- *Corresponding Author:
- Q. Chen
The First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui Province 233000, China
E-mail: chuiji5579199@163.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “264-269” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze the clinical efficacy and safety of monotherapy and dual therapy in patients with early ischemic stroke. The study was conducted from August 2020 to August 2021, and 80 patients with early ischemic stroke who received treatment at our hospital during this period were selected as the study population. They were randomly divided into a control group and an observation group, with 40 patients in each group. The control group received conventional monotherapy, while the observation group received conventional dual therapy. The efficacy and safety indicators of the two groups were compared. The treatment efficacy, functional recovery, blood indicators and inflammation markers in the observation group were all superior to those in the control group (all, p<0.05); there was no significant difference in the drug safety profile between the two groups (p>0.05). The dual antiplatelet therapy is more ideal than monotherapy for treating patients with early ischemic stroke. It can further promote the recovery of neurological and cognitive function, improve hematological and inflammatory response indicators in patients and the safety of this r egimen is comparable to monotherapy.
Keywords
Monotherapy, dual therapy, early ischemic stroke, efficacy, safety, hypoxia
Ischemic stroke is a common neurological disorder in clinical practice. It refers to the abnormality of cerebral blood supply arteries, leading to localized ischemia and hypoxia of brain tissue, resulting in ischemic necrosis and neurological syndromes[1]. Patients with acute ischemic stroke are in a relatively critical condition, with higher disability and mortality rates. Moreover, the pre symptoms of this disease are easily confused with other conditions, leading to a potential missed treatment window[2]. Additionally, the occurrence of this disease can affect patient’s neurological function, which not only impacts their daily life but also poses a certain threat to their life safety[3].
Currently, for early ischemic stroke patients, thrombolysis or mechanical thrombectomy is recommended for treatment. However, studies have shown that although thrombolysis and arterial thrombectomy have been widely used in the treatment of early ischemic stroke, these approaches have strict time window limitations[4]. Therefore, the main treatment options in clinical practice for early ischemic stroke still involve antiplatelet aggregation, anticoagulation, intracranial pressure reduction, and neurotrophic drugs[5]. The onset of early ischemic stroke is often associated with factors such as long- term arterial atherosclerosis leading to vascular stenosis, thrombus formation and vessel occlusion. Therefore, timely and effective regulation of patient’s coagulation mechanisms is of great significance in promoting their microcirculation and improving prognosis[6]. Antiplatelet aggregation is an important measure for the treatment of ischemic stroke patients. In recent years, the dual therapy regimen combining aspirin and clopidogrel has been highly praised in clinical practice, as both drugs are commonly used antiplatelet medications[7]. Studies have shown that the dual therapy regimen combining aspirin and clopidogrel is effective in treating patients with acute ischemic stroke, and it further improves the hypercoagulable state compared to the conventional monotherapy regimen. However, there are still concerns about the safety of dual therapy according to some clinical studies[8,9].
Materials and Methods
Basic information:
The study was conducted from August 2020 to August 2021 and included a total of 80 patients with early ischemic stroke admitted during this period as the study population. Basic information of the patients was collected.
Inclusion: Patients diagnosed with early ischemic stroke confirmed by relevant tests and with cerebral infarction lesions clearly identified by imaging examination; adult patients; time from onset of symptoms to hospital admission ≤24 h; patients experiencing their first stroke; patients voluntarily participating in the study and signing the relevant informed consent.
Exclusion: Allergic reactions or relevant contraindications to the drugs, methods, or devices used in this study; recurrent stroke; concomitant malignant tumor disease; concomitant severe organ dysfunction; presence of congenital or autoimmune diseases; presence of hematological disorders or coagulation dysfunction; concomitant psychiatric disorders or pre-existing cognitive or behavioral disorders and intracranial hemorrhage. The patients were randomly divided into a control group and an observation group, with 40 patients in each group.
