- *Corresponding Author:
- J. H. Zhang
Department of Pharmacy, Zhangpu County Hospital, Zhangzhou, Fujian 363200, China
E-mail: 15080350679@139.com
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “179-185” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To compare the safety and efficacy of clopidogrel and ticagrelor among patients with cytochrome P450 2C19*2 and *3 mutations, and to assess the necessity of pharmacogenetic testing is the objective of the study.The study is a two-center retrospective, observational cohort study of 425 patients. Compare the differences of patients with different genotypes in the rehospitalization owing to original disease, major adverse cardiovascular events, initial readmission duration, frequency and all-cause mortality, dyspnea, minor hemorrhagic incidents, as well as significant hemorrhagic incidents.No significant differences were observed between the two groups in terms of rehospitalization incidence and duration due to the primary disease, recurrent myocardial infarction, revascularization, stent thrombosis, as well as ischemic stroke (p>0.05). Nevertheless, ticagrelor-treated patients experienced a higher incidence of dyspnea events compared to those treated with clopidogrel (43.1 % vs. 31.7 %, p=0.039). Furthermore, the incidence of minor bleeding was similar between both groups (6.6 % vs. 2.8 %, p=0.184) and no major bleeding events were reported. In patients with cytochrome P450 2C19 gene deletion, clopidogrel and ticagrelor have similar efficacy, but clopidogrel has less dyspnea. Therefore, there is no more benefit from replacing clopidogrel with ticagrelor.
Keywords
Clopidogrel, ticagrelor, cytochrome P450 2C19 gene detection, mutations
In Cytochrome P450 2C19 (CYP2C19) gene polymorphisms, Loss-of-Function Single Nucleotide Polymorphisms (LoF SNPs) are most commonly found in CYP2C19*2 and *3. Among them, CYP2C19*2 mutation outcomes from the G681A mutation in exon 5, whereas *3 arises from the G636A mutation in exon 4, which eventually leads to the expression of inactive enzymes[1-4]. The rate of presence of LoF SNPs varies from race to race, reaching as high as 65 % among East Asians and about 30 % among whites[5]. The metabolism of clopidogrel slows down due to the degradation or inactivity of the corresponding protein in individuals with CYP2C19*2 and *3 mutations, and cannot make clopidogrel fully converted into active metabolites by CYP2C19 metabolism, thus reducing its antiplatelet effect[4,6]. But ticagrelor did not undergo CYP2C19 metabolism and the data from the platelet function test showed that it worked faster[7]. Therefore, there is a literature recommending that patients with gene deletion switch to other antiplatelet drugs such as ticagrelor[4]. However, a study of patients with coronary heart disease showed no difference in net clinical benefits between patients with CYP2C19 gene dysfunction receiving clopidogrel and ticagrelor treatment[8]. And there are relevant literatures that the conventional implementation of CYP2C19 gene typography to guide the choice of antiplatelet therapy is still controversial[9].
The purpose of this study is to discuss the need for genetic testing before selecting clopidogrel or ticagrelor by comparing their clinical benefits among patients with CYP2C19 gene deletion.
Materials and Methods
Study design and participants:
This is a two-center retrospective, observational cohort study from Fujian Medical University Union Hospital and Zhangzhou Hospital of traditional Chinese Medicine to collect all patients with CYP2C19 gene deletion using clopidogrel or ticagrelor from June 1, 2018 to June 30, 2020. Patients who continue to use clopidogrel are set as observation groups and patients whose genetic results are medium-slow metabolism are changed to use ticagrelor are set as control groups. Individuals carrying one or two copies of CYP2C19 without functional variation are considered medium or slow metabolites. Individuals with normal function and functional enhancement or two features to increase the copy of the variant are called fast or ultrafast metabolites. This study has been approved by the Institute’s Hospital Ethics Committee (2020KY0135).
Inclusion and exclusion criteria of patients:
Inclusion criteria: Patients insisting on taking clopidogrel or ticagrelor for at least half a year after discharge from the hospital; patients whose CYP2C19 gene test results show slow or medium metabolism; patients whose case data are sufficient for follow-up study; patients who agree to the follow-up and patients with at least 18 y of age.
