- *Corresponding Author:
- W. Fu
The First Clinical Medical College,
Nanjing University of Chinese Medicine,
Nanjing 210023,
P.R.China
E-mail: dr.wysh@163.com
|
This article was originally published in a special issue, “Novel Therapeutic Approaches in Biomedicine and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2021:83(6) Spl Issue “145-150” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
We aimed to evaluate the effects of mecobalamin on the cerebral ischemia-reperfusion injury of spontaneously hypertensive stroke prone rats. 72 male rats were randomly divided into control group, model group, nimodipine group, high-dose mecobalamin group, middle-dose mecobalamin group and low-dose mecobalamin group (n=12). All groups were administrated intragastrically every morning once a day for 7 continuous days. The model of 2 h of focal cerebral ischemia and 24 h of reperfusion was established by blocking middle cerebral artery. Neurological deficits were graded with reference to the Longa method. Cerebral edema was determined using the wet-dry weighing method. The volume of cerebral infarction was measured by triphenyltetrazolium chloride staining and the levels of tumour necrosis factor alpha, interleukin-1 beta and interleukin-8 were measured by radioimmunoassay. High and middle-dose mecobalamin significantly reduced the volume of cerebral infarction, alleviated cerebral edema and improved nerve function compared with those of the model group (p<0.05). Compared with the model group, high and middle-dose mecobalamin significantly reduced the levels of tumour necrosis factor alpha, interleukin-1 beta and interleukin-8 in cerebral tissue (p<0.05). Mecobalamin protected spontaneously hypertensive stroke prone rats from cerebral ischemia-reperfusion injury, which may be related to the decreased levels of tumour necrosis factor alpha, interleukin-1 beta and interleukin-8 in cerebral tissue.
Keywords
Cerebral ischemia, interleukin, mecobalamin, reperfusion, tumour necrosis factor alpha
Ischemic cerebrovascular disease, which is a common complication of hypertension with high incidence, morbidity and mortality rates, has seriously threatened the health of human. The treatment mainly aims to restore cerebral perfusion and prevent cerebral injury [1]. The reperfusion after cerebral ischemia can save dying cells, but it aggravates the injury of ischemic cells simultaneously [2]. Therefore, preventing ischemia reperfusion injury is crucial in the treatment of hypertension complicated with ischemic cerebrovascular disease [3]. Spontaneously Hypertensive Stroke Prone (SHRSP) rats, based on the pathological changes of cerebrovascular disease similar to those of human primary hypertension, have similar pathophysiological process to that of people after cerebral ischemia [4]. Mecobalamin, the chemical component of which is Alpha (α)-(5,6-dimethyl- benzimidazol-yl)-Co-methyl-cobamide, promotes the synthesis of nucleic acid and protein, axonal transport and regeneration. This study, on the basis of a thrombotic cerebral ischemia-reperfusion injury model of middle cerebral artery of SHRSP rats, aims to observe the effects of mecobalamin on cerebral ischemia- reperfusion injury and the levels of Tumour Necrosis Factor Alpha (TNF-α), Interleukin-1 Beta (IL-1β) and Interleukin-8 (IL-8) in the cerebral tissue of SHRSP rats after cerebral ischemia-reperfusion injury, so as to provide experimental evidence for the prevention and treatment of cerebral ischemia-reperfusion injury with mecobalamin.
Materials and Methods
Drugs and reagents:
Mecobalamin (0.5 mg/tube, batch No.:110615) was purchased from Shandong Haishan Medicine Co., Ltd. Nimodipine (5 mg/tube, batch No.: 100828) was bought from Tianjin Pharmaceutical Group Xinzheng Co., Ltd. Triphenyltetrazolium Chloride (TTC) was acquired from Sigma (USA). TNF-α kit had a measurement accuracy of 2.5 ng/l with intra-assay variation <10 % and inter-assay variation <15 %. IL-1β kit had a measurement accuracy of 1.5 ng/l with intra-assay variation <12 % and inter-assay variation <13 %. IL-8 kit had a measurement accuracy of 1.5 ng/l with intra- assay variation <7 % and inter-assay variation <15 %.
