p on the transplanted tumor growth. The expression of long non-coding RNA TRIM52 antisense RNA 1 in colorectal cancer tissues and cell lines was increased, while the expression of microRNA-361-5p was decreased (p<0.05). After transfection of small interfering long non-coding RNA TRIM52 antisense RNA 1 or microRNA-361-5p mimics, cell viability and cell survival score were decreased, the number of cell clone formation was decreased and cell apoptosis rate was increased (p<0.05). Transfection of small interfering long non-coding RNA TRIM52 antisense RNA 1 could inhibit the growth of transplanted tumors in nude mice. Long non-coding RNA TRIM52 antisense RNA 1 could target the microRNA-361-5p expression. The expression of long non-coding RNA TRIM52 antisense RNA 1 could inhibit colorectal cancer cell proliferation and clone formation and promote cell apoptosis by targeted regulation of microRNA-361-5p expression and could enhance the radiosensitivity of colorectal cancer cells.
Keywords
Colorectal cancer, long non-coding RNA TRIM52 antisense RNA 1, microRNA-361-5p, cell proliferation, apoptosis, radiosensitivity
Colorectal cancer is a common malignant tumor in China, with high morbidity. Some patients develop radiation resistance, which leads to reduced therapeutic effect and poor prognosis[1,2]. Long Non-Coding Ribonucleic Acid (lncRNA) plays an important regulatory role in the occurrence and development of colorectal cancer, mainly through competitively binding MicroRNA (miRNA) to regulate the expression of their target genes and promote or inhibit the biological behavior of colorectal cancer cells[3,4]. LncRNA TRIM52 Antisense RNA 1 (TRIM52-AS1) is up-regulated in hepatocellular carcinoma tissues and inhibition of its expression can inhibit the proliferation, migration and invasion of hepatocellular carcinoma cells, as well as the growth of transplanted tumors in vivo[5]. However, the expression of lncRNA TRIM52-AS1 in colorectal cancer and its effect on cell biological behavior remain unknown. StarBase prediction showed that lncRNA TRIM52-AS1 and MicroRNA-361-5p (miR-361-5p) had complementary sequences. Studies showed that miR-361-5p was down-regulated in colorectal cancer and up-regulation of miR-361-5p expression could inhibit cell proliferation, migration and invasion[6]. However, the mechanism of lncRNA TRIM52-AS1/ miR-361-5p in the development and progression of colorectal cancer has not been clarified. Therefore, this study mainly investigates whether lncRNA TRIM52- AS1 could affect the proliferation, apoptosis and radiosensitivity of colorectal cancer cells by targeting the expression of miR-361-5p.
Materials and Methods
Materials and reagents:
A total of 51 patients with colorectal cancer who received treatment in our hospital from January 2019 to May 2020 were collected and stored in a -80° refrigerator. All patients were diagnosed with colorectal cancer by pathological diagnosis, including 31 males and 20 females, aged 52-66 y, with an average age of (60.32±4.11) y.
Exclusion criteria: Patients who were combined with other malignant tumors; those with autoimmune diseases. The informed consents of the patients or their relatives were obtained and the study complied with the relevant requirements of the Declaration of Helsinki of the World Medical Association.
Human normal colorectal mucosal cells, Fetal Human Cells (FHC), Human Colorectal Cancer Cell Line (SW480), Colorectal Adenocarcinoma Cell Line (DLD- 1) and Colorectal Carcinoma Cell Line (HCT116) were purchased from American Type Culture Collection (ATCC), United States of America (USA). Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum, trypsin, 3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyltetrazolium Bromide (MTT) reagent were purchased from Beyotime Biotechnology, Shanghai. Apoptosis detection reagent and luciferase activity detection kit were purchased from Solarbio, Beijing; LipofectamineTM 3000 transfection reagent, TRIzol reagent, lncRNA TRIM52-AS1 overexpression vector (Plasmid Cloning Deoxyribonucleic Acid (pcDNA)- lncRNA TRIM52-AS1) and its control empty vector (pcDNA) were purchased from Invitrogen, USA. Reverse transcription reagent and SYBR fluorescent quantitative reagent were purchased from Thermo Fisher, USA. Small Interfering lncRNA TRIM52-AS1(silncRNA TRIM52-AS1) and Small Interfering RNANegative Control (si-NC), miR-361-5p Oligonucleotide Mimics (miR-361-5p mimics) and negative control mimic NC sequence (miR-NC), miR-361-5p specific oligonucleotide inhibitor (anti-miR-361-5p) and its negative control (anti-miR-361-5p) were purchased from RiboBio, Guangzhou. Rabbit anti-human cleaved caspase-3, Gamma-H2A Histone Family Member X (γ-H2AX) antibody and Horseradish Peroxidase (HRP) labeled goat anti-rabbit Immunoglobulin G (IgG) were purchased from Zhongshan Jinqiao Bio, Beijing.
45 Specific-Pathogen Free (SPF) male Sprague Dawley (SD) rats were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., with age of 6 w old, body weight of 180-200 g, animal license Number: SXCK (Beijing): 2018-0006. All rats were free to eat and drink and the culture environment was sterile, under temperature of 37°, in atmosphere of 5 % CO2, under illumination condition of 12 h light/12 h darkness and the operation method meet the animal laboratory standards.
