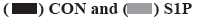- *Corresponding Author:
- Hui Xu
Department of Internal Medicine, Clinical Medical College, Changsha Health Vocational College, Changsha 410100, PR China
E-mail: xulu434912211@163.com
| This article was originally published in a special issue,“Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “127-135” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effects and mechanism of sphingosine-1-phosphate/sphingosine-1-phosphate receptor on pro-angiogenesis in human colon cancer cells is the main objective. The effects of sphingosine-1-phosphate on the pro-angiogenesis in human colon cancer cells HT-29 and SW480 were assessed by tube formation assay. Quantitative real-time polymerase chain reaction was used to detect the expression of interleukin-8, interleukin-6 and vascular endothelial growth factor in HT29 and SW480 cells after sphingosine-1-phosphate treatment. Small interfering ribonucleic acid was designed to silence the expression of sphingosine-1- phosphate receptor 1, sphingosine-1-phosphate receptor 2 and sphingosine-1-phosphate receptor 3 in HT-29 and SW480 cells. Western blot and quantitative real-time polymerase chain reaction were used to assess the down-regulation efficiency of sphingosine-1-phosphate receptor in HT-29 and SW480 cells. Later, the effects of sphingosine-1-phosphate receptor were detected by quantitative real-time polymerase chain reaction. Tube formation assay showed that the number of formed tubes in human umbilical vein endothelial cell-fused cells (EA.hy926) resuspended in the culture supernatant from HT-29 and SW480 cells treated with sphingosine-1-phosphate was significantly more than that of the control group (t=3.667 and 4.881, p=0.021 and 0.013), indicating that sphingosine-1-phosphate promoted the pro-angiogenic ability of colon cancer cells. Moreover, the messenger ribonucleic acid expression of interleukin-8, interleukin-6 and vascular endothelial growth factor were significantly increased after sphingosine-1-phosphate treatment compared to the control group (p<0.05) where sphingosine-1-phosphate receptor 1 gene silence could significantly decrease the messenger ribonucleic acid expression in HT-29 and SW480 cells (p<0.05), while sphingosine-1-phosphate receptor 2 and sphingosine-1-phosphate receptor 3 gene silence did not lead to significant messenger ribonucleic acid changes. Sphingosine-1-phosphate up-regulated the expression of interleukin-8, interleukin-6 and vascular endothelial growth factor in human colon cancer cells through sphingosine-1-phosphate receptor 1 and thereby enhancing the proangiogenic effect of colon cancer cells. The sphingosine-1-phosphate/sphingosine-1-phosphate receptor pathway is a promising novel therapeutic target for inhibiting colon cancer growth.
Keywords
Colon cancer, angiogenesis, sphingosine-1-phosphate, sphingosine-1-phosphate receptor
Angiogenesis, the process of forming new blood vessels and branches in tissues and gradually becomes mature, plays an important role in the biological process of tumors and is one of the hallmarks of malignant tumors[1,2]. Accumulative studies have found that the development of colon cancer is closely associated with the process of angiogenesis[3,4]. Angiogenesis in colon cancer provides nutritional support for tumor development and promotes the continuous growth of tumors. During tumor growth, colon cancer also promotes angiogenesis by secreting a series of factors into the tumor microenvironment[5]. However, the present studies on the mechanism of colon cancer and angiogenesis are far from perfect. Sphingosine-1-Phosphate (S1P), a well-known important pro-angiogenic factor, plays a crucial role in angiogenesis and maturation by binding to its receptor (Sphingosine-1-Phosphate Receptor, (S1PR))[6,7]. Studies have shown that S1P is critically involved in the development of colon cancer[8-10]. Based on the above research, it is speculated that the S1P/S1PR signaling pathway may be involved in the regulation of angiogenesis in colon cancer. Herein, in this study, we investigated the effects of S1P on the proangiogenic ability in human colon cancer cells in vitro and explored the effects of S1PR on the secretion of angiogenic factors in human colon cancer cells, thereby exploring the role and possible mechanism of S1P/S1PR pathway and angiogenesis in colon cancer.
Materials and Methods
Main materials:
Cells and main reagents: Human colon cancer cells like human colorectal adenocarcinoma cell line (HT-29), human colon adenocarcinoma cell line (SW480) and human umbilical vein endothelial cell-fused cells (EA.hy926) were purchased from the Cell Resource Center of the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. Roswell Park Memorial Institute (RPMI)-1640 medium and high-glucose Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone); Fetal Bovine Serum (FBS, Hyclone); antibiotics (penicillin, streptomycin, Hyclone); TRIzol (Invitrogen); S1P (Cayman Chemical Company); Prime ScriptTM Reverse Transcriptase (RT) reagent kit (Takara); SYBR Green Real-time Polymerase Chain Reaction (PCR) kit (Takara); Matrigel (BD); Calcein-AM (Dojindo Chemical Company); Rabbit anti-human Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), S1PR1, S1PR2, S1PR3, Cluster of Differentiation 34 (CD34) antibody (Cell Signaling Technology), cell culture dish (Corning) and culture plate (Corning) were purchased. Isopropanol, absolute ethanol, sodium chloride, etc. were domestic analytical reagent, PCR apparatus was Quant Studio Real Time PCR System (Applied Biosystems).