Exclusion criteria: Patients with a history of major bleeding within the first 6 mo of admission, example; confirmed gastrointestinal bleeding, intracranial bleeding; patients unable to provide complete information for the study during follow-up; patients suffering from mental illness; patients whose case data are recorded as suffering from moderate and severe liver insanity and patients who miss the service more than or equal to 4 times a month.
Research outcomes:
The main efficacy endpoints of this study are when patients are hospitalized again for the original disease (re-hospitalization due to the original disease, e.g., coronary heart disease, stroke, reoccurrence hospitalization) and Major Adverse Cardiovascular Events (MACE) including recurrent infarction, blood transport reconstruction (Acute Coronary Syndrome, ACS), patients are hospitalized again for chest tightness and imaging found infarction in other areas and Percutaneous Coronary Intervention (PCI) surgery, stent thrombosis (imaging diagnosed stent thrombosis) and ischemic stroke. The endpoint of secondary efficacy is the time and number of first readmissions and all-cause deaths. Safety outcome indicators include breathing difficulties, minor bleeding events and severe bleeding events due to any cause. Dyspnea is a condition during follow-up that is known to the patient or his family about chest tightness and breathing difficulties. Hemorrhage is defined as fatal bleeding; bleeding from important areas or organs, such as intracranial, intraocular, peritoneal, intra-joint, heart bag or osteofascial compartment syndrome; hemoglobin reduction ≥20 g/l (1.24 mmol/l), or infusion ≥2 units of red blood cells, minor bleeding and other bleeding events that do not meet the criteria for hemorrhage[10].
Data collection:
Extract relevant information from the hospital information system, demographic information includes patient sex, age, height, weight and history of smoking or drinking; clinical information includes combined diseases including diabetes, hypertension, heart failure, arrhythmia and past medical history; high uric acid, digestive tract disease (acute and chronic gastroenteritis, ulcers, atrophic gastritis, etc.) and patient history of drug combination.
Clinical-related events are obtained through follow-up. 425 selected patients were followed up by telephone to understand and record the occurrence of patient outcome indicators. Follow-up to this study ends on December 31, 2020.
Statistical analysis:
The statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) 25.0 statistical software. Continuous variables such as age, height, weight, Body Mass Index (BMI) are expressed in mean±standard deviation. Intergroup mean comparisons are used in two independent sample t-tests. Classified variables (e.g., gender and combined disease, etc.) are expressed in numbers and percentages. Intergroup comparisons are expressed using the chi square test or Fisher’s accurate probability test. The difference between p<0.05 is statistically significant. Kaplan-Meier analysis was employed to estimate the relationship between various clinical events and time over a 30 mo period, and to determine the total number of events observed at each endpoint within the study. Kaplan-Meier estimates were plotted for incidence at the time of the primary therapeutic endpoint (whether or not to be hospitalized again and the MACE).
Results and Discussion
Follow-up data was shown here. In this study, we followed up to 425 patients, 44 in the clopidogrel group and 42 patients in ticagrelor group with incomplete information respectively, and 10 and 20 patients stopped taking their medications. These patients were excluded from further analysis. No significant difference was observed between the efficacy rates of the two patient groups (72.9 % vs. 72.4 %; p=0.912). Ultimately, the study included 167 patients in the ticagrelor group and 142 in the clopidogrel group. The comparison between the two groups, ticagrelor and clopidogrel, revealed no statistically significant difference (14.24±7.28 vs. 15.25±6.81; p=0.209).