Methods:
Experimental grouping: FHC cells, SW480, DLD-1 and HCT116 cells were cultured in DMEM complete medium containing 10 % fetal bovine serum. SW480 cells in logarithmic growth phase were prepared and inoculated in 6-well plates (1×104 cells/well) and transfected to 70 % by referring to the LipofectamineTM 3000 transfection reagent instruction. si-NC, silncRNA TRIM52-AS1, miR-NC, miR-361-5p mimics, anti-miR-NC and si-lncRNA TRIM52-AS1, anti-miR- 361-5p and si-lncRNA TRIM52-AS1 were transfected into SW480 cells, respectively and cultured at 37° in 5 % CO2 for 6 h, before discarding and replacing the culture medium with DMEM medium containing 10 % fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin for another 48 h, respectively. They were named as si-NC group, si-lncRNA TRIM52-AS1 group, miR-NC group, miR-361-5p group, anti-miR-NC+silncRNA TRIM52-AS1 group and anti-miR-361-5p+silncRNA TRIM52-AS1 group. In contrast, SW480 cells in normal culture were recorded as NC group.
Detection of the expression levels of lncRNA TRIM52-AS1 and miR-361-5p by Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR): TRIzol reagent was used to extract total RNA from paracancerous tissues, colorectal cancer tissues, FHC cells, SW480, DLD-1, HCT116 cells and SW480 cells in each group. The RNA concentration was detected by NanoDrop 2000c ultrafine spectrophotometer and complementary DNA (cDNA) was synthesized by reverse transcription. Polymerase Chain Reaction (PCR) amplification reaction system adopted was SYBR Green Master Mix 10 μl. The volume of positive and negative primer was 0.8 μl, volume of cDNA was 2 μl and the volume of Double Distilled Water (ddH2O) system was set to 20 μl. Reaction conditions were 95° for 2 m, 95° for 30 s, 60° for 30 s, 72° for 30 s (Repeat cycle for 40 times). The relative expression levels of lncRNA TRIM52-AS1 (internal reference Beta actin (β-actin) and (internal reference U6) were calculated by 2-ΔΔCt method.
Detection of cell proliferation by MTT: SW480 cells from each group were inoculated into 96-well plates (2×103 cells/well) and 20 μl of 5 mg/ml MTT solution was added to each well. The cells were cultured for 4 h in an incubator with 5 % CO2 at 37°, followed by centrifugal treatment at 3000 r/min for 5 min to discard supernatant. Then, 150 μl of DMSO was added to each well and the cells were incubated with vibration for 5 min away from light. The absorbance of each well was detected by a microplate reader (A 490 nm).
Plate clone formation experiment: SW480 cells in each group were inoculated in 6-well plates (1000 cells/well) and cultured at 37° in a 5 % CO2 incubator for 14 d before discarding the medium. Then, the cells were washed with Phosphate Buffered Saline (PBS) and fixed with methanol for 20 min, stained with 1 % crystal violet staining solution for 15 min, washed with distilled water and dried, and the number of cell clone formation was counted under a microscope.
Detection of cell apoptosis rate by flow cytometry: SW480 cells were collected and washed with pre-cooled PBS, centrifuged at 3000 r/min for 6 min to discard the supernatant. After that, 500 μl of binding buffer was added to resuspended cells and cell apoptosis rate was detected according to the instruction of apoptosis detection kit.
Detection of radiosensitivity by clonogenic assay: SW480 cells in each group were inoculated into 6-well plates (1×104 cells/well) and irradiated with different doses of 0, 2, 4, 6 and 8 Gray (Gy), with the source target distance of 100 cm, irradiation field of 10 cm×10 cm and dose rate of 5 Gy/min. If cell clone group appeared, the culture was terminated, washed with PBS, fixed with methanol for 20 min and stained with 1 % crystal violet staining solution for 40 min. The number of effective clones was observed under a microscope. The cell survival fraction is the percentage of the ratio of the clone formation rate of the irradiated cells to that of the control cells.
Detection of the targeting relationship between lncRNA TRIM52-AS1 and miR-361-5p using dual luciferase reporting assay: StarBase prediction showed that lncRNA TRIM52-AS1 had a binding site with miR-361-5p. The binding site was mutated using the point mutation kit and the Wild Type (WT) vector, WT-lncRNA TRIM52-AS1 and mutated vectors MUT-lncRNA TRIM52-AS1, WT-lncRNA TRIM52- AS1 MUT-lncRNA TRIM52-AS1 were constructed respectively and then co-transfected into SW480 cells miR-NC or miR-NC, respectively. After 48 h of transfection, discard the old medium, wash with PBS, add 20 μl 1× Passive Lysis Buffer (PLB) to each well, add 100 μl of luciferase assay reagent Ⅱ, then add PLB lysis solution, so as to measure the firefly luciferase activity with fluorescence spectrometer. After addition of Stop and Glo® Reagent, the activity of Renilla luciferase was determined by fluorescence luminescence analyzer. The activity of luciferase reporter gene in sea kidney was used as internal reference to calculate the activity of luciferase.
Detection of the expression levels of cleaved caspase-3 and γ-H2AX by western blot: SW480 cells in each group were collected and added with 400 μl of Radioimmunoprecipitation assay (RIPA) lysate to extract total protein. Protein concentration was detected by Bicinchoninic Acid (BCA) method and Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) was performed according to the density of 30 μg of protein samples per well. After membrane transfer, cells were placed in 5 % skim milk for 2 h, followed by addition of cleaved caspase-3 (1:800), γ-H2AX primary antibody (1:800) and internal reference -actin antibody (1:1000) to dilute solution and incubated at 4° for 24 h for Tris Buffered Saline with Tween 20 (TBST) membrane washing. Subsequently, second antibody diluent (1:3000) was added and incubated at 37° for 1 h. Emitter-Coupled Logic (ECL) was dropped and exposed to develop in darkroom. ImageJ software was used to analyze the gray values of each strip.