Sample collection:
A total of 67 post-operative paraffin-embedded tissue samples were collected from patients who underwent surgery in Xiangya Hospital of Central South University from 2012 to 2015. Detailed general information, tumor stage, differentiation, lymph node metastasis and distant metastasis were recorded in all samples. The tumor stage was assessed according to the Union for International Cancer Control (UICC). Among the 67 patients, there were 42 male patients, 25 females, with age ranging from 19 y to 85 y old (Mean age: 56.9 y old). In addition, there were 30 cases of well differentiated adenocarcinomas, 28 cases of moderately differentiated adenocarcinomas and 9 cases of poorly differentiated adenocarcinoma; 22 cases of Tumor, Nodes and Metastases (TNM) stage I, 17 cases of stage II, 18 cases of stage III and 10 cases of stage IV; 41 cases of lymph node metastasis and 26 cases without lymph node metastasis; 12 cases with distant metastasis and 55 cases without distant metastasis.
Methods:
Cell culture: Human colon cancer cells (HT-29, SW480) were cultured in RPMI-1640 medium containing 10 % FBS and human umbilical vein endothelial cell-fused cells were maintained in high-glucose DMEM containing 10 % FBS in a 37°, 5 % Carbon dioxide (CO2) incubator until cells reached 80 % to 90 % confluency. Cells were subsequently digested using 0.25 % trypsin digestion and cells in the logarithmic growth phase were used for subsequent assays.
Real-time quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR): Cells were washed with Phosphate Buffered Saline (PBS) to remove the residual medium and total Ribonucleic Acid (RNA) was extracted by TRIzol. The Prime ScriptTM RT reagent kit was used for the reverse transcription of messenger RNA (mRNA) into complementary Deoxyribonucleic Acid (cDNA) according to the manufacturer’s instruction. The mRNA level of each gene was detected by qRT-PCR using the SYBR method, with three replicate wells set for each sample. The reaction conditions were shown as follow: 95° for 30 s, 95° for 5 s, 60° for 30 s (the last two steps were conducted for 40 cycles). The above experiments were conducted in triplicate.
Tube formation assay: The 96-well plates were pre-cooled at -20° and each well was pre-coated with 50 μl Matrigel, followed by incubation at 37° for 30 min to solidify the gel. EA.hy926 cells in logarithmic growth phase were digested by trypsin, further resuspended in culture supernatant from HT29 cells, which were then seeded in Matrigel pre-coated 96-well plates at a cell number of 4×104 in 100 μl per well. The culture plate was placed in an incubator. After 6 h, Calcein Acetoxymethyl (Calcein-AM)/PBS solution was added, incubated at 37° in dark for 30 min, washed twice with PBS and followed by observation and photography under fluorescence microscope.
Cell transfection: HT-29 and SW480 cells were seeded at a density of 2×105/well in 6-well culture plates and used for transfection when the cell density reached 50 % to 70 %. Small interfering RNA (siRNA)-liposome complex transfection solution was prepared and incubated at room temperature for 20 min. 1.5 ml of serum-free RPMI-1640 medium was added to the 6-well plate, followed by addition of 500 μl transfection mixture and incubation for 4-6 h. Afterwards, the culture medium was changed into fresh RPMI-1640 medium. Subsequent assays were performed 48 h after cell transfection.
Western blot: After discarding culture medium, cells were washed with PBS for 2-3 times, added with Radioimmunoprecipitation Assay (RIPA) lysis containing 10 % Phenylmethylsulfonyl Fluoride (PMSF) and lysed on ice for 30 min, centrifuged at 12 000 r/min at 4° for 10 min, followed by collection of the supernatant and subsequent determination of the protein concentration by Bicinchoninic Acid (BCA) kit. Protein samples were separated by 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) with an initial voltage of 80 V, which was adjusted to 120 V when the markers were separated. The protein samples were transferred to the Polyvinylidene Fluoride (PVDF) membrane for 90 min at constant current of 200 mA. Afterwards, the membranes were blocked with 5 % Bovine Serum Albumin (BSA) at room temperature for 2 h, incubated with proper diluted primary antibody at 4° overnight according to the instructions. After washing with Tris Buffered Saline with Tween 20 (TBST) buffer, the membranes were incubated with secondary antibody at room temperature for 1 h and visualized by a fluorescent scanner.
Immunohistochemistry (IHC): The paraffin-embedded tissue samples were cut into 4 μm-thick sections and prepared for staining. The expression of CD34 and S1PR1 in tissues was determined by IHC (Support Pack (SP) method) by two senior pathologists. S1PR1 were positively stained in the cytoplasm. The criteria of the number of positive tumor cells were shown as follows. No positive cells: 0 points; <10 % positive: 1 point; 10 %-34 % positive: 2 points; 35 %-70 % positive: 3 points and >70 % positive: 4 points. The criteria of staining intensity of tumor cells were shown as follows. No color: 0 points; light yellow: 1 point; brown yellow: 2 points; brown: 3 points. Yellow or brown indicated positive staining, while no color suggested negative staining. The proportion of positive cells and staining intensity were multiplied: ≤4 points indicated low expression and ≥6 points indicated high expression. The CD34-labeled Microvessel Density (MVD) was counted under light microscope. First, neovascular active areas (hot spots) mainly consisting of capillaries and venules (microvessels) was selected under low magnification (100×), followed by counting the number of blood vessels in five consecutive fields of view for average value under high magnification (200×).