Patient demographic and baseline characteristics are shown in Table 1.The ticagrelor group consisted of 167 patients, including 154 men and 13 women, with a mean age of 60.80±9.88 y. In the clopidogrel group, there were 142 patients, comprising 127 men and 15 women, with a mean age of 67.05±11.21 y. No significant differences were found in the demographic characteristics of the two patient groups, such as gender, height, weight, BMI, smoking history and alcohol consumption (p>0.05). However, the age of patients in the clopidogrel group was significantly higher than that in the ticagrelor group (p<0.001). The patients in two groups had no statistical significance in the comparison of combined medications. For example, the comparison of p values between the patient’s history of hypertension, hyperglycemia, arrhythmia, heart failure and previous Transient Ischemic Attack (TIA), ACS, PCI and high uric acid and digestive tract disease were greater than 0.05, without statistical significance (p>0.05). Aside from statistically significant differences between the two groups of combined aspirin (p<0.01), there was no statistically significant difference between the two groups, such as diuretics, sugar-lowering drugs, Angiotensin Converting Enzyme Inhibitors (ACEI)/Angiotensin Receptor Blockers (ARBs), calcium antagonists/calcium channel inhibitors, statins, nitrates, whose comparison of p values were greater than 0.05, as shown in Table 1.
| Baseline features | Ticagrelor (n=167) | Clopidogrel (n=142) | p-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 60.80±9.88 | 67.05±11.21 | <0.001 |
| Male gender, n (%) | 154 (92.2) | 127 (89.4) | 0.396 |
| Height, cm | 165.7±11.07 | 165.41±7.40 | 0.816 |
| Weight, kg | 69.62±13.44 | 66.78±10.30 | 0.071 |
| BMI, kg/m2 | 24.85±3.10 | 24.37±3.10 | 0.227 |
| Current smoking | 69 (41.3) | 44 (31.0) | 0.060 |
| Current drinking | 68 (40.7) | 43 (30.3) | 0.057 |
| Follow-up time | 14.24±7.28 | 15.25±6.81 | 0.029 |
| Cardiovascular history and risk factors, n (%) | |||
| Diabetes mellitus | 56 (33.5) | 43 (30.3) | 0.542 |
| Hypertension | 101 (60.5) | 92 (64.8) | 0.436 |
| ACS | 25 (31.1) | 43 (30.3) | 0.871 |
| PCI | 29 (17.4) | 23 (16.2) | 0.784 |
| TIA | 14 (8.4) | 21 (14.8) | 0.077 |
| Arrhythmia | 37 (22.2) | 27 (19.0) | 0.497 |
| Congestive heart failure | 2 (1.2) | 2 (1.4) | 0.626 |
| Hyperuricemia | 45 (26.9) | 28 (19.7) | 0.136 |
| Disease of digestive tract | 27 (16.2) | 22 (15.5) | 0.871 |
| Combined medications, n (%) | |||
| Diuretic | 37 (22.2) | 22(15.5) | 0.138 |
| Blood glucose-lowering drugs | 41 (24.6) | 39 (27.5) | 0.560 |
| Aspirin | |||
| ≤6 mo | 137 (82.0) | 61 (43.0) | <0.001 |
| 6-12 mo | 20 (12.0) | 20 (14.1) | 0.582 |
| >12 mo | 1 (0.6) | 6 (4.2) | 0.051 |
| ACE-inhibitor and/or ARB | 71 (42.5) | 50 (35.2) | 0.190 |
| Calcium channel inhibitors | 22 (13.2) | 27 (19.0) | 0.161 |
| Statin | 134 (80.2) | 123 (86.6) | 0.135 |
| Nitrates | 16 (9.6) | 16 (11.3) | 0.628 |
Table 1: Patient Demographic and Baseline Characteristics.