Animal grouping and administration:
72 Specific Pathogen-Free (SPF) male SHRSP rats aged 12 w with the body weights of 230 to 260 g were purchased from the Experimental Animal Center of Lanzhou University, Animal certificate: GWXA (Gan) 20103100. The rats were randomly divided into 6 groups (n=12): A control group, a model group, a nimodipine group 5 mg/(kg/d)], a high-dose Mecobalamin group [MecoH, 5 mg/(kg/d)], a middle- dose Mecobalamin group [MecoM, 2 mg/(kg/d)] and a low-dose Mecobalamin group [MecoL, 1 mg/ (kg/d)]. The control and model groups were given the same amounts of normal saline. All groups were administrated intragastrically with normal saline or corresponding drugs every morning, once a day for 7 continuous days.
Rat tail artery blood pressure measurement:
The rat tail artery systolic pressure was measured non- invasively in the first administration as well as after 2 h of cerebral ischemia and 24 h of reperfusion by using an RBP-Ñ computer blood pressure and heart rate meter.
Establishment of the cerebral ischemia-reperfusion injury model:
The cerebral artery occlusion model was established according to the suture method in literature 1 h after the final administration [5]. The rats were weighed and then intraperitoneally injected with 350 mg/kg 10 % chloral hydrate. After anesthesia, the rats were fixed on the autopsy table for rats in a supine position. An incision was made in the neck midline and the neck skin was cut open to separate cephalic artery, external and internal carotid arteries respectively. A 0.2 mm small cut was sheared open in the external carotid artery about 2 cm away from its proximal end, an embolism line was inserted from the small cut in the external carotid artery to gently push the end of the line and slowly push the line by 8 mm ~22 mm in the direction of the internal carotid artery into the cranium along the internal carotid artery via the cephalic artery bifurcation, which was stopped in case of slight resistance. Then the middle cerebral artery was blocked, when the time was recorded. The embolism line was pulled out slowly 2 h later for re-canalizing the cephalic artery with the internal carotid artery, achieving middle cerebral artery ischemia reperfusion for 24 h. The control group was only subjected to being exposed and separated the cephalic artery, internal and external carotid arteries and the middle cerebral artery was not embolized. The room temperature was strictly controlled at 23°~25° to maintain the rectal temperature of the rats at about 37°. After cerebral ischemia for 2 h and reperfusion for 24 h, the rats survived are 8, 4, 6, 6, 7 and 6 respectively in the control group, model group, nimodipine group, MecoH group, MecoM group and MecoL group. Two rats were randomly selected from the surviving rats of each group for future experiments.
Neurological deficit grading:
After 24 h of reperfusion, neurological deficits were graded referring to the Zea-Longa score method. 0 points: Rats were not observed with symptoms of neurological deficit and their activities were normal; 1 point: Rats could not fully extend the contralateral forelimb to the surgery; 2 points: Rats made circles towards the contralateral side at the time of crawling;
3 points: The body of rats inclined towards the contralateral side when walking; 4 points: Rats could not spontaneously walk, progressing to coma.
Determination of cerebral edema using the wet-dry weighting method:
After neurological deficits grading, the rats were decapitated to remove the whole brain and then the ischemic side of the brain was taken to remove the cerebellum, low brainstem and olfactory bulb and the water content of cerebral tissue was calculated using the wet-dry weighting method.
Cerebral water content (%)=(Brain wet weight-dry weight)/Brain wet weight×100 %. The extent of cerebral edema was expressed by the percentage of cerebral water content.
Determination of infarct volume percentage:
After 24 h of reperfusion, the rats were decapitated to remove the brain and then the cerebellum, low brainstem and olfactory bulb were removed. The brain was cut along the coronal plane into five slices with the thickness basically the same. The slices were placed in 3 ml of 2 % TTC solution and incubated in dark for 30 min. The normal brain tissue was red and the infarcted tissue was white. Pictures were taken by a digital camera and analyzed using an image processing software to obtain the percentage of infarct volume to the total volume of slice of the five brain slices [6].
Determination of TNF-α, IL-1β and IL-8 levels:
After 24 h of reperfusion, the rats were decapitated to remove the brain. Approximately 100 mg cerebral cortex was taken from the ischemic area, made into 10 % tissue homogenate with normal saline in an ice bath and centrifuged at 2000 r/min at 4° for 10 min. The levels of TNF-α, IL-1β and IL-8 were measured by radioimmunoassay [7], which was in strict accordance with the kit instructions.