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 statistical software was used for statistical analysis. Quantitative data with normal distribution were shown as mean±standard deviation (x?±s), the difference between the two groups was compared by t-test. All data in the experiment were obtained after three independent repeated experiments, p<0.05 indicated statistical significance.
Results and Discussion
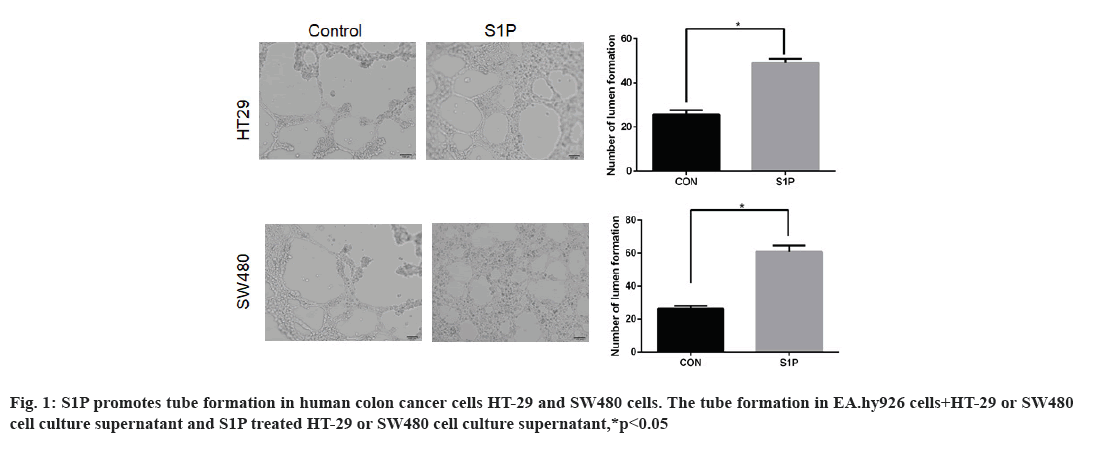
Effects of S1P on angiogenesis in human colon cancer cells HT-29 and SW480 is shown here. HT-29 and SW480 cells were treated with S1P (1 μmol/l for 6 h), further changed into fresh serum-free medium for 24 h. In addition, EA.hy926 cells were resuspended in blank Control (CON group) and culture supernatant from S1P (S1P group), cultured in Matrigel. The results of tube formation assay were shown in fig. 1. The number of formed tubes in the S1P group was significantly more than that in the CON group (52.8±9.1 vs. 28.3±4.7, t=3.667, p=0.021 in HT-29 cells, 68.4±5.2 vs. 27.6±3.1, t=4.881, p=0.013 in SW480 cells). The results indicated that S1P promoted the tube formation ability in human colon cancer cells.
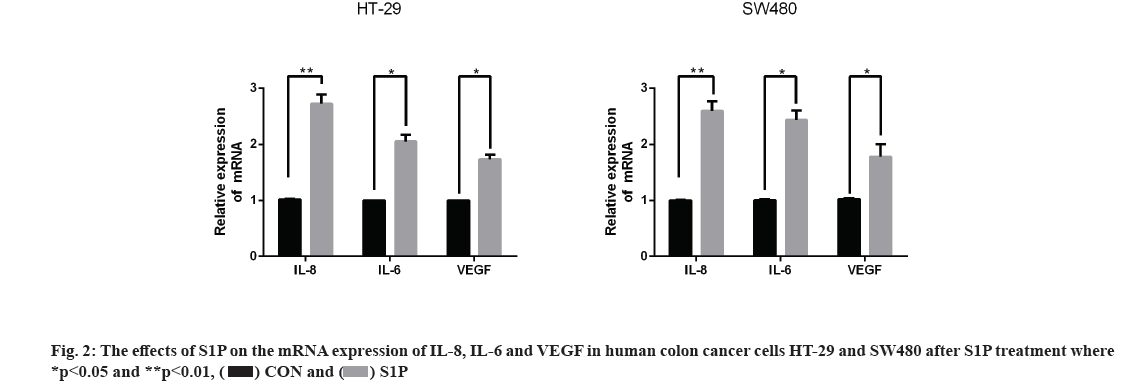
Effects of S1P on the mRNA expression of Interleukin (IL)-8, IL-6 and Vascular Endothelial Growth Factor (VEGF) in human colon cancer cells HT-29 and SW480 is as follow. qRT-PCR assay was used to detect the expression of angiogenic factors in HT-29 and SW480 cells after treatment of S1P (1 μmol/l for 6 h). As a result, the mRNA expression of IL-8, IL-6 and VEGF was significantly higher than the control group (p<0.05) as shown in fig. 2, indicating that S1P up-regulated the expression levels of IL-8, IL-6 and VEGF in HT-29 and SW480 cells.
