- *Corresponding Author:
- H. B. Qu
Department of Orthopedics, Zhejiang Hospital, Hangzhou, Zhejiang 310012, China
E-mail: cai75459@126.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “215-219” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
To investigate the clinical efficacy and mechanism of action of combined medication of nonsteroidal antiinflammatory drugs and thalidomide on the axial ankylosing spondylitis. A total of 134 patients were randomly selected and divided into the observation group (n=67) and the control group (n=67). Finally, there were 60 patients in the observation group and 56 in the control group. Patients in the control group took nonsteroidal anti-inflammatory drugs (etoricoxib tablets) for treatment, while those in the observation group received the combined medication of nonsteroidal anti-inflammatory drugs and thalidomide. In the observation group, the total effectiveness rate was significantly higher than that in the control group (p<0.05). At 6 w and 12 w after treatment, erythrocyte sedimentation rate and C-reactive protein levels in the observation group were significantly reduced when compared with levels in the control group and before treatment (all p<0.05). However, at 2 w after treatment, the level of C-reactive protein in the observation group was significant decreased as compared with the levels in the control group and before treatment (all p<0.05), whereas a great decrease of the level of C-reactive protein in the control group was only found at 12 w after treatment (p<0.05). At 6 w and 12 w after treatment, bath ankylosing spondylitis disease activity index, bath ankylosing spondylitis functional index and visual analog scale scores of patients in two groups were ameliorated significantly, and the amelioration in the observation group was more evident than that in the control group (p<0.05). No statistical significance was shown in differences of the incidence rates of the gastrointestinal tract response, abnormal liver function and complications of infection (all p>0.05). In treatment of axial ankylosing spondylitis, combined medication of nonsteroidal anti-inflammatory drugs and thalidomide performs better than the single medication of nonsteroidal anti-inflammatory drugs. Thalidomide mainly ameliorates the condition of axial ankylosing spondylitis patients by inhibiting tumour necrosis factor alpha, with little adverse effect, high safety and effectiveness and low cost. Thus, combined medication of nonsteroidal antiinflammatory drugs and thalidomide is worthy of being promoted in clinical practice.
Keywords
Nonsteroidal anti-inflammatory drugs, etoricoxib tablets, thalidomide, axial ankylosing spondylitis
Ankylosing Spondylitis (AS), a chronic inflammatory disease frequently seen in young males, may involve the sacroiliac joint, spine and surrounding soft tissues as well as peripheral joint, thereby leading to the loss of somatic function. According to the difference in the involved joints the AS is classified into the axial and peripheral types. For treatment of axial AS, conventional Disease-Modifying Antirheumatic Drugs (DMARDs) performs worse with slow onset [1,2]. The rapid development in the biologicals has gained the evident control over the condition of axial AS patients, among which Tumor Necrosis Factor Alpha (TNF-α) inhibitors has been extensively applied with the exact efficacy.