Tumor transplantation in nude mice: si-NC, silncRNA TRIM52-AS1, anti-miR-NC and si-lncRNA TRIM52-AS1 (co-transfection), anti-miR-361- 5p and si-lncRNA TRIM52-AS1 (co-transfection) were transfected into SW480 cells (1×106 cells/ml). FHC cells and SW480 cells transfected above were subcutaneously injected into nude mice, with 9 cells in each group and cultured for 4 w. The nude mice were executed by cervical dissection and the transplanted tumor tissue was removed to detect the volume and weight of the transplanted tumor.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 statistical software was used to analyze the data and the measurement data were expressed as (x̄±s) and were in accordance with normal distribution. Independent sample t-test was used for comparison between the two groups, one-way Analysis of Variance (ANOVA) was used for comparison between multiple groups. The difference was considered statistically significant when p<0.05.
Results and Discussion
The expression levels of miR-361-5p and lncRNA TRIM52-AS1 in colorectal cancer patients is explained here. Compared with para-cancer tissues, the expression level of lncRNA TRIM52-AS1 in colorectal cancer tissues was increased (p<0.05) and the expression level of miR-361-5p was decreased (p<0.05), as shown in Table 1.
| Group | miR-361-5p | lncRNA TRIM52-AS1 |
|---|---|---|
| Para-carcinoma tissue | 1.00±0.13 | 1.00±0.15 |
| Colorectal cancer tissue | 0.38±0.05* | 2.86±0.31* |
| t | 31.789 | 38.571 |
| p | 0.000 | 0.000 |
Note: Compared with para-cancer tissues, *p<0.05
Table 1: Expression Levels of miR-361-5p and lncRNA TRIM52-AS1 in Colorectal Cancer Patients (x̄±s, n=51)
Compared with FHC cells, the expression of lncRNA TRIM52-AS1 in SW480, DLD-1 and HCT116 cells was increased (p<0.05), while the expression of miR- 361-5p was decreased (p<0.05). Among them, the expression level of lncRNA TRIM52-AS1 in colorectal cancer cells SW480 was relatively higher, so SW480 cells were selected for subsequent experiments, as shown in Table 2.
| Group | miR-361-5p | lncRNA TRIM52-AS1 |
|---|---|---|
| FHC | 1.00±0.10 | 1.00±0.09 |
| SW480 | 0.31±0.03* | 2.38±0.18* |
| DLD-1 | 0.42±0.04* | 2.01±0.15* |
| HCT116 | 0.49±0.05* | 1.82±0.13* |
| F | 224.400 | 153.323 |
| p | 0.000 | 0.000 |
Note: Compared with FHC,*p<0.05
Table 2: Expression Levels of miR-361-5p and lncRNA TRIM52-AS1 in Colorectal Cancer Cell Lines (x̄±s, n=9)
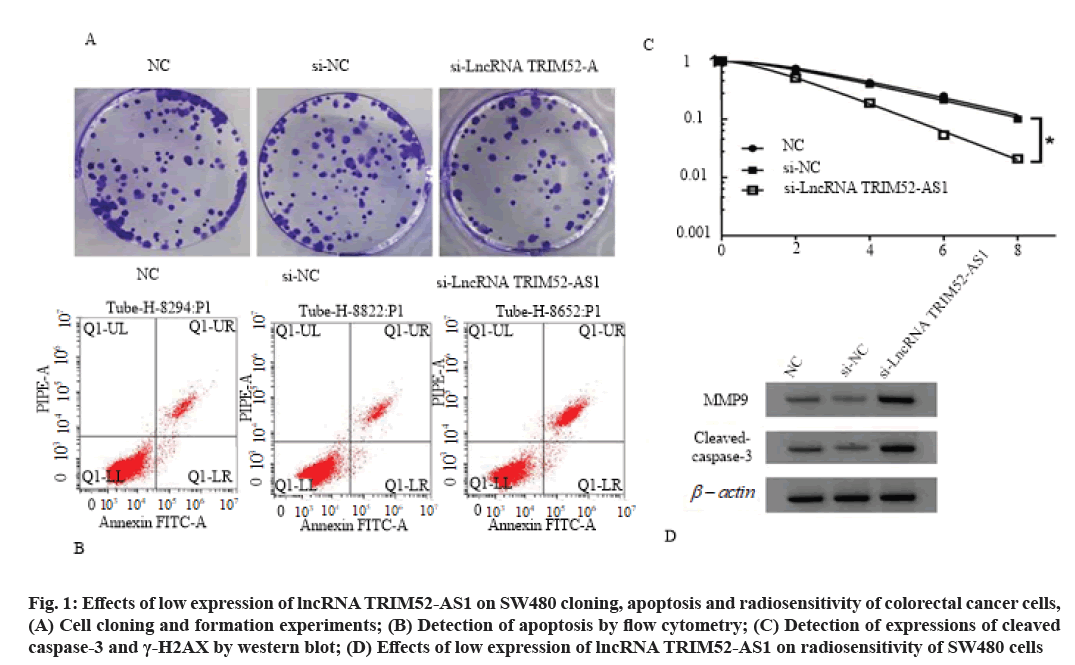
Effects of low expression of lncRNA TRIM52-AS1 on proliferation, cloning, apoptosis and radiosensitivity of colorectal cancer cell SW480 is shown here. Compared with si-NC group, the cell viability of si-lncRNA TRIM52-AS1 group was decreased (p<0.05), cell clone formation number was decreased (p<0.05), cell apoptosis rate and cleaved caspase-3 and γ-H2AX protein levels were increased (p<0.05), cell survival fraction was decreased (p<0.05), and Sensitization Enhancement Ratio (SER) was 1.607 (>1), as shown in fig. 1, Table 3 and Table 4.