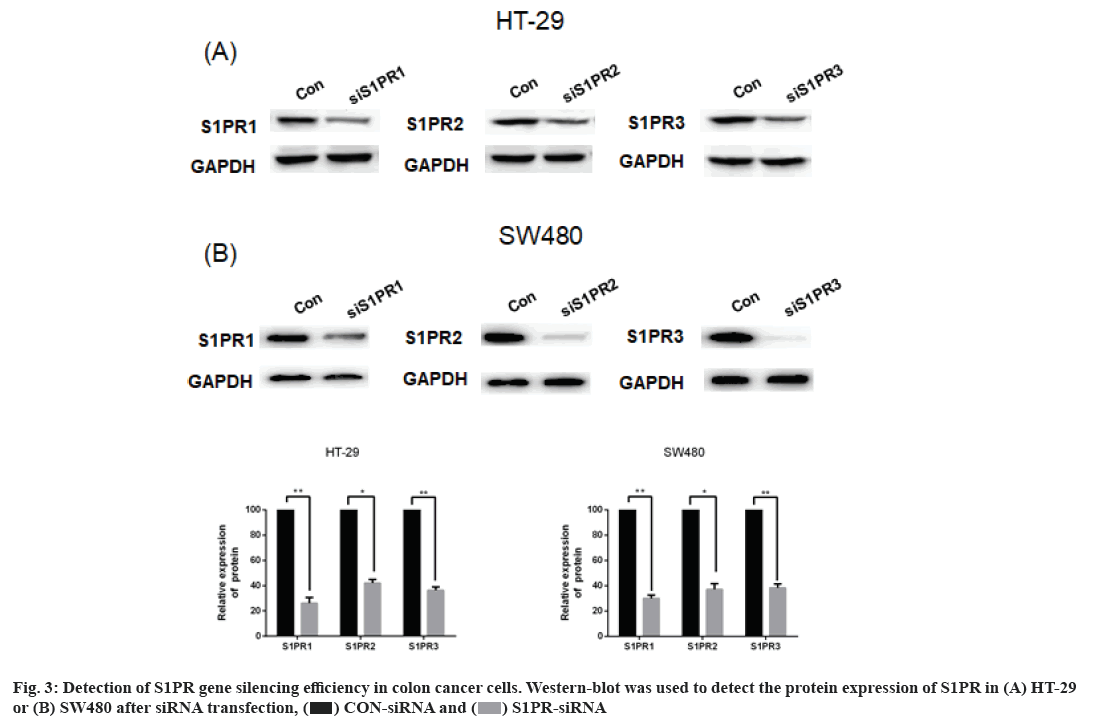
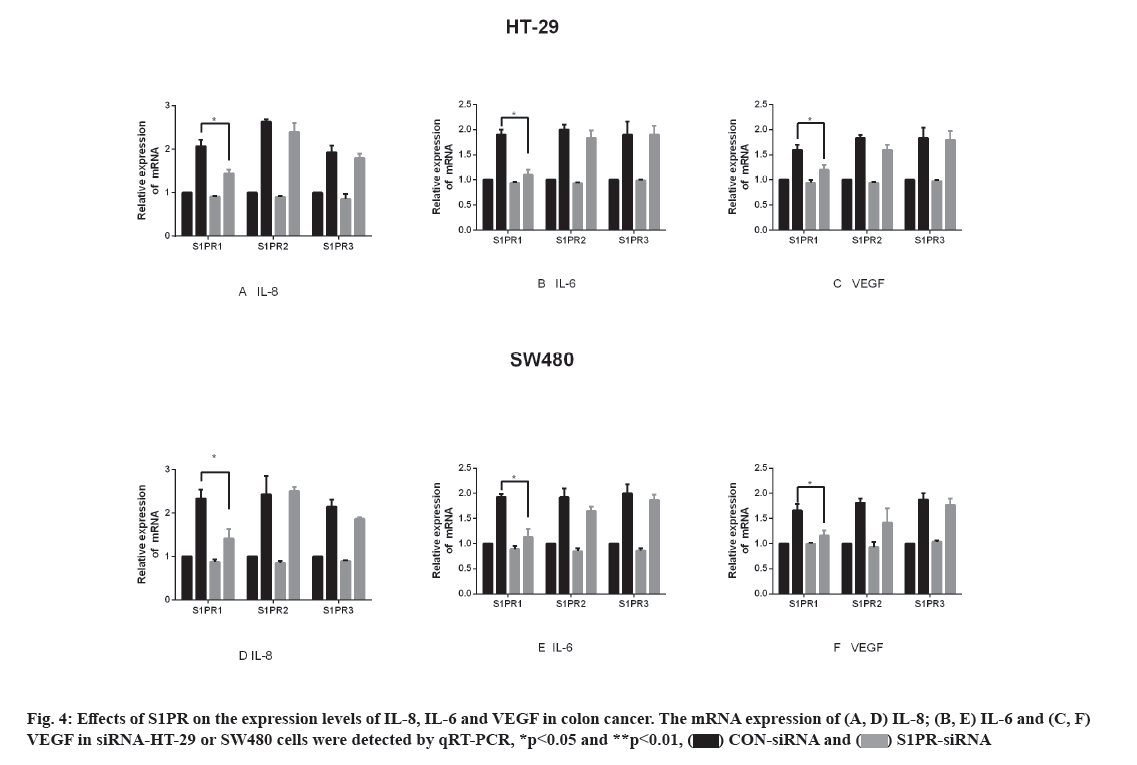
Effects of S1PR on the mRNA expression of IL-8, IL-6 and VEGF in human colon cancer cells are explained below. S1PR-siRNA was used to silence the expression of S1PR1, S1PR2 and S1PR3 in HT-29 or SW480, negative CON-siRNA was transfected into HT-29 or SW480 cells as control group, aiming to construct transiently transfected siS1PR-HT-29 or siS1PR-SW480 cell line. Afterwards, Western blot and qRT-PCR assays showed that the expression of S1PR was significantly down-regulated in siS1PR-HT-29 and siS1PR-SW480 group than that in CON-siRNA control group (fig. 3). S1P (1 μmol/l, 6 h) was used to treat siRNA-transfected HT-29 or SW480 cell line. qRT-PCR was subsequently used to detect the mRNA expression of IL-8, IL-6 and VEGF in S1P-treated siRNA-HT-29 or si-SW480, showing that the