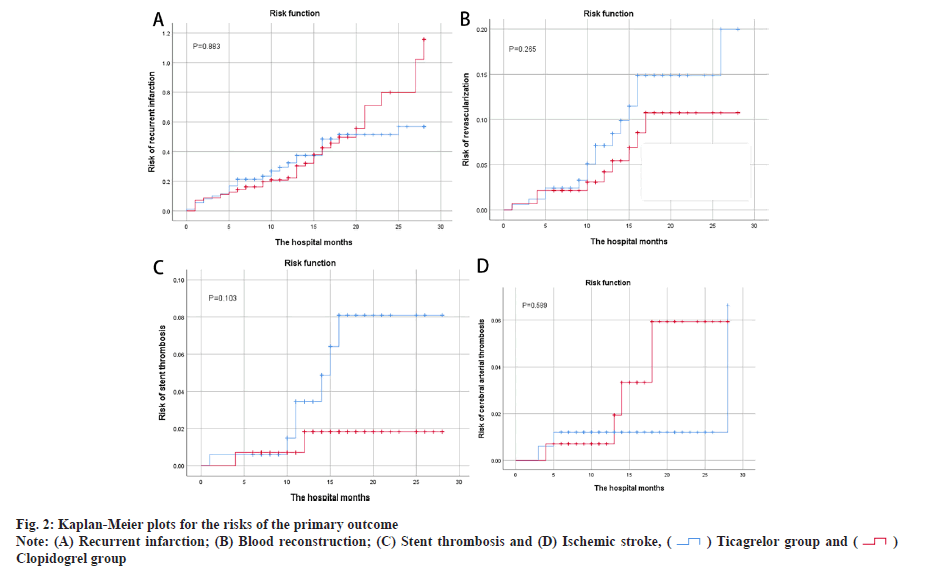
Main outcome assessment was shown here. Table 2 summarizes the overall primary treatment outcomes, as indicated in the Kaplan-Meier risk ratio curves in fig. 1 and fig. 2. Re-hospitalization incidence between groups was not statistically significant (40.7 % vs. 36.6 %; p=0.685). There were 74 MACE cases (44.3 %) in the ticagrelor group and 63 MACE cases (44.4 %) in the clopidogrel group, indicating no statistical differences between the primary efficacy endpoint incidence groups. The comparison of recurrence incidence was not statistically significant (30.5 % vs. 32.4 %; p=0.883), nor the comparison of blood transport reconstruction incidence (9.0 % vs. 6.3 %; p=0.265), or the comparison of stent thrombosis incidence (4.2 % vs. 1.4 %; p=0.103). Fig. 2A-fig. 2D shows no difference in recurrent infarction, blood reconstruction, stent thrombosis or ischemic stroke risks in both groups.
| Outcomes | Ticagrelor (n=167) | Clopidogrel (n=142) | p value | HR (95 % CI) |
|---|---|---|---|---|
| Re-hospitalizations for the original disease, n (%) | 68 (40.7) | 52 (36.6) | 0.286 | 0.685 (16.345, 19.031) |
| MACE | ||||
| Myocardial infarction, n (%) | 51 (30.5) | 46 (32.4) | 0.883 | 0.658 (18.711, 21.289) |
| Coronary revascularization, n (%) | 15 (9.0) | 9 (6.3) | 0.265 | 0.390 (25.228, 26.758) |
| Stent thrombosis, n (%) | 7 (4.2) | 2 (1.4) | 0.103 | 0.255 (26.720, 27.720) |
| Ischemic stroke, n (%) | 3 (1.8) | 4 (2.8) | 0.589 | 0.235 (27.004, 27.926) |
Note: HR: Hazard Ratio; CI: Confidence Interval and MACE: Major Adverse Cardiovascular Events
Table 2: Assessment of Primary Efficacy Endpoint.