Statistical analysis:
The measurement data were expressed as (x±s). The model and control groups were compared by the t test. The model and other dose groups were compared by one-way Analysis of Variance (ANOVA). The means of all groups were compared by the Least Significant Difference (LSD)-t method, p<0.05 was considered statistically significant.
Results and Discussion
Effects of mecobalamin on the systolic pressure of tail artery were explained here. There were no statistically significant differences in the tail artery blood pressures between the rats of all groups before the first administration (p>0.05). After 2 h of cerebral ischemia and 24 h of reperfusion, the tail artery blood pressures of the rats in the MecoL, MecoM and MecoH groups were not significantly different from that in the model group (p>0.05), but the tail artery blood pressure of the nimodipine group slightly decreased (p<0.05) (Table 1).
| Group | Before first administration | After 24 h of reperfusion |
|---|---|---|
| Control | 222.34±9.55 | 227.62±9.08 |
| Model | 219.13±7.36 | 230.92±8.04 |
| MecoL group | 217.47±10.48 | 245.17±11.53 |
| MecoM group | 211.12±9.29 | 237.63±12.57 |
| MecoH group | 225.35±7.57 | 247.44±7.07 |
| Nimodipine | 221.42±10.58 | 200.56±6.44a |
Note: Compared with the model group, ap<0.05
Table 1: Effects of Mecobalamin on the Systolic Pressure Of Tail Artery (x±s) (mmHg)
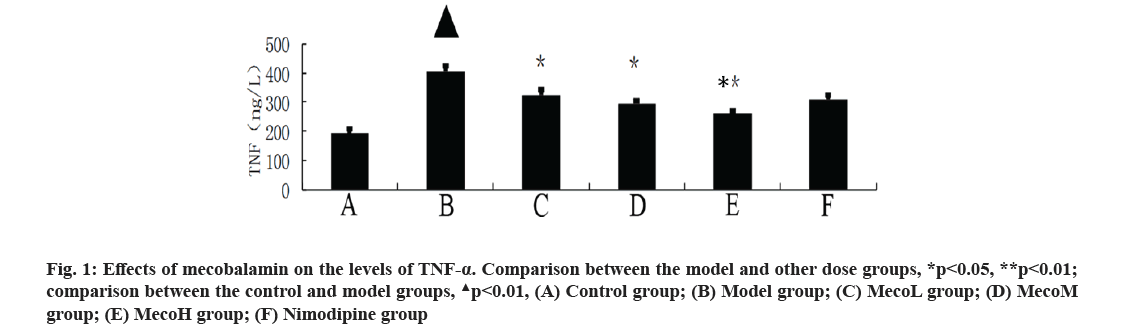
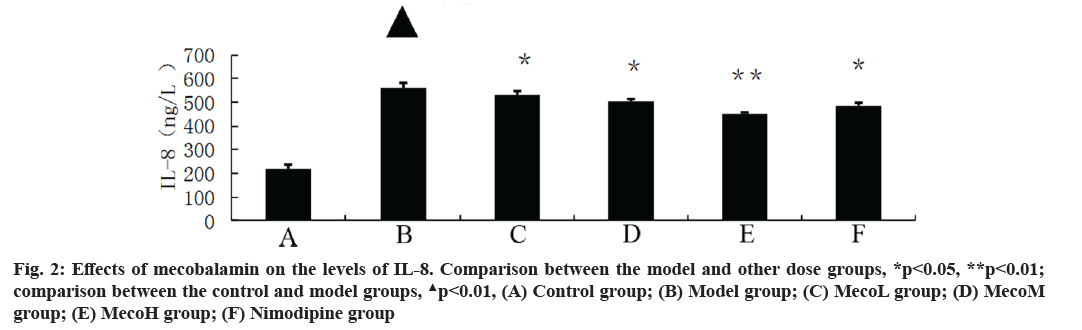
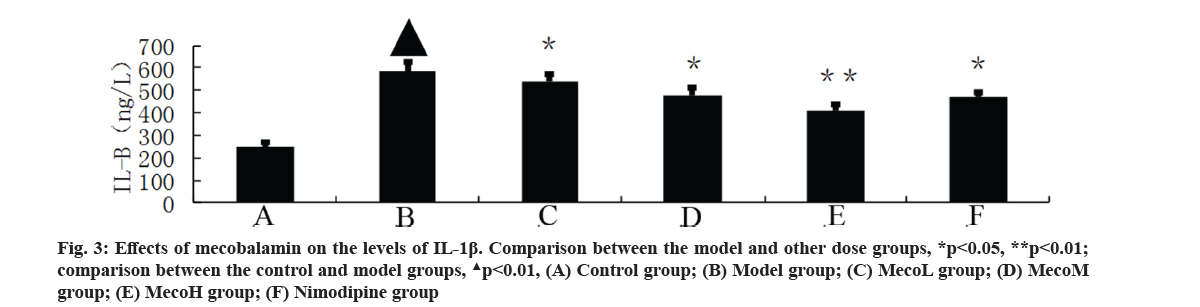
Effects of mecobalamin on nerve function and cerebral edema were explained here. The neurological defect score and brain water content in percentage (%) were significantly higher in the model group than those in the control group (p<0.01). The neurological defect scores and brain water contents (%) were significantly decreased in the MecoM and MecoH groups and the nimodipine group compared with those in the model group (p<0.01 or 0.05) (Table 2). The volume of cerebral infarction of the model group was higher than that of the control group (p<0.01). The volume of cerebral infarction were significantly lower in the MecoM and MecoH groups and the nimodipine group compared with those in the model group (p<0.01 or 0.05) (fig. 1-fig. 3).
| Group | Neurological deficit grading (score) | Brain water content (%) | Cerebral infarction volume (%) |
|---|---|---|---|
| Control | 0 | 78.54±3.41 | 0 |
| Model | 2.20±0.51a | 91.43±2.52a | 53.56±3.84a |
| MecoL group | 2.09±0.34 | 88.46±2.73 | 43.85±4.15 |
| MecoM group | 1.71±0.40b | 78.93±1.95b | 25.22±3.42b |
| MecoH group | 0.52±0.31a | 64.53±2.81a | 10.35±3.74a |
| Nimodipine | 1.39±0.82b | 82.26±2.27b | 19.84±2.40b |
Note: Compared with the control group, ap<0.01; compared with the model group, bp<0.05
Table 2: Effects of Mecobalamin on Neurological Deficit Grading, Brain Water Content and Infarction Volume (x±s, n=12)