mRNA expression of IL-8, IL-6 and VEGF in S1PR1-silenced HT-29 or SW480 cell line were significantly decreased than that in the CON-siRNA control group (p<0.05), while the mRNA expression of IL-8, IL-6 and VEGF was not significantly changed after S1PR2 and 3 silencing compared with the control group (fig. 4). These above results indicated that S1P up-regulated the expression of IL-8, IL-6 and VEGF via S1PR1 in human colon cancer cell line HT-29 or SW480.
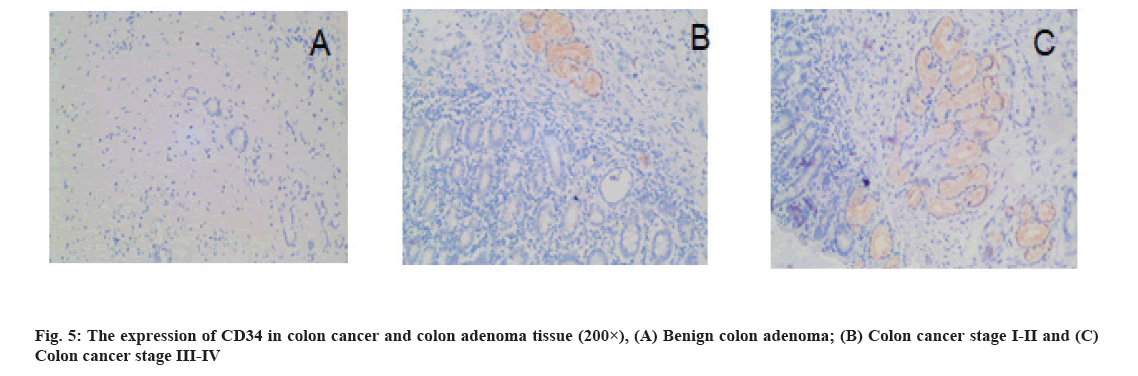
Results of MVD detection in colon cancer tissues is as follow. The CD34-labeled MVD in colon cancer tissues (25.18±9.25) was significantly higher than that in benign colon adenoma tissues (14.88±3.90) (p=0.000) (fig. 5). MVD was significantly associated with TNM stage, pathological grade, lymph node metastasis and distant metastasis (p<0.05) is shown in Table 1.