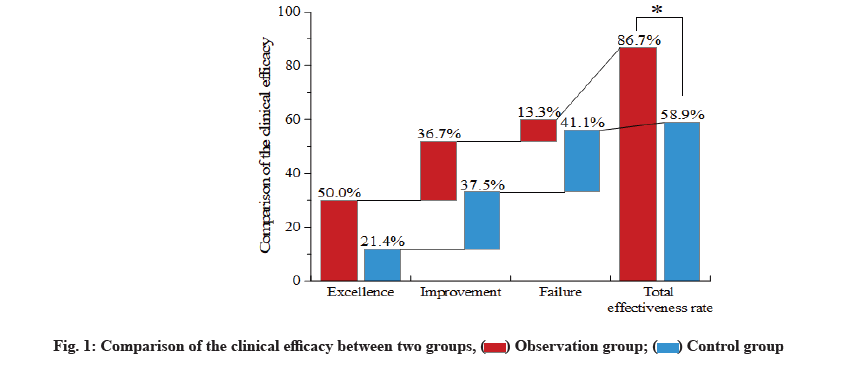
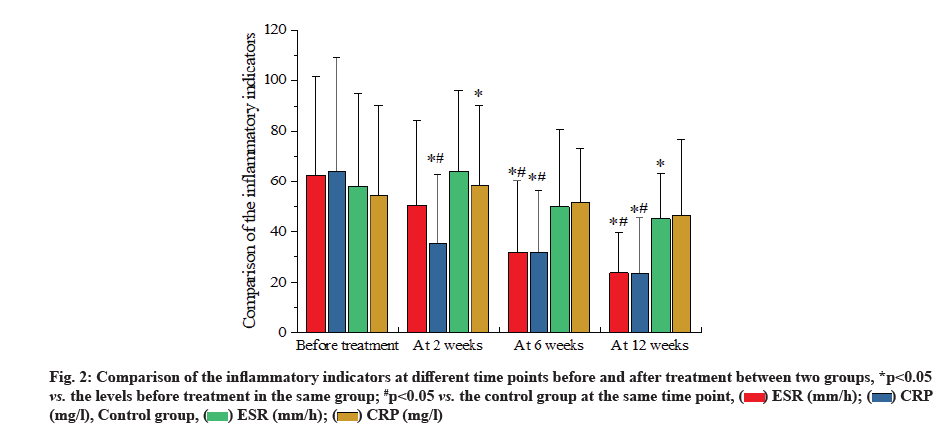
However, the expensive cost and high risk of hepatitis B or tuberculosis increase the difficulty in clinical application [3]. Nonsteroid Anti-Inflammatory Drugs (NSAIDs) are listed as the first-line drug for treatment of AS. Accumulating evidence has shown that thalidomide tablet has the antagonistic effect on TNF-α [4]. Previous findings reveal that the combined medication of NSAIDs and TNF-α inhibitor works more rapidly in treatment of AS, with more evident amelioration [5], but there remains little information regarding the efficacy of NSAIDs in combination with thalidomide tablet. Thus, the aim of this study is to explore the clinical efficacy of combined medication of NSAIDs and thalidomide on axial AS. From the axial AS patients who were admitted to this hospital between March 2018 and February 2020, 134 patients were randomly selected and divided into the observation group and control group, with 67 patients in each group. Following the further selection, 9 patients with gastrointestinal responses, somnolence or poor response and 9 patients loss to the follow-up or with incomplete clinical data, were excluded from this study. Finally, there were 60 patients in the observation group and 56 in the control group. In the observation group, there were 51 males and 9 females aged between 20 y and 55 y old, with an average age of (33.09±8.47) y; their disease course ranged from 0.1 to 27.9 y, with an average of (8.46±5.07) y. In the control group, there were 46 males and 10 females aged between 20 y and 54 y old, with an average age of (31.78±8.31) y; their disease course ranged from 0.5 y to 23.8 y, with an average of (7.76±4.55) y. No statistical significance was shown in the differences of the general data, including sex, age and disease course, between two groups (all p>0.05). This study was implemented according to the risk management standard of thalidomide in Europe [5]. This study was reviewed and approved by the Ethics Committee of the hospital and gained the written informed consents from all subjects that promised to quit the trials only in the case of severe adverse reaction. Inclusion criteria include patients conforming to the diagnostic criteria of AS (1984 edition) and the classification criteria of axial Spondyloarthritis (SpA) issued by the International Collaborative Team of SpA in 2009 [6]. Exclusion criteria include patients complicated with the diseases in heart, liver or kidney; patients with active tuberculosis or in close contact with the active tuberculosis patients; patients with hepatitis A, B or C, or carriers; patients with Acquired Immunodeficiency Syndrome (AIDS), malaria or other contagious diseases; patients with the history of severe allergy; patients with completely AS; patients complicated with severe mental diseases; patients complicated with the coagulation disorders [3]; patients in pregnancy or lactation period or with the desire of pregnancy. Patients in the control group took NSAIDs (etoricoxib tablets; MSD China; Saudi Food and Drug Authority (SFDA) approval number: J20180057; specification: 60 mg) once per day (120 mg) for treatment, while those in the observation received the combined medication of NSAIDs and thalidomide (Changzhou Pharmaceutical Factory Co., Ltd; SFDA approval number: H32026129; specification: 25 mg) at an initial dose of 50 mg/time, once per day,which would be doubled to 100 mg/time, once per day, for patients with no severe adverse reaction. During 12 w of medication, calcium was supplemented by oral administration, while vitamin D by Alfacalcidol soft capsules. Evaluation of efficacy of inflammatory indicators is described in detail. Before treatment and at 2 w, 6 w and 12 w after treatment, fasting venous blood was drawn from patients of two groups in the morning for analyzing the levels of Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) by use of the automatic blood sedimentation analyzer and automatic CRP analyzer. Evaluation of disease activity and functions is explained below. Prior to the treatment and at 2 w, 6 w and 12 w after treatment, we compared the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the functional indicators of AS joints. BASDAI and Bath Ankylosing Spondylitis Functional Index (BASFI) were evaluated by 6 and 10 questions, respectively, and after the Visual Analog Scale (VAS) evaluation, final scores were calculated by the specific formula, within 0 to 10 points. Higher points represent more severe condition [7]. Pains were assessed by VAS. A line in length of 10 cm was drawn on a paper, 0 cm representing no pain, 1 to 3 cm representing mild pain, 4 to 6 cm representing moderate pain, 7 to 9 cm representing severe pain and 10 cm representing acute pain. Markers on the paper suggested the pain degree of patients [8]. Prior to the treatment and at 2 w, 6 w and 12 w after treatment, we measured the ESR, CRP, BASDAI, BASFI and VAS scores. Patients with amelioration ≥50 % were thought as reaching the standard. Standard for efficacy evaluation is calculated as excellence for patients reaching all of the standards; improvement for patients reaching 3 or 4 of standards and failure or deterioration for patients failing to reach 3 or more standards. Total effectiveness rate=Rate of excellence+Rate of improvement. Safety evaluation was also calculated. After medication, the incidence of gastrointestinal responses, abnormal liver function, somnolence, complications of infection and other adverse events of patients was compared between two groups, and regular examinations were carried out to detect the indicators in blood, urine, faeces, occult blood, hepatic and renal indicators of patients in two groups. Statistical Package for the Social Sciences (SPSS) 22.0 software was used to process the data in this study. Enumeration data were presented in n/% and compared by chi-square test. Measurement data were presented in mean±standard deviation (x̄±s) and compared by use of t-test. Difference at p<0.05 suggested the statistical significance. Comparison of the clinical efficacy between two groups was explained below. In the observation group, the total effectiveness rate was significantly higher than that in the control group (p<0.05, fig. 1). Comparison of the inflammatory indicators at different time points before and after treatment between two groups was shown below. At 6 w and 12 w after treatment, ESR and CRP levels in the observation group were significantly reduced when compared with the levels in the control group and before treatment (all p<0.05). However, at 2 w after treatment, the level of CRP in the observation group was significant decreased as compared with the levels in the control group and before treatment (all p<0.05), whereas a great decrease of the level of ESR in the control group was only found at 12 w after treatment (p<0.05, fig. 2). Comparison of the disease activity,function evaluation indicators and VAS scores at different time points before and after treatment between two groups (x̄±s) were shown here. At 2 w, 6 w and 12 w after treatment, BASDAI, BASFI and VAS scores of patients in two groups were ameliorated significantly and the amelioration in the observation group was more evident than that in the control group at 6 w and 12 w after treatment (p<0.05, Table 1). Comparison of the incidence of adverse events of patients between two groups was shown below. No statistical significance was shown in differences of the incidence rates of the gastrointestinal tract response, abnormal liver function and complications of infection (all p>0.05). In the observation group, the incidence rate of the somnolence was 15.0 %, significantly higher than 3.6 % in the control group (p<0.05, Table 2).