| Group | lncRNA TRIM52-AS1 | Cleaved caspase-3 | γ-H2AX | A value | Cell clone number | Apoptosis rate (%) |
|---|---|---|---|---|---|---|
| NC | 1.00±0.08 | 0.42±0.03 | 0.32±0.02 | 1.135±0.10 | 118±8.13 | 8.21±0.61 |
| si-NC | 0.97±0.10 | 0.43±0.11 | 0.33±0.03 | 1.131±0.09 | 115±6.02 | 8.19±0.53 |
| si-lncRNA TRIM52-AS1 | 0.42±0.04* | 0.81±0.04* | 0.75±0.07* | 0.531±0.04* | 61±4.14* | 23.76±1.67* |
| F | 159.950 | 91.418 | 262.306 | 165.571 | 232.539 | 633.088 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with si-NC group, *p<0.05
Table 3: Effects of Low Expression of lncRNA TRIM52-AS1 on Proliferation, Cloning, Apoptosis and Radiosensitivity of Colorectal Cancer Cells SW480 (x̄±s, n=9)
| Group | D0 (Gy) | Dq (Gy) | N | SF2 | k | SER (D0 ratio) |
|---|---|---|---|---|---|---|
| NC | 2.837 | 2.072 | 2.076 | 0.757 | 0.353 | / |
| si-NC | 2.850 | 1.708 | 1.821 | 0.713 | 0.351 | / |
| si-lncRNA TRIM52-AS1 | 1.773 | 1.090 | 1.849 | 0.515 | 0.564 | 1.607 |
Note: D0 is mean lethal dose of cells; Dq is quasi-threshold dose; N is extrapolation number; SF2 is surviving fraction at 2 Gy; k is passivation constant; SER is sensitization enhancement ratio
Table 4: Effects of Low Expression of lncRNA TRIM52-AS1 on Radiosensitivity of SW480 Cells
Fig. 1: Effects of low expression of lncRNA TRIM52-AS1 on SW480 cloning, apoptosis and radiosensitivity of colorectal cancer cells, (A) Cell cloning and formation experiments; (B) Detection of apoptosis by flow cytometry; (C) Detection of expressions of cleaved caspase-3 and γ-H2AX by western blot; (D) Effects of low expression of lncRNA TRIM52-AS1 on radiosensitivity of SW480 cells
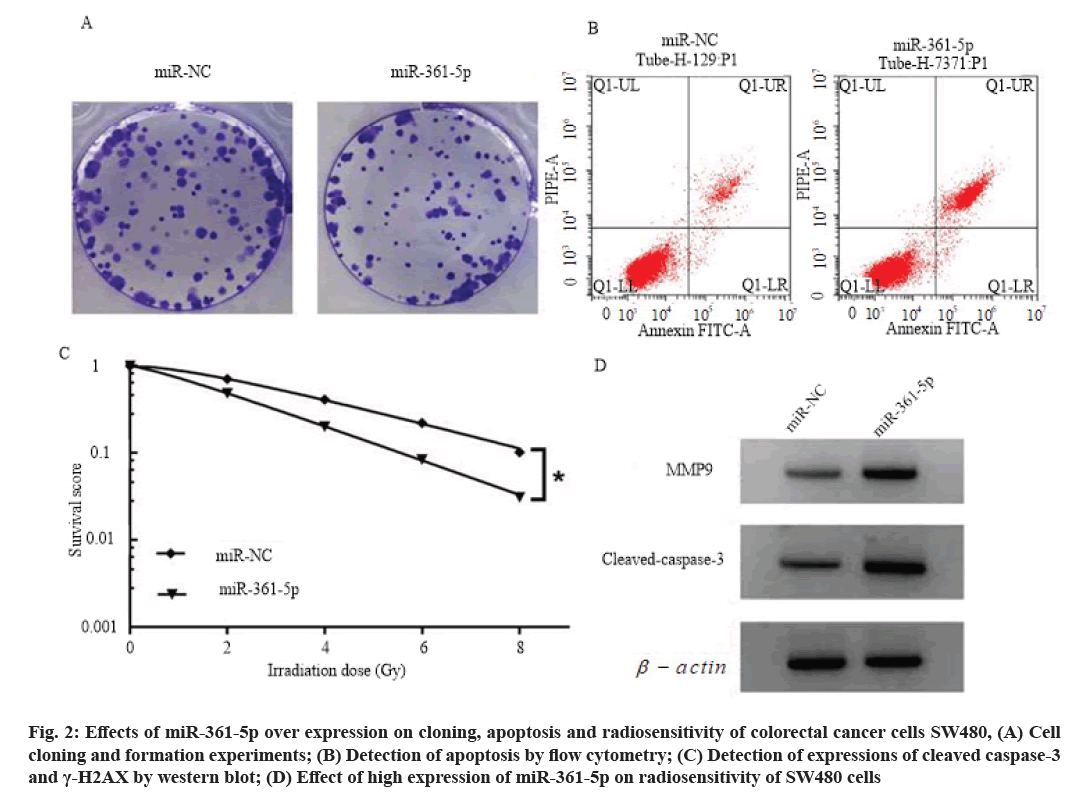
Effects of high expression of miR-361-5p on proliferation, cloning, apoptosis and radiosensitivity of colorectal cancer cells SW480 is explained here. Compared with the miR-NC group, the cell viability of miR-361-5p group was decreased (p<0.05), the number of cell clone formation was decreased (p<0.05), the cell apoptosis rate and cleaved caspase-3 and γ- H2AX protein levels were increased (p<0.05), and the cell survival score was decreased (p<0.05). SER is 1.349 (>1), as shown in fig. 2, Table 5 and Table 6.