Methods:
Control group: Within 48 h of onset, patients received monotherapy with a monoclonal antibody (Bayer aspirin (Bayer Healthcare Ltd., J20130078)) at a dose of 300 mg/day. After 7 d of continuous use, the maintenance dose was reduced to 100 mg/day, with total treatment duration of 4 w.
Observation group: Within 48 h of onset, patients received combination therapy with dual antibodies (aspirin (Bayer Pharmaceutical, J20130078)+clopidogrel (Lepu Pharmaceutical, H20123115)). On the 1st d, the dose was aspirin 300 mg+clopidogrel 75 mg and from the 2nd d onwards, it was switched to a maintenance dose, i.e., aspirin 100 mg/day+clopidogrel 75 mg/day. After 3 w, patients switched to monotherapy with aspirin 100 mg/day, with total treatment duration of 4 w.
Observation indicators:
Clinical efficacy: Cure means complete disappearance of symptoms and signs; significant improvement means improvement in symptoms and signs; effective means some improvement in symptoms and signs; ineffective means no significant change in symptoms and signs and loss of vital signs leads to death.
Changes in neurological and cognitive function: Neurological function of patients was assessed using the Glasgow Coma Scale (GCS) and National Institutes of Health Stroke Scale (NIHSS) at T1, T2 and T3. Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA). The total score of GCS is 15, with higher scores indicating a lighter level of consciousness. The total score of NIHSS is 42, with lower scores indicating milder neurological impairment. The total score of MoCA is 30, with higher scores indicating better cognitive function recovery.
Levels of hematological indicators: Plasma levels of D-dimer, fibrinogen and peak systolic velocity were measured before and after treatment using an automated biochemical analyzer.
Levels of inflammatory markers: Serum levels of C-Reactive Protein (CRP), Interleukin-6 (IL-6) and Tumor Necrosis Factor-Alpha (TNF-α) were measured before and after treatment using the Enzyme-Linked Immunosorbent Assay (ELISA) method.
Adverse reactions: Adverse reactions observed in this study included gastrointestinal reactions, nausea and vomiting, abnormal liver and kidney function, skin symptoms, mental symptoms and recurrence within 90 d.
Statistical analysis:
Graph Pad Prism 8 was used for graphic software; Statistical Package for the Social Sciences (SPSS) 26.0 was used for data analysis. The t-test was used for comparison of quantitative data and (x͞ ±s) represents the mean±standard deviation. The Chi-square (χ2) test was used for comparison of categorical data and n (%) represents the number and percentage. A significance level of p<0.05 was considered statistically significant.
Results and Discussion
The basic information of the two groups of patients was comparable and there were no significant differences in the comparison (p>0.05) as shown in Table 1.
| Control group (n=40) | Observation group (n=40) | t/χ2 | p | |
|---|---|---|---|---|
| Gender | 0.208 | 0.648 | ||
| Male | 23 | 25 | ||
| Female | 17 | 15 | ||
| Age) | 56-73 | 53-76 | ||
| Average age (years) | 63.12?5.67 | 63.24?5.73 | -0.094 | 0.925 |
| Onset time (h) | 2.3-11.4 | 3.3-11.5 | ||
| Average time (h) | 8.34?1.23 | 8.29?1.25 | 0.18 | 0.858 |
| Infarction site | ||||
| Basal ganglia | 23 | 21 | 0.202 | 0.653 |
| Brain lobe | 14 | 15 | 0.054 | 0.816 |
| Cerebellum | 2 | 2 | 0.0 | 1.0 |
| Brainstem | 1 | 2 | 0.346 | 0.556 |
| Combined underlying disease | ||||
| Hypertension | 31 | 30 | 0.069 | 0.793 |
| Diabetes | 20 | 22 | 0.201 | 0.654 |
| Coronary heart disease | 25 | 24 | 0.053 | 0.818 |
Table 1: Comparison of Basic Information
The overall treatment effectiveness in the observation group was significantly higher than that in the control group (p<0.05) as shown in Table 2.