Effects of mecobalamin on levels of TNF-α, IL-1β and IL-8 were shown here. The levels of TNF-α, IL- 1β and IL-8 in the cerebral tissue of the model rats were significantly elevated compared with those of the control rats (p<0.01). However, those levels in the cerebral tissue of the rats in the MecoM and MecoH groups and the nimodipine group were significantly lower than those in the model group (p<0.01 or 0.05) Inflammatory factors play an important role in cerebral ischemia-reperfusion injury and over-expression of inflammatory factors may aggravate cerebral hypoxic-ischemic injury. The cascade effect formed by uncontrolled release of local IL-8, IL-1β and TNF-α in early cerebral ischemia-reperfusion is the main reason for brain cell damage [8]. IL-8, which is a leukocyte synthesized and released under the induction of hypoxia and ischemia by multiple cells such as monocytes, neutrophils, polymorphonuclear leukocytes, endothelial cells, etc., is a key factor mediating neutrophil aggregation. It can, not only serve as a chemotactic factor and activated factor of neutrophils, increase neutrophils expression of adhesion molecules and enhance the activity of cell adhesion on neutrophil surface, but also can make neutrophil activation and chemotaxis and induce respiratory burst, release lysosomal enzymes, to produce large amounts of free radicals, proteolytic enzymes, superoxide, hydrogen peroxide and other inflammatory mediators [9]. IL-1β, which mainly exists in cerebral tissue [10] and is synthesized and secreted by glial cells, neuron cells and endothelial cells [11], can not only promote B and T cell activation in synergy with other cytokines, but also can induce the generation of other inflammatory mediators and strengthen the adhesion of leukocytes with endothelial cells [12]. IL- 1β can also promote inflammatory reaction, increase the release of excitatory amino acids and oxygen free radicals, activate endothelial cells to produce a variety of tissue factors to participate in cerebral ischemia- reperfusion injury [13]. TNF-α, as a cytokine with a wide range of biological activities [14], is a key inflammatory mediator in ischemic cerebral injury that is secreted by activated macrophages and monocytes. It is mainly associated with inflammation and immune response, performing the function of increasing the permeability of vascular endothelial cells and inducing the expression of cell adhesion molecules [15]. TNF-α can activate polymorphonuclear leukocytes, increase the expression of leukocyte-endothelial cell adhesion molecules and promote leukocyte adhesion in capillaries and gradually penetrate into the cerebral tissue [16]. TNF-α has an effect of chemotaxis on neutrophils, monocytes and lymphocytes, which can make aggregation and infiltration of leukocytes in cerebral tissue and produce toxic products such as superoxide ion [17]. TNF-α can promote nerve cells to express inducible nitric oxide synthase to produce excessive amounts of nitric oxide and induce the formation of free radicals to exacerbate oxidative damage [18]. TNF-α can induce the release of excitatory amino acids and promote cascade reaction of calcium overloading injury after cerebral ischemia [19]. IL-1β and TNF-α can also activate other cells to produce a variety of cytokines, such as induction of IL-8 synthesis [20]. Interaction among IL-1β, TNF-α and IL-8 forms network