| Group | miR-361-5p | Cleaved caspase-3 | γ-H2AX | A value | Cell clone number (individual) | Apoptosis rate (%) |
|---|---|---|---|---|---|---|
| miR-NC | 1.00±0.10 | 0.41±0.03 | 0.31±0.03 | 1.135±0.10 | 117±8.31 | 8.23±0.61 |
| miR-361-5p | 2.37±0.21* | 0.86±0.07* | 0.80±0.07* | 0.613±0.05* | 52±3.31* | 27.86±1.93* |
| t | 17.670 | 17.726 | 19.302 | 14.007 | 21.800 | 29.094 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with miR-NC group, *p<0.05
Table 5: Effects of miR-361-5p Over Expression on Proliferation, Cloning, Apoptosis and Radiosensitivity of Colorectal Cancer Cell SW480 (x̄±s, n=9)
| Group | D0 (Gy) | Dq (Gy) | N | SF2 | k | SER (D0 ratio) |
|---|---|---|---|---|---|---|
| miR-NC | 2.942 | 1.643 | 1.748 | 0.709 | 0.340 | / |
| miR-361-5p | 2.181 | 0.569 | 1.298 | 0.484 | 0.459 | 1.349 |
Note: D0 is mean lethal dose of cells; Dq is quasi-threshold dose; N is extrapolation number; SF2 is surviving fraction at 2 Gy; k is passivation constant; SER is sensitization enhancement ratio
Table 6: Effects of High Expression of miR-361-5p on Radiosensitivity of SW480 Cells
Fig. 2: Effects of miR-361-5p over expression on cloning, apoptosis and radiosensitivity of colorectal cancer cells SW480, (A) Cell cloning and formation experiments; (B) Detection of apoptosis by flow cytometry; (C) Detection of expressions of cleaved caspase-3 and γ-H2AX by western blot; (D) Effect of high expression of miR-361-5p on radiosensitivity of SW480 cells
StarBase prediction showed that lncRNA TRIM52- AS1 had a binding site with miR-361-5p, as shown in fig. 3. In cell experiment of co-transfected WT-lncRNA TRIM52-AS1, the relative luciferase activity of cells in the miR-361-5p group was decreased compared with that in the miR-NC group (p<0.05), as shown in Table 7. These results indicate that lncRNA TRIM52-AS1 can bind miR-361-5p in a targeted manner. Compared with pcDNA group, the expression of miR-361-5p in pcDNA-lncRNA TRIM52-AS1 group was decreased (p<0.05). Compared with the si-NC group, the expression of miR-361-5p in the si group was increased (p<0.05), as shown in Table 8.
| Group | Luciferase activity | |
|---|---|---|
| WT-lncRNA TRIM52-AS1 | MUT-lncRNA TRIM52-AS1 | |
| miR-NC | 1.00±0.10 | 1.00±0.08 |
| miR-361-5p | 0.42±0.04* | 1.03±0.11 |
| t | 16.155 | 0.662 |
| p | 0.000 | 0.518 |
Note: Compared with miR-NC group, *p<0.05
Table 7: Double Luciferase Activity Detection (x̄±s, n=9)
| Group | miR-361-5p |
|---|---|
| pcDNA | 1.00±0.08 |
| pcDNA-lncRNA TRIM52-AS1 | 0.45±0.04* |
| si-NC | 1.02±0.08 |
| si-lncRNA TRIM52-AS1 | 1.93±0.16# |
| F | 338.340 |
| p | 0.000 |
Note: Compared with pcDNA group, *p<0.05; compared with si-NC group, #p<0.05
Table 8: Expression of lncRNA TRIM52-AS1 Detected by Western Blot (x̄±s, n=9)