| Group | Number of cases | Cure | Markedly effective | Efficient | Invalid | Die | Total effective rate (%) |
|---|---|---|---|---|---|---|---|
| Control | 40 | 4 | 10 | 14 | 10 | 2 | 70 |
| Observation | 40 | 10 | 12 | 15 | 2 | 1 | 92.5 |
| χ2 | 6.646 | ||||||
| p | 0.01 |
Table 2: Comparison of Clinical Efficacy
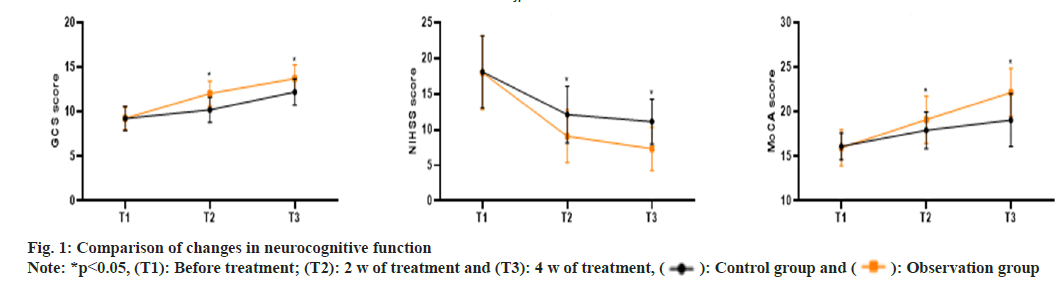
As shown in fig. 1, the GCS scores in the control group at T1, T2 and T3 were (9.26±1.34, 10.23±1.42, 12.23±1.48), the NIHSS scores were (18.09±5.06, 12.12±3.97, 11.14±3.18) and the MoCA scores were (16.13±1.53, 17.94±2.05, 19.08±2.97). In the observation group, the GCS scores at T1, T2 and T3 were (9.28±1.29, 12.06±1.38, 13.72±1.56), the NIHSS scores were (17.98±5.16, 9.07±3.72, 7.32±3.04) and the MoCA scores were (15.98±2.03, 19.12±2.65, 22.17±2.71). The GCS and MoCA scores at 2 w and 4 w of treatment were significantly higher in the observation group compared to the control group, while the NIHSS scores were significantly lower in the observation group (p<0.05).
As shown in fig. 2, the Drug-Drug Interactions (DDIs) levels before and after treatment in the control group was (1.23±0.38, 0.84±0.31), Fibonacci (FIB) levels were (4.48±1.21, 3.75±1.06) and Pressure Support Ventilation (PSV) levels were (3.58±1.13, 2.43±1.08). The DDI levels before and after treatment in the observation group was (1.21±0.39, 0.48±0.27), FIB levels were (4.51±1.18, 3.08±0.92) and PSV levels were (3.62±1.17, 1.31±1.01). The DDI, FIB and PSV levels in the observation group after treatment were significantly lower than those in the control group (p<0.05).
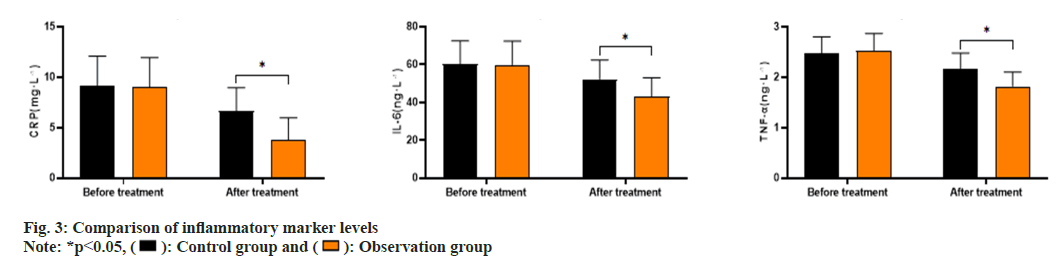
As shown in fig. 3, the CRP levels before and after treatment in the control group were (9.13±2.95, 6.62±2.34), IL-6 levels were (60.15±12.37, 52.32±10.08) and TNF-α levels were (2.48±0.32, 2.16±0.32). The CRP levels before and after treatment in the observation group were (9.07±2.86, 3.75±2.21), IL-6 levels were (59.89±12.52, 43.18±9.85) and TNF-α levels were (2.53±0.34, 1.82±0.28). The CRP, IL-6, and TNF-α levels in the observation group after treatment were significantly lower than those in the control group (p<0.05). The comparison of the incidence of adverse reactions between the two groups (p>0.05) is shown in Table 3.