| Clinicopathological parameters | n | MVDCD34 | p | S1PR1 | χ2 | p | |
|---|---|---|---|---|---|---|---|
| Low expression | High expression | ||||||
| Age (y) | |||||||
| ≤54 | 15 | 24.93±10.7 | 0.757 | 8 | 7 | 0.047 | 0.828 |
| >54 | 36 | 25.83±8.83 | 18 | 18 | |||
| TNM stage | |||||||
| I~II | 24 | 22.42±9.57 | 0.021* | 18 | 6 | 10.47 | 0.00** |
| III~IV | 27 | 28.37±8.28 | 8 | 19 | |||
| Pathological classification | |||||||
| I | 13 | 19.62±7.48 | 0.006** | 11 | 2 | 7.898 | 0.005** |
| II~III | 38 | 27.61±9.08 | 15 | 23 | |||
| Lymph node metastasis | |||||||
| Yes | 9 | 34.44±12.28 | 0.031* | 1 | 8 | 5.149 | 0.023* |
| No | 42 | 23.67±7.44 | 25 | 17 | |||
| Distant metastasis | |||||||
| Yes | 8 | 37.63±7.07 | 0.000** | 1 | 7 | 3.944 | 0.047* |
| No | 43 | 23.33±7.88 | 25 | 18 | |||
Note: p<0.05 and **p<0.01
Table 1: The Expression of MVD and S1PR1 Colorectal Cancer Tissue
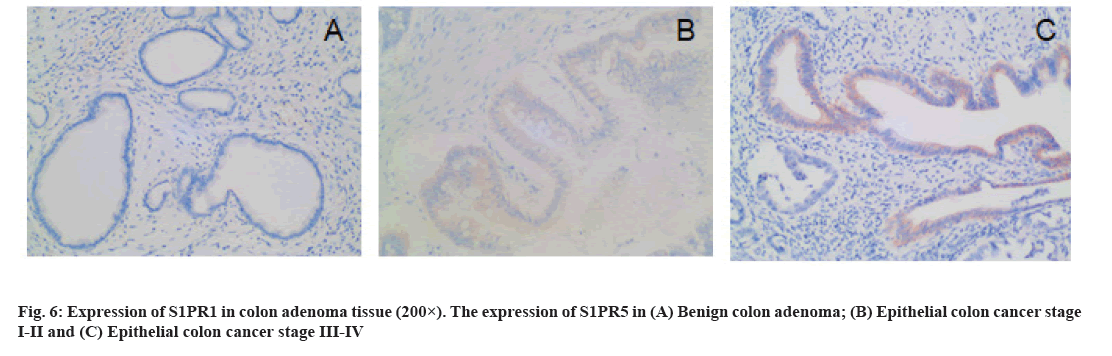
The expression of S1PR1 in colon cancer tissues is shown below. There were 25 cases and 3 cases of high expression of S1PR1 in patients with colon cancer and benign colon adenoma, respectively, with statistical significance (χ2=4.587, p=0.032). In colon cancer tissues, the expression of S1PR1 was significantly associated with TNM stage, pathological grade, lymph node metastasis and distant metastasis (p<0.05) (fig. 6 and Table 1).
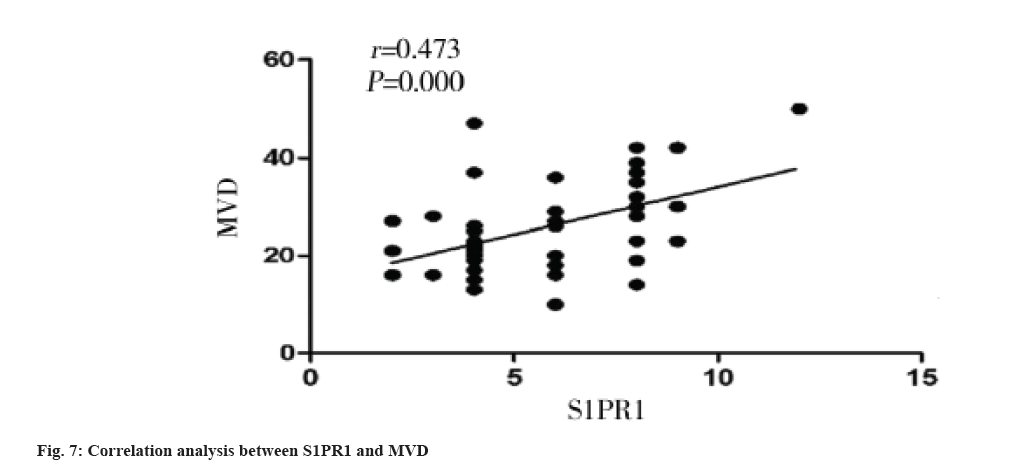
Correlation between S1PR1 expression and MVD in colon cancer tissues is shown here. The MVD was significantly higher in S1PR1 high expression group than that in S1PR1 low expression group in colon cancer tissue (p=0.005) (Table 2). MVD was significantly and positively correlated with S1PR1 expression (r=0.473, p=0.000) (fig. 7).
| Expression | n | MVDCD34 | p | |
|---|---|---|---|---|
| S1PR1 | Low expression | 26 | 22.08±8.18 | 0.005** |
| High expression | 25 | 29.20±9.17 |
Note: **p<0.01
Table 2: The Correlation Between S1PR1 Expression and MVD in Colon Cancer Tissue
Colon cancer is a common malignancy of the digestive tract[11], due to the insidious symptoms in the early stage and lack of typical clinical manifestations and diagnostic methods with satisfactory specificity and sensitivity. Approximately 75 % of colon cancer patients are burdened with advanced stage (TNM III-IV stages) at the initial diagnosis, therefore, the prognosis of these patients is extremely poor, with the 5 y survival rate of only about 30 %[12]. At present, the low survival rate of colon cancer is mainly caused by the easy to metastasize and wide spread. The growth, infiltration and metastasis of tumors depend on angiogenesis and tumor angiogenesis is a complicated process involving multiple types of cells and molecules[13,14].