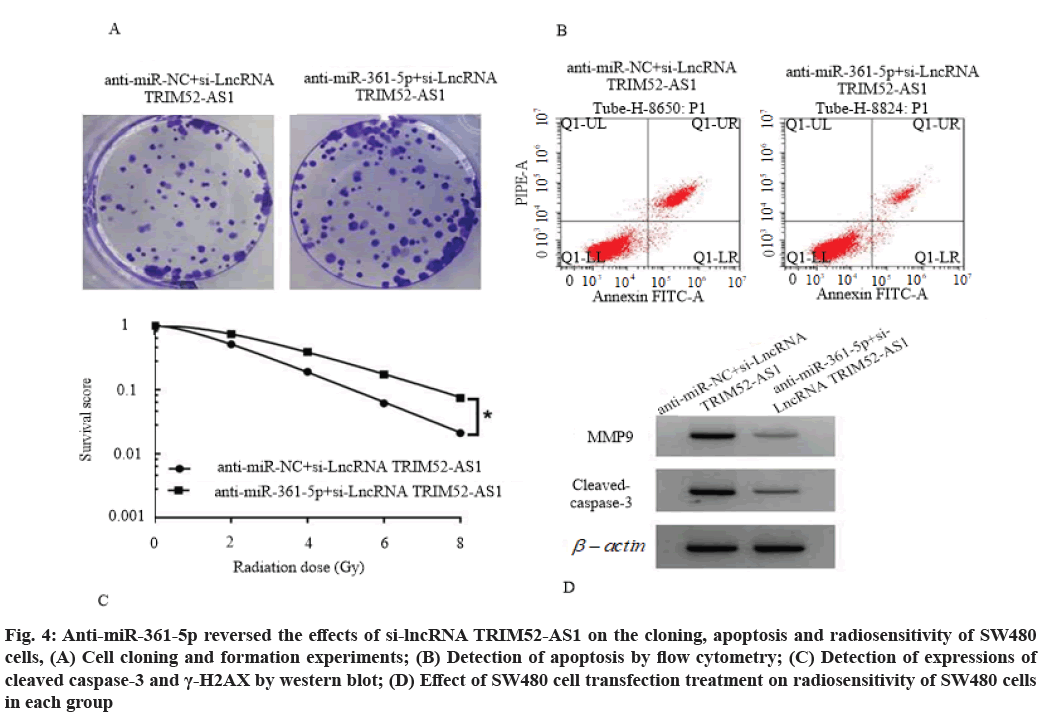
Anti-miR-361-5p reversed the effects of si-lncRNA TRIM52-AS1 on proliferation, cloning, apoptosis and radiosensitivity of SW480 cells. Compared with the anti-miR-NC+si-lncRNA TRIM52-AS1 group, the cell viability of anti-miR-361-5p+si-lncRNA TRIM52-AS1 group was increased (p<0.05) and the number of cell clone formation was increased (p<0.05), cell apoptosis rate, cleaved caspase-3 and γ-H2AX protein levels were decreased (p<0.05), cell survival score was increased (p<0.05), SER was 0.782 (<1), as shown in fig. 4, Table 9 and Table 10.
| Group | lncRNA TRIM52-AS1 | Cleaved caspase-3 | γ-H2AX | A value | Cell clone number | Apoptosis rate (%) |
|---|---|---|---|---|---|---|
| Anti-miR-NC+si-lncRNA TRIM52-AS1 | 1.00±0.10 | 0.82±0.07 | 0.76±0.06 | 0.534±0.05 | 64±4.86 | 23.68±1.91 |
| Anti-miR-361-5p+si-lncRNA TRIM52-AS1 | 0.43±0.03* | 0.38±0.03* | 0.43±0.03* | 1.086±0.10* | 127±10.02* | 8.05±0.62* |
| t | 16.379 | 17.332 | 14.758 | 14.812 | 16.971 | 23.350 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 9: Anti-miR-361-5p Reversed the Effects of si-lncRNA TRIM52-AS1 on Proliferation, Cloning, Apoptosis and Radiosensitivity of SW480 Cells (x̄±s, n=10)
| Group | D0 (Gy) | Dq (Gy) | N | SF2 | k | SER (Do ratio) |
|---|---|---|---|---|---|---|
| Anti-miR-NC+si-lncRNA TRIM52-AS1 | 1.810 | 1.058 | 1.794 | 0.514 | 0.552 | / |
| Anti-miR-361-5p+si-lncRNA TRIM52-AS1 | 2.315 | 2.113 | 2.491 | 0.744 | 0.432 | 0.782 |
Note: D0 is mean lethal dose of cells; Dq is quasi-threshold dose; N is extrapolation number; SF2 is surviving fraction at 2 Gy; k is passivation constant; SER is sensitization enhancement ratio
Table 10: Parameter Values of the Single-Click Multi-Target Model of SW480 Cells Transfected with X-Ray Irradiation in Each Group
Fig. 4: Anti-miR-361-5p reversed the effects of si-lncRNA TRIM52-AS1 on the cloning, apoptosis and radiosensitivity of SW480 cells, (A) Cell cloning and formation experiments; (B) Detection of apoptosis by flow cytometry; (C) Detection of expressions of cleaved caspase-3 and γ-H2AX by western blot; (D) Effect of SW480 cell transfection treatment on radiosensitivity of SW480 cells in each group
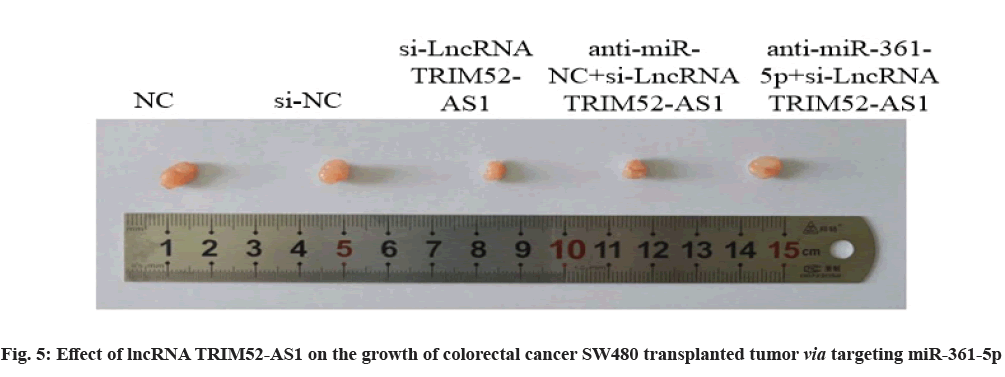
Effect of lncRNA TRIM52-AS1 on the growth of colorectal cancer transplanted tumor SW480 via targeting miR-361-5p is explained clearly. Compared with si-NC group, the volume and weight of transplanted tumor in si-lncRNA TRIM52-AS1 group were decreased (p<0.05). Compared with the anti-miRNC+ si-lncRNA TRIM52-AS1 group, the volume and weight of the transplanted tumor in the anti-miR-361-5p+si-lncRNA TRIM52-AS1 group were increased (p<0.05), as shown in fig. 5 and Table 11.