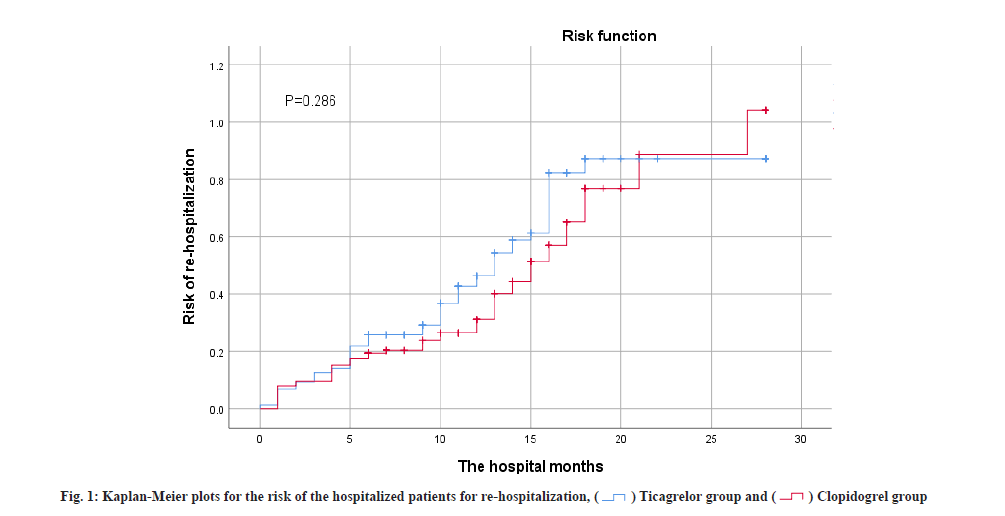
Table 3 summarizes the overall secondary efficacy outcomes and safety assessments. No statistical difference was found in re-hospitalization frequency and timing between the two groups (p>0.05). As shown in fig. 1, curve shows no difference in re-hospitalization risk, at 3 %. A total of 84 safety-related adverse events (50.3 %) occurred in patients in the ticagrelor group of which 73 (43.7 %) were difficult to breathe for any cause, 0 (0 %) has severe bleeding and 11 (6.6 %) have minor bleeding. A total of 49 (34.5 %) patients safety-related adverse events were documented in the clopidogrel group, including 45 patients (31.7 %) of dyspnea from various causes, 0 instances of major bleeding (0 %) and 4 occurrences of minor bleeding (2.8 %). Patients in the ticagrelor group exhibited a significantly higher incidence of dyspnea compared to the clopidogrel group (43.1 % vs. 31.7 %; p=0.039). However, there was no statistically significant difference between the two groups regarding the likelihood of minor bleeding (6.6 % vs. 2.8 %; p=0.184). Furthermore, no major bleeding events were detected in either group during the follow-up period. The overall mortality rate was lower in the ticagrelor group, with no recorded deaths during follow-up, while 7 patients in the clopidogrel group succumbed by the end of the follow-up period (p=0.004).
| Outcomes | Ticagrelor (n=167) | Clopidogrel (n=142) | p value |
|---|---|---|---|
| Number of re-hospitalizations for the original disease, n (%) | |||
| 0 | 99 (59.3) | 90 (63.4) | 0.461 |
| 1 | 62 (37.1) | 44 (31.0) | 0.257 |
| 2 | 6 (3.6) | 8 (5.6) | 0.390 |
| Re-hospitalization time for the original illness, n (%) | |||
| ≤1 mo | 10 (13.7) | 4 (6.7) | 0.189 |
| 1-12 mo | 37 (50.7) | 32 (53.3) | 0.761 |
| >12 mo | 26 (35.6) | 24 (40.0) | 0.604 |
| Adverse events, n (%) | |||
| Dyspnea | 72 (43.1) | 45 (31.7) | 0.039 |
| Slight bleeding | 11 (6.6) | 4 (2.8) | 0.184 |
| Severe bleeding | 0 (0.0) | 0 (0.0) | 0.000 |
| All-cause death | 0 (0.0) | 7 (4.9) | 0.004 |
Table 3: Secondary Efficacy Endpoint and Adverse Events.
Through this retrospective cohort study, we found that at the end of follow-up, patients who used ticagrelor and clopidogrel are similar in time and number of re-hospitalizations due to the original disease. In terms of major adverse reactions, in the ticagrelor group, breathing difficulties caused by any cause were more prevalent than in the clopidogrel group, but minor bleeding was similar in both groups and severe bleeding was not reported.
Baseline differences between the two groups in this study included age and concomitant aspirin use. Patients in the clopidogrel group were older and had a higher prevalence of aspirin co-administration for over 6 mo compared to the ticagrelor group. However, we believe that the results of this study are still reliable for the following reasons. In the studies of Baber et al. age was associated with severe bleeding and for every 1 y of age, the risk of hemorrhage increased by 0.02. However, age was not a risk factor for coronary thrombotic events[11]. Older patients in the clopidogrel group faced a heightened risk of bleeding, regardless of concurrent aspirin use. Despite the older patients in the ticagrelor group and a higher proportion of aspirin use, there was no difference in bleeding events between the two groups. Additionally, aspirin administration in both groups was similar over a 6 mo period. From the Kaplan-Meier risk prediction graph (fig. 2), it can be found that the four MACE curves only showed a significant increase in 5 mo. As a result, data are still comparable in 6 mo after discharge, but more research is needed to further assess the safety and efficacy of patients within 6 mo. The Kaplan-Meier risk prediction (fig. 2) shows that MACE occurs less often within 6 mo.