S1P is an important sphingomyelin metabolite of cell membrane, which is produced by Sphingosine Kinase (SphK) via the catalyzing sphingosine, thereby involving in cell proliferation, apoptosis, angiogenesis, etc. and subsequently regulating tumorigenesis and tumor progression[15,16]. S1PR is a member of the G Protein-Coupled Receptor (GPCR) family. The S1P/S1PR pathway plays an important role in angiogenesis and S1P/S1PR has been confirmed to play regulatory roles in the process of angiogenesis under physiological conditions, as well as other pathological processes including tumor and inflammation[17,18]. S1P is involved in the proliferation, migration and survival of endothelial cells, capillary formation and maturation of new vessels by binding its receptor S1PR to subsequently regulate downstream molecular pathways. However, at present, the role and mechanism of S1P/S1PR pathway in angiogenesis of colon cancer have not been reported at home and abroad.
In this study, EA.hy926 cell line was used as a model in the tube formation assay, HT-29 and SW480 cells were treated with S1P, whose culture supernatant could promote the tube formation of EA.hy926 cell line, that is to say, S1P enhanced the angiogenic ability of HT-29 and SW480 cells. Studies have shown that a variety of biological active substances can regulate tumor angiogenesis, these pro-angiogenic factors are mainly polypeptides of growth factors or cytokines, some small molecules of lipids, nucleotides and vitamins[19]. Among them, VEGF plays an important role in the angiogenesis, S1P has been shown to promote the overexpression of VEGF in human umbilical vein endothelial cells[20]. Apart from VEGF, IL-8 is another important pro-angiogenic factor in tumor and S1P antagonists effectively inhibits IL-8 secretion in colon cancer, indicating that S1P is closely associated with IL-8 in colon cancer. According to the results of previous experiments, it is speculated that S1P might act on colon cancer cells to over-express certain angiogenic factors, thereby promoting the tube formation in EA.hy926 cells. In this study, qRT-PCR was used to detect the changes of certain angiogenic factors after S1P treatment in HT-29 and SW480 cells. As a result, the expression levels of IL-8, IL-6 and VEGF were significantly increased, indicating that S1P may promote the overexpression of IL-8, IL-6 and VEGF in HT-29 and SW480 cells, thereby enhancing the pro-angiogenic ability of HT-29 and SW480 cells.
S1P exerts most biological effects mainly by binding to S1PR. Currently, five subtypes of S1PR have been discovered, including S1PR1 and S1PR5[21]. S1PR1, S1PR2 and S1PR3 are widely expressed in various types of cells, while S1PR4 and S1PR5 are mainly found in tissues such as the immune system and nervous system[22]. Multiple studies have shown that S1PR1, S1PR2 and S1PR3 regulate angiogenesis[23]. In this study, we constructed a transiently transfected siS1PR-HT-29 or SW480 cell line to silence the expression of S1PR to investigate the possible roles of S1PR in angiogenesis of colon cancer. As a result, when the expression of S1PR1 was inhibited, the expression levels of IL-8, IL-6 and VEGF were decreased; while the silence of S1PR2 and S1PR3 gene did not affect the overexpression of IL-8, IL-6 and VEGF induced by S1P treatment in HT-29 and SW480. Therefore, S1P promoted the overexpression of pro-angiogenic factors IL-8, IL-6 and VEGF via S1PR1 in human colon cancer cells, thereby promoting tumor angiogenesis.
In this study, we found that CD34-labeled MVD was significantly higher in colon cancer tissues than that in benign colon adenomas and MVD was significantly associated with clinical stage, pathological grade, lymph node metastasis, and distant metastasis of colon cancer. The correlation between S1PR and tumor angiogenesis was first found in lung cancer model that S1PR1 was involved in tumor angiogenesis[24]. S1PR1 deletion has been reported to cause maturation disorder of angiogenesis in breast cancer, thereby effectively inhibiting tumor growth and distant metastasis[25]. In this study, we demonstrated that S1PR1 was highly expressed in colon cancer tissues, S1PR1 expression was significantly correlated with clinical stage, pathological grade, lymph node metastasis and distant metastasis of colon cancer. A large number of previous studies have shown that S1PR1 can promote tumor angiogenesis[26]. In this study, we found that S1PR1 expression was significantly and positively correlated with MVD in colon cancer tissue, indicating the important correlation between S1PR1 expression and microvessel formation in colon cancer tissue, however, the specific molecular mechanism remains largely undefined. The poor prognosis of colon cancer is associated with tumor angiogenesis, indicating that S1PR1 is a promising prognostic marker for colon cancer and that it is of great therapeutic significance to explore novel anti-tumor angiogenesis drugs targeting S1PR1 in colon cancer.
In summary, S1P might promote the pro-angiogenic ability of colon cancer by overexpression of IL-8, IL-6 and VEGF through S1PR1. The growth, metastasis and invasion of colon cancer depend on tumor angiogenesis. Our present study provides a new research direction for inhibiting angiogenesis in colon cancer and the S1P/S1PR pathway is a promising new therapeutic target to suppress colon cancer growth.
Acknowledgements:
The present study was supported by the science and education Joint project of Natural Science Foundation of Hunan Province (No: 2018JJ5073) and Hunan Provincial Health Commission Project (No: 202203033582).
Conflict of interests:
The authors declare that they have no competing interests.
References
- Hoff PM, Machado KK. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat Rev 2012;38(7):825-33.
[Crossref] [Google scholar] [PubMed]
- Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines 2017;5(2):34.