| Group | Volume/mm3 | Weight/mg |
|---|---|---|
| NC | 315.26±18.23 | 435.25±38.16 |
| si-NC | 320.53±28.13 | 431.02±29.63 |
| si-lncRNA TRIM52-AS1 | 163.25±10.53* | 243.26±18.43* |
| Anti-miR-NC+si-lncRNA TRIM52-AS1 | 158.06±14.13 | 248.06±16.03 |
| Anti-miR-361-5p+si-lncRNA TRIM52-AS1 | 375.02±31.95# | 405.61±34.25# |
| F | 180.979 | 106.021 |
| p | 0.000 | 0.000 |
Note: Compared with si-NC group, *p<0.05; Compared with si-lncRNA TRIM52-AS1 group, #p<0.05
Table 11: Effects of lncRNA TRIM52-AS1 on the Growth of Colorectal Cancer Transplanted Tumor SW480 via Targeting miR-361-5p (x̄±s, n=9)
LncRNA is widely present in colorectal cancer and plays a role in the occurrence and development of colorectal cancer by regulating gene transcription. For example, lncRNA Neighbor of BRCA1 Gene 2 (NBR2) inhibits the migration and invasion of colorectal cancer cells by down-regulating the expression of miR-21[7]. LncRNA Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) promotes the proliferation, invasion and migration of colorectal cancer cells by regulating SRY-Box Transcription Factor 9 (SOX9) [8]. LncRNA AC009022.1 promotes the expression of Actin Related Protein 3B (ACTR3B) by inhibiting the expression of miR-497-5p, thereby promoting the proliferation, migration and invasion of colorectal cancer cells[9]. LncRNA Small Nucleolar RNA Host Gene 5 (SNHG5) affects the proliferation and migration of colorectal cancer cells by regulating miR-132-3p/ CAMP Responsive Element Binding Protein 5 (CREB5) [10]. LncRNA SNHG7 can act as a sponge molecule of miR-216b to promote the proliferation of colorectal cancer cells[11]. LncRNA Special AT-Rich Sequence- Binding Protein 2 (SATB2)-AS1 inhibits colorectal cancer cell metastasis by regulating SATB2[12].
LncRNA TRIM52-AS1 is over expressed in Hepatocellular Carcinoma (HCC) and inhibition of its expression can inhibit the proliferation and metastasis of HCC cells[13]. However, the expression of lncRNA TRIM52-AS1 in colorectal cancer remains unknown. The results of this study showed that the expression level of lncRNA TRIM52-AS1 increased in colorectal cancer tissues and cell lines and interfering the expression of lncRNA TRIM52-AS1 can reduce the viability of colorectal cancer cells and the number of cell clone formation. These results suggest that interfering lncRNA TRIM52-AS1 expression can inhibit the proliferation and clonal formation of colorectal cancer cells. Cytochrome C can be released after activation of the mitochondrial pathway, which can activate caspase-3 to form cleaved caspase-3 and induce apoptosis[14]. Results of this study showed that apoptosis rate and cleaved caspase-3 protein levels were increased after interfering lncRNA TRIM52-AS1 expression, suggesting that interfering lncRNA TRIM52-AS1 expression can promote apoptosis of colorectal cancer cells. H2AX is a member of H2A family. Ionizing radiation can promote the phosphorylation of H2AX to form γ-H2AX and H2AX can play an anti-tumor role by inducing cell cycle arrest. Increased expression of γ-H2AX indicates enhanced radiosensitivity. The results of this study showed that after interfering with lncRNA TRIM52- AS1 expression, colorectal cancer cell survival score was reduced, sensitization ratio was higher than 1 and γ-H2AX protein level was increased, which suggest that interfering lncRNA TRIM52-AS1 expression can enhance the radiosensitivity of colorectal cancer cells.
This study further confirmed that lncRNA TRIM52- AS1 can target miR-361-5p and negatively regulate the expression of miR-361-5p. Studies have shown that miR-361-5p inhibits gastric cancer cell migration by inhibiting Wingless-Related Integration Site (Wnt)/β- catenin signaling pathway[15,16]. miR-361-5p inhibits the proliferation and metastasis of lung cancer cell by targeting Forkhead Box M1 (FOXM1)[17]. miR- 361-5p inhibits retinoblastoma cell proliferation and induces apoptosis by negatively regulating Claudin 8 (CLDN8)[18]. The results of this study showed that the expression of miR-361-5p was decreased in colorectal cancer tissues and cell lines, and the up-regulation of miR-361-5p could inhibit the proliferation and clonal formation of colorectal cancer cells, promote cell apoptosis, and enhance the radiosensitivity of cells. However, inhibiting lncRNA TRIM52-AS1 expression restored the effect of interfering the proliferation, apoptosis and radiosensitivity of colorectal cancer cells. At the same time, in vivo animal experiments confirmed that interfering lncRNA TRIM52-AS1 expression could inhibit the growth of colorectal cancer transplanted tumor in nude mice, while inhibiting miR- 361-5p expression could reduce the inhibitory effect of interfering lncRNA TRIM52-AS1 expression on the growth of colorectal cancer transplanted tumor in nude mice.