All 7 deaths in this study occurred in the clopidogrel group, but none of these patients were hospitalized again in the month prior to death due to the associated primary disease. Two of the seven patients who died were of terminal lung cancer, one with chronic renal failure, one who refused PCI surgery and three who were older with a variety of underlying diseases (75 y, 76 y and 90 y old, respectively), and the above analysis showed that the patient’s death was not significantly related to the drug choice.
This study has shown that ticagrelor has no protective effect on reducing ischemic events in patients with gene loss compared to the use of clopidogrel, aligning with a recent study in ACS patients aged 70 y and older, wherein researchers observed no significant difference within the results of CYP2C19, non-carriers of functional loss alleles received clopidogrel or ticagrelor treatment in net clinical benefits (all-cause death, myocardial infarction, stroke and platelet inhibition, as well as atherosclerotic thrombosis comprising cardiovascular death, myocardial infarction and stroke). In terms of effectiveness[8], this investigation align with those of the Tailored Antiplatelet Initiation to Lessen Outcomes due to Decreased Clopidogrel Response after PCI (TAILOR-PCI) randomized clinical trial[9], as well as two recent small studies[12,13]. These studies demonstrated no considerable disparity in the occurrence of hemorrhagic events with ticagrelor and even in two investigations conducted by Xi et al.[14] and Chen et al.[15] in East Asia, a heightened risk of bleeding in the ticagrelor group was observed.
To sum up, for patients with CPY2C19 gene deletion, clopidogrel is no worse than ticagrelor in terms of first admission time and number of times, MACE events, and severe, minor bleeding, or even less breathing difficulties. In addition, in terms of compliance, the way of taking ticagrelor is administered twice a day and the higher cost are also important factors in the patient’s inability to adhere to the drug[16]. Studies by Romagnoli et al.[17], show that patients who use clopidogrel have higher compliance. As a result of the latest study, there does not appear to have a greater clinical benefit to take ticagrelor for patients with slow or medium metabolism of CYP2C19 gene deficiency. Due to limited human and financial resources, more advanced equipment is used effectively in large general hospitals. Because of the limitation of equipment, high testing cost, shortage of medical testing resources, low patient acceptance capacity, the primary hospitals cannot popularize genetic testing. Whether it is clinically necessary to monitor the relevant genes before taking clopidogrel needs to consider the patient's financial level and willingness. The limitations of this study are, this study is a retrospective cohort study, so there is no randomization of patient inclusion, which may be a bias in choice; some minor clinical events occurring in the predischarge period may be forgotten in patients with long follow-up time and discharge time.
In patients with CYP2C19 gene deletion, patients with clopidogrel have the same or even fewer breathing difficulties compared to ticagrelor in terms of time and number of first admissions, as well as the risk of MACE events and severe bleeding and minor bleeding. In this study, patients with slow or medium metabolism of clopidogrel do not appear to have a greater clinical benefit, so in primary hospitals where genetic testing is not possible, doctors could choose antiplatelet drugs based on the patient’s financial situation and wishes, and clopidogrel is seemed to be a better option for patients.
Funding:
This work was supported by Natural Science Foundation of Fujian Province of China (2018Y0037).
Author’s contributions:
Xiaofang Cai, Wenlong Wang, Shaojun Jiang drafted the manuscript; Jinhua Zhang, Xiaofang Cai and Wenlong Wang participated in the design of the study; Jinhua Zhang, Xiaofang Cai, Wenlong Wang, Shaojun Jiang, Xiujuan Cai, Jiandong Huang and Jiana Chen coordinated the study and all authors read and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interest.
References
- Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 2006;108(7):2244-7.