| Adverse reactions | Control group (n=40) | Observation group (n=40) | χ2 | p |
|---|---|---|---|---|
| Gastrointestinal reaction | 3 | 4 | ||
| Feel sick and vomit | 1 | 2 | ||
| Abnormal liver and kidney function | 0 | 1 | ||
| Skin symptoms | 1 | 0 | ||
| Mental symptoms | 1 | 1 | ||
| Stroke recurrence | 6 | 7 | ||
| Total incidence (%) | 30 | 37.5 | 0.503 | 0.478 |
Table 3: Comparison of Adverse Reactions
To date, the pathogenesis of early acute mild ischemic stroke has not been fully elucidated in clinical practice. Most studies suggest that the disease is caused by the combined effect of multiple factors[10,11]. Currently, no specific drugs have been discovered for the treatment of early acute ischemic stroke, so the clinical approach to treating this condition primarily focuses on reducing patient mortality and mitigating neurological damage[12]. Intravenous thrombolysis, arterial thrombectomy, and drug interventions are commonly used clinical methods for treating early acute mild ischemic stroke. However, the clinical application of these two treatment methods is limited due to factors such as the time window for thrombolysis and the technical requirements for arterial thrombectomy[13]. Given the limitations of thrombolysis or thrombectomy, antiplatelet therapy with medications has become the most commonly used measure in clinical practice. Aspirin and clopidogrel are both evidence-based antiplatelet drugs and studies have shown that the dual antiplatelet regimen of aspirin and clopidogrel can effectively improve the clinical symptoms of ischemic stroke patients and reduce the risk of disease recurrence[14]. Research conducted by Yang et al.[15] compared the efficacy and safety of dual antiplatelet therapy with single antiplatelet therapy in 4139 cases of early acute ischemic stroke or Transient Ischemic Attack (TIA) patients with a high risk of recurrence. The results showed that the dual antiplatelet therapy group had better clinical efficacy compared to the single antiplatelet therapy group, and the incidence of adverse reactions and the risk of stroke recurrence within 90 d were relatively lower in the dual antiplatelet therapy group. Subsequent relevant studies have further supported this conclusion[16]. The clinical efficacy results in this study are consistent with domestic and international research[17]. However, there are certain differences between this study and previous research in terms of adverse reactions and recurrence.