In conclusion, lncRNA TRIM52-AS1 expression is up-regulated in colorectal cancer tissues and cell lines. However, the down-regulated expression of miR-361- 5p and interfering with the expression of lncRNA TRIM52-AS1 can inhibit the proliferation, clonal formation and apoptosis of colorectal cancer cells by promoting the expression of miR-361-5p and enhance the radiosensitivity of colorectal cancer cells. LncRNA TRIM52-AS1 may be a potential therapeutic target for colorectal cancer and a potential target for reversing the radiosensitivity of colorectal cancer cells. However, the effects of lncRNA TRIM52-AS1 on the biological behavior of other colorectal cancer cell lines and the radiosensitivity of cells still need to be further explored.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bian Z, Zhang J, Li M, Feng Y, Wang X, Zhang J, et al. LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res 2018;24(19):4808-19.
[Crossref] [Google Scholar] [PubMed]
- Ding D, Li C, Zhao T, Li D, Yang L, Zhang B. LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on Wnt signaling. Mol Cells 2018;41(5):423-35.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Lai Q, He J, Li Q, Ding J, Lan Z, et al. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int J Med Sci 2019;16(1):51-9.
- Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu XY, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol Cancer 2017;16(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Zhou C, Chen Z, Peng C, Chen C, Li H. Long noncoding RNA TRIM52-AS1 sponges miR-514a-5p to facilitate hepatocellular carcinoma progression through increasing MRPS18A. Cancer Biother Radiopharm 2021;36(2):211-9.
[Crossref] [Google Scholar] [PubMed]
- Ma F, Song H, Guo B, Zhang Y, Zheng Y, Lin C, et al. MiR-361-5p inhibits colorectal and gastric cancer growth and metastasis by targeting staphylococcal nuclease domain containing-1. Oncotarget 2015;6(19):17404-16.
[Crossref] [Google Scholar] [PubMed]
- Bai J, Xu J, Zhao J, Zhang R. LncRNA NBR2 suppresses migration and invasion of colorectal cancer cells by downregulating miRNA-21. Hum Cell 2020;33(1):98-103.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Zhang X, Hu X, Zhou W, Zhang P, Zhang J, et al. The effects of lncRNA MALAT1 on proliferation, invasion and migration in colorectal cancer through regulating SOX9. Mol Med 2018;24(1):52-62.
[Crossref] [Google Scholar] [PubMed]
- Yu C, Zhang F. LncRNA AC009022.1 enhances colorectal cancer cells proliferation, migration, and invasion by promoting ACTR3B expression via suppressing miR‐497‐5p. J Cell Biochem 2020;121(2):1934-44.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Li Y, Wang H, Yu W, Lin S, Guo J. LncRNA SNHG5 affects cell proliferation, metastasis and migration of colorectal cancer through regulating miR-132-3p/CREB5. Cancer Biol Ther 2019;20(4):524-36.
[Crossref] [Google Scholar] [PubMed]
- Shan Y, Ma J, Pan Y, Hu J, Liu B, Jia L. LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis 2018;9(7):1-3.
- Xu M, Xu X, Pan B, Chen X, Lin K, Zeng K, et al. LncRNA SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol Cancer 2019;18(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Wu Y, Liu S, Dai Y. Long non-coding RNA TRIM52-AS1 promotes growth and metastasis via miR-218-5p/ROBO1 in hepatocellular carcinoma. Cancer Manag Res 2021;13:547-58.
[Crossref] [Google Scholar] [PubMed]
- Zhang P, Li R, Xiao H, Liu W, Zeng X, Xie G, et al. BRD4 Inhibitor AZD5153 suppresses the proliferation of colorectal cancer cells and sensitizes the anticancer effect of PARP inhibitor. Int J Biol Sci 2019;15(9):1942-54.
[Crossref] [Google Scholar] [PubMed]
- Liu YJ, Guo RX, Han LP, Gu H, Liu MZ. Effect of CASC19 on proliferation, apoptosis and radiation sensitivity of cervical cancer cells by regulating miR-449b-5p expression. Chin J Obstet Gynecol 2020;55(1):36-44.
[Crossref] [Google Scholar] [PubMed]
- Tian L, Zhao Z, Xie L, Zhu J. MiR-361-5p inhibits the mobility of gastric cancer cells through suppressing epithelial-mesenchymal transition via the Wnt/β-catenin pathway. Gene 2018;675:102-9.
[Crossref] [Google Scholar] [PubMed]
- Hou XW, Sun X, Yu Y, Zhao HM, Yang ZJ, Wang X, et al. miR-361-5p suppresses lung cancer cell lines progression by targeting FOXM1. Neoplasma 2017;64(4):526-34.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Lu B, Wang X, Jiang H, Kuang W. MiR-361-5p inhibits cell proliferation and induces cell apoptosis in retinoblastoma by negatively regulating CLDN8. Childs Nerv Syst 2019;35(8):1303-11.
[Crossref] [Google Scholar] [PubMed]