[Crossref] [Google scholar] [PubMed]
- Gurbel PA, Shuldiner AR, Bliden KP, Ryan K, Pakyz RE, Tantry US. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J 2011;161(3):598-604.
[Crossref] [Google scholar] [PubMed]
- Scott S, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther 2011;90(2):328-32.
[Crossref] [Google scholar] [PubMed]
- Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 2013;94(3):317-23.
[Crossref] [Google scholar] [PubMed]
- Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014;11(10):597-600.
[Crossref] [Google scholar] [PubMed]
- Hagihara K, Kazui M, Kurihara A, Yoshiike M, Honda K, Okazaki O, et al. A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Metab Dispos 2009;37(11):2145-52.
[Crossref] [Google scholar] [PubMed]
- McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment and outcomes of patients with STEMI and NSTEMI. Am J Med 2011;124(1):40-7.
[Crossref] [Google scholar] [PubMed]
- Claassens DM, Gimbel ME, Bergmeijer TO, Vos GJ, Hermanides RS, van der Harst P, et al. Clopidogrel in non-carriers of CYP2C19 loss-of-function alleles versus ticagrelor in elderly patients with acute coronary syndrome: A pre-specified sub analysis from the popular genetics and popular age trials CYP2C19 alleles in elderly patients. Int J Cardiol 2021;334:10-7.
[Crossref] [Google scholar] [PubMed]
- Klein MD, Williams AK, Lee CR, Stouffer GA. Clinical utility of CYP2C19 genotyping to guide antiplatelet therapy in patients with an acute coronary syndrome or undergoing percutaneous coronary intervention. Arterioscler Thromb Vasc Biol 2019;39(4):647-52.
[Crossref] [Google scholar] [PubMed]
- Schulman S, Kearon C, Subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non?surgical patients. J Thromb Haemost 2005;3(4):692-4.
[Crossref] [Google scholar] [PubMed]
- Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: Risk scores from PARIS. J Am Coll Cardiol 2016;67(19):2224-34.
[Crossref] [Google scholar] [PubMed]
- Zhang Y, Shi XJ, Peng WX, Han JL, Lin BD, Zhang R, et al. Impact of implementing CYP2C19 genotype-guided antiplatelet therapy on P2Y12 inhibitor selection and clinical outcomes in acute coronary syndrome patients after percutaneous coronary intervention: A real-world study in China. Front Pharmacol 2021;11:582929.
[Crossref] [Google scholar] [PubMed]
- Lee CR, Sriramoju VB, Cervantes A, Howell LA, Varunok N, Madan S, et al. Clinical outcomes and sustainability of using CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Circ Genom Precis Med 2018;11(4):e002069.
[Crossref] [Google scholar] [PubMed]
- Xi Z, Zhou Y, Zhao Y, Liu X, Liang J, Chai M, et al. Ticagrelor versus clopidogrel in patients with two CYP2C19 loss-of-function alleles undergoing percutaneous coronary intervention. Cardiovasc Drugs Ther 2020;34:179-88.
[Crossref] [Google scholar] [PubMed]
- Chen S, Zhang Y, Wang L, Geng Y, Gu J, Hao Q, et al. Effects of dual-dose clopidogrel, clopidogrel combined with tongxinluo capsule and ticagrelor on patients with coronary heart disease and CYP2C19* 2 gene mutation after Percutaneous Coronary Interventions (PCI). Med Sci Monit 2017;23:3824.
[Crossref] [Google scholar] [PubMed]
- Dayoub EJ, Seigerman M, Tuteja S, Kobayashi T, Kolansky DM, Giri J, et al. Trends in platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence among antiplatelet-naive patients after percutaneous coronary intervention, 2008-2016. JAMA Intern Med 2018;178(7):943-50.
[Crossref] [Google scholar] [PubMed]
- Romagnoli A, Santoleri F, Costantini A. Adherence and persistence analysis in patients treated with Double Antiplatelet Therapy (DAPT) at two years in real life. Patient Educ Couns 2021;104(8):2012-7.
[Crossref] [Google scholar] [PubMed]
 .
.