Aspirin is a commonly used derivative of salicylic acid in clinical practice. It effectively inhibits the activity of cyclooxygenase in platelets, thereby blocking the generation of thromboxane A2 and exerting effects such as inhibiting platelet aggregation and thrombus formation[18]. Clopidogrel is a platelet membrane adenosine diphosphate receptor inhibitor that effectively inhibits the generation of adenosine diphosphate, blocks platelet aggregation and binding to adenosine diphosphate and, activates and amplifies already aggregated platelets and adenosine diphosphate[19]. The combined use of these two drugs can further promote thrombus dissolution, inhibit platelet aggregation and adhesion, and thereby prevent thrombus formation[20]. Studies have confirmed the superior effectiveness and safety of dual antiplatelet therapy compared to monotherapy. However, there is relatively limited research on the impact of early application of dual antiplatelet therapy on neurological and cognitive function, hematological indicators and inflammatory markers in patients with acute ischemic stroke. In this study, the observation group showed superior improvement in neurocognitive function, indicating that the dual therapy approach resulted in more significant improvement in both neurological and cognitive functions compared to the monotherapy approach. Related research has shown that the blood viscosity of patients with ischemic stroke is significantly increased compared to the normal population, leading to persistent thrombotic status and subsequent secondary vascular and neural damage even after treatment[21]. FIB is one of the indicators reflecting high blood viscosity and patients in the acute phase of stroke generally exhibit abnormal FIB levels. Elevated levels of FIB further increase the risk of thrombus formation[22]. In this study, the observation group showed superior improvement in hematological parameters, indicating that early application of the dual therapy approach effectively improves the hypercoagulable state in patients with acute ischemic stroke. Related research has found that inflammatory reactions are involved in the occurrence and development of acute ischemic stroke and as the patient's condition progresses, the body's inflammatory response also intensifies[23]. Serum CRP, IL-6 and TNF-α are commonly seen inflammatory factors in clinical practice, and in patients with acute ischemic stroke, these inflammatory mediators are significantly elevated[24]. In this study, the observation group demonstrated superior improvement in inflammation markers, which may be attributed to the effective blockade of inflammatory factor release by the dual therapy approach, thereby further alleviating excessive expression of inflammatory responses in patient’s bodies.
In summary, compared to the single antiplatelet regimen, the dual antiplatelet regimen is more effective in treating early ischemic stroke. It can further promote the recovery of neurological and cognitive functions, improve hematology and inflammatory response indicators and its safety is comparable to the single antiplatelet regimen.
Ethics approval:
This study has been approved by Baozhang Hospital's ethics committee and, patients and their families were informed of the research content and voluntarily signed the informed consent. All the methods were carried out in accordance with the Declaration of Helsinki.
Authors' contributions:
Qiming Chen wrote the main manuscript text and Huanli Ma prepared figures and tables. All authors reviewed, read and approved the manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rabinstein AA. Update on treatment of acute ischemic stroke. Continuum 2020;26(2):268-86.
[Crossref] [Google Scholar] [PubMed]
- Stack CA, Cole JW. Ischemic stroke in young adults. Curr Opin Cardiol 2018;33(6):594-604.
[Crossref] [Google Scholar] [PubMed]
- Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med 2020;48(11):1654.
- Jolugbo P, Ariëns RA. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke 2021;52(3):1131-42.
- Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp Neurol 2021;335:113518.
[Crossref] [Google Scholar] [PubMed]
- Silva GS, Nogueira RG. Endovascular treatment of acute ischemic stroke. Continuum 2020;26(2):310-31.
[Crossref] [Google Scholar] [PubMed]
- Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379(3):215-25.
[Crossref] [Google Scholar] [PubMed]
- Pan Y, Elm JJ, Li H, Easton JD, Wang Y, Farrant M, et al. Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: A pooled analysis of clopidogrel in high-risk patients with acute non-disabling cerebrovascular events (CHANCE) and platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trials. JAMA Neurol 2019;76(12):1466-73.
[Crossref] [Google Scholar] [PubMed]
- Paciaroni M, Ince B, Hu B, Jeng JS, Kutluk K, Liu L, et al. Benefits and risks of clopidogrel vs. aspirin monotherapy after recent ischemic stroke: A systematic review and meta-analysis. Cardiovasc Ther 2019;2019:1607181.
[Crossref] [Google Scholar] [PubMed]
- Farina M, Vieira LE, Buttari B, Profumo E, Saso L. The Nrf2 pathway in ischemic stroke: A review. Molecules 2021;26(16):5001.
[Crossref] [Google Scholar] [PubMed]
- Ajoolabady A, Wang S, Kroemer G, Penninger JM, Uversky VN, Pratico D, et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol Ther 2021;225:107848.
[Crossref] [Google Scholar] [PubMed]
- Datta A, Sarmah D, Mounica L, Kaur H, Kesharwani R, Verma G, et al. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res 2020;11(6):1185-202.
[Crossref] [Google Scholar] [PubMed]
- Joundi RA, Menon BK. Thrombus composition, imaging, and outcome prediction in acute ischemic stroke. Neurology 2021;97(20):68-78.