| Group | Item | Before treatment | After treatment | ||
|---|---|---|---|---|---|
| At 2 w | At 6 w | At 12 w | |||
| Observation group (n=60) | BASDAI scores | 6.9±1.9 | 3.5±2.4*# | 2.6±1.7*# | 2.6±1.5*# |
| BASFI scores | 5.3±2.8 | 3.6±2.0* | 2.2±1.3*# | 1.9±1.1*# | |
| VAS scores | 5.3±3.3 | 3.6±2.5* | 2.3±1.7*# | 2.2±1.9*# | |
| Control group (n=56) | BASDAI scores | 6.7±1.9 | 5.6±2.0* | 3.8±1.5* | 3.6±1.7* |
| BASFI scores | 5.1±2.4 | 4.2±2.2* | 3.3±1.8* | 2.8±1.7* | |
| VAS scores | 4.9±3.2 | 4.1±2.2* | 3.5±1.7* | 3.6±1.4* | |
Note: *p<0.05 vs. the levels before treatment in the same group; #p<0.05 vs. the control group at the same time point, BASDAI means Bath Ankylosing Spondylitis Disease Activity Index; BASFI means Bath Ankylosing Spondylitis Functional Index; VAS means Visual Analog Scale
Table 1: Comparison of The Disease Activity, Function Evaluation Indicators And Vas Scores Before and After Treatment Between Two Groups (x̄±s)
| Group | N | Gastrointestinal responses | Abnormal liver function | Somnolence | Complicated infections |
|---|---|---|---|---|---|
| Observation group | 60 | 8 (13.3) | 5 (8.3) | 9 (15.0) | 2 (3.3) |
| Control group | 56 | 6 (10.7) | 4 (7.1) | 2 (3.6) | 1 (1.8) |
| X2 | 0.181 | 0.056 | 4.403 | 0.273 | |
| p | 0.671 | 0.813 | 0.039 | 0.601 |
Table 2: Comparison of The Incidence of Adverse Events of Patients Between Two Groups (x̄±s)
Previous strategies for treatment of axial AS mainly include conventional DMARDs+NSAIDs, biologicals+conventional DMARDs or biologicals+NSAIDs. The first combination performs poorly in treatment, while the efficacy of latter two combinations is much better, but with a high cost. Clinical application of biologicals is less extensive in China, while in 2010, European Alliance of Associations for Rheumatology (EULAR) emphasized the importance of NSAIDs in treatment of AS and NSAIDs as such, was recommended as the first-line drugs for treatment of pains or morning stiffness of AS [4]. Thalidomide tablet, as an artificially synthesized glutamic derivative can antagonize the inflammation by suppressing the release of TNF-α, Interleukin-1 (IL-1) and Interleukin-12 (IL-12) from mononuclear cells [9]. Thalidomide tablet can stabilize the lysosome membrane and decrease the chemotaxis of neutrophils to achieve the efficacy of immunoregulation and antiinflammation [10]. Thalidomide can not only suppress the release of TNF-α from mononuclear cells and facilitate the messenger Ribonucleic Acid (mRNA) degradation of TNF-α, but also enhance antagonistic effect on TNF-α by binding to α1-acid glycoprotein [11]. Thus, thalidomide generates the satisfying efficacy in treatment of axial joint and peripheral joint of AS patients. NSAIDs, having been regarded as the first-line drugs for treatment of AS, can suppress the activity of epoxidase and block the generation of prostaglandin, which is thought to be the pharmaceutical basis. As indicated in multiple Randomized Controlled Trials (RCTs), about 15 % of axial AS patients in active phase could reach the standard of partial remission of Assessment of SpondyloArthritis International Society (ASAS) after sufficient medication of NSAIDs [3]. However, there are few studies focusing on the efficacy of NSAIDs (etoricoxib tablets) in combination with thalidomide on the axial AS or comparative studies with the single medication of NSAIDs. In this study, we compared the efficacy of these two strategies on the axial AS and found that VAS scores, disease activity and functional score of joints in two groups were all ameliorated, but at 6 w and 12 w after treatment, the amelioration of these indicators in the observation group was more evident than that in the control group (p<0.05); as for the inflammatory indicators, at 6 w and 12 w after treatment, the levels of ESR and CRP of patients in the observation group were much lower than those in the control group or before treatment (all p<0.05); at 12 mo after treatment, ESR in the control group was much lower than that before treatment (p<0.05); during the whole treatment, no significant variation was observed in the level of CRP in the control group, whereas in the observation group, this variation was more evident as the treatment went on. In the observation group, the total effectiveness rate was significantly higher than that in the control group (p<0.05); no statistical significance was shown in differences of the incidence rates of the gastrointestinal tract response, abnormal liver function and complications of infection (all p>0.05). In the observation group, the incidence rate of the somnolence was 15.0 %, significantly higher than 3.6 % in the control group (p<0.05). Thus, combined medication works better than the single medication, but for specific populations (aloft work or driving), medication of thalidomide should be prudent. Taken together, combined medication of NSAIDs and thalidomide performs more significantly in treatment of axial AS and from different aspects, these two drugs could block or inhibit the pathogenesis of axial AS, thereby achieving the efficacy of combined efficacy. In comparison with the single medication, the efficacy of combined medication is further improved as the treatment goes on. Moreover, the reliable safety and low medical cost make it more worthy of being promoted in clinical work.
Author’s contributions:
Wubin Shu and Xingpeng Xiao contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Palazzi C, D’Angelo S, Gilio M, Leccese P, Padula A, Olivieri I. Pharmacological therapy of spondyloarthritis. Expert Opin Pharmacother 2015;16(10):1495-504.
[Crossref] [Google Scholar] [PubMed]
- Zhou LG, Jiang B. Progress in clinical application of anti-tumor necrosis factor-alpha inhibitors for ankylosing spondylitis. Chin Pharm 2018;27(1): 59-63.
- Braun JV, Van Den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70(6):896-904.
[Crossref] [Google Scholar] [PubMed]
- Kiltz U, Sieper J, Braun J. ASAS/EULAR recommendations for the management of ankylosing spondylitis: Evaluation of the 2010 update in the German-speaking area. Z Rheumatol 2013;72(1):71-80.
[Crossref] [Google Scholar] [PubMed]
- Wang Y. Thalidomide risk management plan in Europe. China Licensed Pharm 2008;5(11):21-2.
- Linden SV, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 1984;27(4):361-8.
[Crossref] [Google Scholar] [PubMed]
- de Andrade KR, de Castro GR, Vicente G, da Rosa JS, Nader M, Pereira IA, et al. Evaluation of circulating levels of inflammatory and bone formation markers in axial spondyloarthritis. Int Immunopharmacol 2014;21(2):481-6.
[Crossref] [Google Scholar] [PubMed]
- Li SR. The influence of different factors on the same rate of visual analogue scale score and digital scale score. J Hebei Med Univ 2015.
- Mitulescu TC, Stavaru C, Voinea LM, Banica LM, Matache C, Predeteanu D. The role of Vitamin D in immuno-inflammatory responses in ankylosing spondylitis patients with and without acute anterior uveitis. J Med Life 2016;9(1):26-33.
[Google Scholar] [PubMed]
- Li YH, Tao LH, Qian KW. Thalidomide in the treatment of ankylosing spondylitis and its effect on the expression of inflammatory factors. Shanxi Med J 2016;45(3):247-9.
- Fakhoury M, Coussa-Charley M, Al-Salami H, Kahouli I, Prakash S. Use of artificial cell microcapsule containing thalidomide for treating TNBS-induced Crohn's disease in mice. Curr Drug Deliv 2014;11(1):146-53.
[Crossref] [Google Scholar] [PubMed]