effects for synergistic proinflammation to cause local inflammation expansion, so as to aggravate cerebral injury [21]. This study showed that the cerebral tissue of rats in the model group suffered from large infarct volume and significant neurological deficits, indicating that the modeling of focal cerebral ischemia was successful. The expression of TNF-α, IL-1β and IL-8 was significantly increased in the rats of the model group, which is consistent with previous literatures [22-24]. The MecoL had not obviously protective effect on cerebral ischemia-reperfusion injury. While MecoM and nimodipine had a certain protective effect on it and could reduce the expression of TNF-α, IL-1β and IL-8 in ischemic brain tissue. The MecoH could significantly alleviate the neurological deficits and brain edema of rats after cerebral ischemia-reperfusion, reduce infarct volume of cerebral tissue and inhibit the expression of TNF-α, IL-1β and IL-8 in cerebral tissue after ischemia reperfusion.
In summary, by inhibiting the expression of TNF-α, IL- 1β and IL-8 in ischemic brain tissue, mecobalamin can suppress the infiltration of inflammatory cells in cerebral tissue after cerebral ischemia-reperfusion, inhibit the generation of adhesion cytokines, reduce the formation of oxygen free radicals and the release of excitatory amino acids, inhibit cascade reaction of inflammation after cerebral ischemia-reperfusion, exerting protective effects on cerebral ischemia-reperfusion injury upon SHRSP. Further in-depth studies are ongoing in our group.
Author’s contributions:
Xiusheng Zhang and Xuanjing Sun contributed equally
to this work.
Acknowledgements:
This study was not financially supported. All procedures were performed in Department of Acupuncture and Encephalopathy, Xuzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine (China) according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH publication No. 85-23, revised in 1996). The protocol was reviewed and approved by the animal’s ethics committee of our hospital and great efforts have been made to minimize animals suffering.
Xiusheng Zhang and Xuanjing Sun are responsible for acquisition of data, analysis and interpretation of data, manuscript preparation, critical revision and final approval; Weimin Fu is responsible for substantive scientific and intellectual contributions to the study, conception and design, critical revision and final approval.
Conflicts of interest:
The authors declared no conflict of interest.
References
- Ginsberg MD. Expanding the concept of neuroprotection for acute ischemic stroke: The pivotal roles of reperfusion and the collateral circulation. Prog Neurobiol 2016;145:46-77.
- Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab 2016;23(2):254-63.
- Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N, et al. Post ischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol 2013;23(1):34- 44.
- Shcherbak N, Popovetsky M, Galagudza M, Barantsevitch E, Shlyakhto E. The infarct?limiting effect of cerebral ischaemic post conditioning in rats depends on the middle cerebral artery branching pattern. Int J Exp Pathol 2013;94(1):34-8.