[Crossref] [Google Scholar] [PubMed]
- Kheiri B, Osman M, Abdalla A, Haykal T, Swaid B, Ahmed S, et al. Clopidogrel and aspirin after ischemic stroke or transient ischemic attack: An updated systematic review and meta-analysis of randomized clinical trials. J Thromb Thrombolysis 2019;47(2):233-47.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Huang Z, Zhang X. Efficacy and safety of clopidogrel and/or aspirin for ischemic stroke/transient ischemic attack: An overview of systematic reviews and meta-analysis. Medicine 2021;100(50):e27804.
[Crossref] [Google Scholar] [PubMed]
- Rahman H, Khan SU, Nasir F, Hammad T, Meyer MA, Kaluski E. Optimal duration of aspirin plus clopidogrel after ischemic stroke or transient ischemic attack: A systematic review and meta-analysis. Stroke 2019;50(4):947-53.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Chen W, Lin Y, Meng X, Chen G, Wang Z, et al. Ticagrelor plus aspirin vs. clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: Open label, blinded endpoint, randomised controlled phase II trial. BMJ 2019;365:l2211.
[Crossref] [Google Scholar] [PubMed]
- Hao Q, Tampi M, O’Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin vs. aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: Systematic review and meta-analysis. BMJ 2018;363:k5108.
- Johnston SC, Elm JJ, Easton JD, Farrant M, Barsan WG, Kim AS, et al. Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke: A secondary analysis from the POINT randomized trial. Circulation 2019;140(8):658-64.
[Crossref] [Google Scholar] [PubMed]
- Albay CE, Leyson FG, Cheng FC. Dual vs. mono antiplatelet therapy for acute non-cardio embolic ischemic stroke or transient ischemic attack, an efficacy and safety analysis-updated meta-analysis. BMC Neurol 2020;20(1):224.
[Crossref] [Google Scholar] [PubMed]
- Wang A, Meng X, Tian X, Johnston SC, Li H, Bath PM, et al. Effect of hypertension on efficacy and safety of ticagrelor-aspirin vs. clopidogrel-aspirin in minor stroke or transient ischemic attack. Stroke 2022;53(9):2799-808.
[Crossref] [Google Scholar] [PubMed]
- Tancin Lambert A, Kong XY, Ratajczak-Tretel B, Atar D, Russell D, Skjelland M, et al. Biomarkers associated with atrial fibrillation in patients with ischemic stroke: A pilot study from the NOR-FIB study. Cerebrovasc Dis Extra 2020;10(1):11-20.
[Crossref] [Google Scholar] [PubMed]
- Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: Focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci 2020;21(18):6454.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Wang Q, Zhao J, Chang H, Zhu R. Inflammation-related circRNA polymorphism and ischemic stroke prognosis. J Mol Neurosci 2021;71:2126-33.
[Crossref] [Google Scholar] [PubMed]
 Observation group
Observation group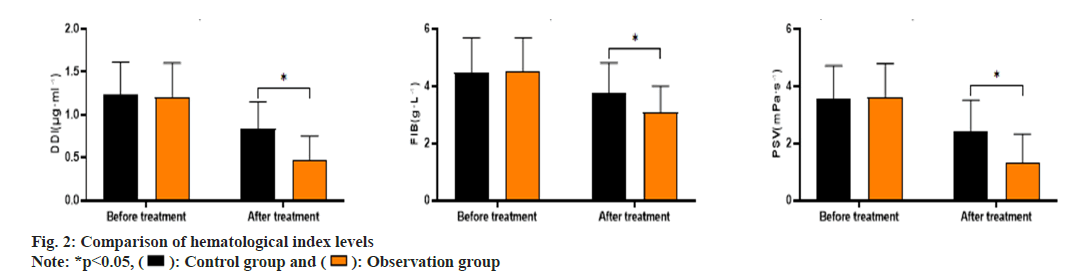
 Observation group
Observation group