[Crossref] [Google scholar] [PubMed]
- Frank RE, Saclarides TJ, Leurgans S, Speziale NJ, Drab EA, Rubin DB. Tumor angiogenesis as a predictor of recurrence and survival in patients with node-negative colon cancer. Ann Surg 1995;222(6):695-9.
[Crossref] [Google scholar] [PubMed]
- Murakami K, Sakukawa R, Sano M, Hashimoto A, Shibata J, Yamada Y, et al. Inhibition of angiogenesis and intrahepatic growth of colon cancer by TAC-101. Clin Cancer Res 1999;5(9):2304-10.
[Google scholar] [PubMed]
- Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem 2011;286(28):25007-15.
[Crossref] [Google scholar] [PubMed]
- Argraves KM, Wilkerson BA, Argraves WS. Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J Biol Chem 2010;1(10):291-7.
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 2000;106(8):951-61.
[Google scholar] [PubMed]
- Schwiebs A, Herrero san Juan M, Schmidt KG, Wiercinska E, Anlauf M, Ottenlinger F, et al. Cancer-induced inflammation and inflammation-induced cancer in colon: A role for S1P lyase. Oncogene 2019;38(24):4788-803.
[Crossref] [Google scholar] [PubMed]
- Suh JH, Saba JD. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: The fat’s in the fire. Transl Cancer Res 2015;4(5):469-83.
[Crossref] [Google scholar] [PubMed]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate is a missing link between chronic inflammation and colon cancer. Cancer Cell 2013;23(1):5-7.
[Crossref] [Google scholar] [PubMed]
- Thapa R, Lakhey M, Yadav PK. Clinico-pathological study of colorectal carcinoma. JNMA J Nepal Med Assoc 2013;52(191):449-52.
[Google scholar] [PubMed]
- Morris M, Platell C, McCaul K, Millward M, van Hazel G, Bayliss E, et al. Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int J Colorectal Dis 2007;22(8):887-95.
[Crossref] [Google scholar] [PubMed]
- Hida K, Maishi N, Torii C, Hida Y. Tumor angiogenesis-Characteristics of tumor endothelial cells. Int J Clin Oncol 2016;21(2):206-12.
[Crossref] [Google scholar] [PubMed]
- Tseng JC, Chen HF, Wu KJ. A twist tale of cancer metastasis and tumor angiogenesis. Histol Histopathol 2015;30(11):1283-94.
[Crossref] [Google scholar] [PubMed]
- Xie Z, Liu H, Geng M. Targeting sphingosine-1-phosphate signaling for cancer therapy. Sci China Life Sci 2017;60(6):585-600.
[Crossref] [Google scholar] [PubMed]
- Nakajima M, Nagahashi M, Rashid OM, Takabe K, Wakai T. The role of sphingosine-1-phosphate in the tumor microenvironment and its clinical implications. Tumor Biol 2017;39(4):1-9.
[Crossref] [Google scholar] [PubMed]
- Takuwa N, Okamoto Y, Yoshioka K, Takuwa Y. Vascular endothelial S1P 2 receptor limits tumor angiogenesis and hyperpermeability. In: Yokomizo T, Murakami M, editors. Bioactive Lipid Mediators. Tokyo: Springer; 2015. p. 237-52.
- Mukhopadhyay P, Ramanathan R, Takabe K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast Cancer Manag 2015;4(5):241-4.
[Crossref] [Google scholar] [PubMed]
- Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: Vascular endothelial growth factor and beyond. Semin Oncol 2014; 41(2):235-51.
[Crossref] [Google scholar] [PubMed]
- Heo K, Park KA, Kim YH, Kim SH, Oh YS, Kim IH, et al. Sphingosine 1-phosphate induces vesicular endothelial growth factor expression in endothelial cells. BMB Rep 2009;42(10):685-90.
[Crossref] [Google scholar] [PubMed]
- Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J Clin Invest 2015;125(4):1379-87.
[Crossref] [Google scholar] [PubMed]
- Patmanathan SN, Wang W, Yap LF, Herr DR, Paterson IC. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell Signal 2017;34:66-75.
[Crossref] [Google scholar] [PubMed]
- Marfe G, Mirone G, Shukla A, di Stefano C. Sphingosine kinases signalling in carcinogenesis. Mini Rev Med Chem 2015;15(4):300-14.
[Crossref] [Google scholar] [PubMed]
- Tabasinezhad M, Samadi N, Ghanbari P, Mohseni M, Saei AA, Sharifi S, et al. Sphingosin 1-phosphate contributes in tumor progression. J Cancer Res Ther 2013;9(4):556-63.
[Crossref] [Google scholar] [PubMed]
- Sarkisyan G, Gay LJ, Nguyen N, Felding BH, Rosen H. Host endothelial S1PR1 regulation of vascular permeability modulates tumor growth. Am J Physiol Cell Physiol 2014;307(1):C14-24.
[Crossref] [Google scholar] [PubMed]
- Gaengel K, Niaudet C, Hagikura K, Laviña B, Muhl L, Hofmann JJ, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 2012;23(3):587-99.
[Crossref] [Google scholar] [PubMed]