- Kim J, Kim MY, Leem KH, Moon S, Jamakattel-Pandit N, Choi H, et al. Key compound groups for the neuroprotective effect of roots of Polygonum cuspidatum on transient middle cerebral artery occlusion in Sprague-Dawley rats. Nat Prod Res 2010;24(13):1214-26.
- Jiang LJ, Zhang SM, Li CW, Tang JY, Che FY, Lu YC. Roles of the Nrf2/HO-1 pathway in the anti-oxidative stress response to ischemia-reperfusion brain injury in rats. Eur Rev Med Pharmacol Sci 2017;21(7):1532-40.
- Ye Y, Li J, Cao X, Chen Y, Ye C, Chen K. Protective effect of n-butyl alcohol extracts from Pinelliae pedatisectae rhizoma against cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol 2016;188:259-65.
- Jiang X, Kuang G, Gong X, Jiang R, Xie T, Tie H, et al. Glycyrrhetinic acid pretreatment attenuates liver ischemia/ reperfusion injury via inhibiting TLR4 signaling cascade in mice. Int Immunopharmacol 2019;76:105870.
- Sumanasinghe RD, Pfeiler TW, Monteiro?Riviere NA, Loboa EG. Expression of proinflammatory cytokines by human mesenchymal stem cells in response to cyclic tensile strain. J Cell Physiol 2009;219(1):77-83.
- Youn YA, Kim SJ, Sung IK, Chung SY, Kim YH, Lee IG. Serial examination of serum IL?8, IL?10 and IL?1Ra levels is significant in neonatal seizures induced by hypoxic–ischaemic encephalopathy 1. Scand J Immunol 2012;76(3):286-93.
- Pott GB, Chan ED, Dinarello CA, Shapiro L. α?1?Antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol 2009;85(5):886-95.
- Herseth JI, Refsnes M, Lag M, Hetland G, Schwarze PE. IL-1β as a determinant in silica-induced cytokine responses in monocyte-endothelial cell co-cultures. Hum Exp Toxicol 2008;27(5):387-99.
- Lin Z, Zhu D, Yan Y, Yu B. Herbal formula FBD extracts prevented brain injury and inflammation induced by cerebral ischemia–reperfusion. J Ethnopharmacol 2008;118(1):140-7.
- Fraczek M, Sanocka D, Kamieniczna M, Kurpisz M. Proinflammatory cytokines as an intermediate factor enhancing lipid sperm membrane peroxidation in in vitro conditions. J Androl 2008;29(1):85-92.
- Dong XR, Wang JN, Liu L, Chen X, Chen MS, Chen J, et al. Modulation of radiation-induced tumour necrosis factor-α and transforming growth factor β1 expression in the lung tissue by Shengqi Fuzheng injection. Mol Med Rep 2010;3(4):621-7.
- Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 2011;152(2):419-27.
- Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, et al. TNF-α in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J Exp Med 2010;222(4):251- 63.
- Mashukova A, Wald FA, Salas PJ. Tumor necrosis factor alpha and inflammation disrupt the polarity complex in intestinal epithelial cells by a post translational mechanism. Mol Cell Biol 2011;31(4):756-65.
- Zou J, Wang YX, Dou FF, Lü HZ, Ma ZW, Lu PH, et al. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int 2010;56(4):577-84.
- Riera KM, Rothfusz NE, Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Interleukin-1, tumor necrosis factor-alpha, and transforming growth factor-beta 1 and integrative meniscal repair: Influences on meniscal cell proliferation and migration. Arthritis Res Ther 2011;13(6):1-20.
- Li F, Wang L, Li JW, Gong M, He L, Feng R, et al. Hypoxia induced amoeboid microglial cell activation in postnatal rat brain is mediated by ATP receptor P2X4. BMC Neurosci 2011;12(1):1-11.
- Ahnstedt H, Stenman E, Cao L, Henriksson M, Edvinsson L. Cytokines and growth factors modify the upregulation of contractile endothelin ETA and ETB receptors in rat cerebral arteries after organ culture. Acta Physiol (Oxf) 2012;205(2):266-78.
- Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Tsuchihashi Y, et al. Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J Neuroinflammation 2011;8(1):1-10.
- Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Inflammation in ischemic stroke subtypes. Curr Pharm Des 2012;18(28):4289-310.